Abstract
Introduction
Emicizumab is an effective new treatment option for people with hemophilia A (PwHA). The approved dosing regimens are based on body weight, without the necessity for laboratory monitoring. This assumes a clear dose–concentration–response relationship, with acceptable variability due to factors other than body weight. To investigate this assumption, a systematic review on the pharmacokinetics (PK) and associated efficacy of emicizumab in humans was conducted.
Methods
The EMBASE, Pubmed and CENTRAL databases were systematically searched to November 2020 to identify studies on the PK data of emicizumab in humans. Data on the study, population, PK and efficacy (annualized bleeding rate of treated [joint] bleeds) were extracted and synthesized, and exposure effects modeling was performed using non-linear least squares regression in a maximum effect (Emax) model.
Results
The 15 included studies reported on data for 140 volunteers and 467 PwHA, including children (0 to <12 years) and adolescents and adults (≥12 years), both with and without factor VIII (FVIII) inhibitors. Emicizumab demonstrated dose-linear PK. The interindividual variability of trough concentrations was moderate (32%) and was similar across various subgroups, such as FVIII inhibitor status, age group and dosing interval. The control of bleeds did not further improve above emicizumab concentrations of 30 µg/mL, potentially enabling lower dosing in a substantial proportion of PwHA.
Conclusion
This review supports body weight-based dosing, although individualized monitoring of emicizumab concentrations may allow for more cost-effective dosing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
After body weight-based dosing, emicizumab shows a linear dose–concentration relationship, with moderate interindividual variability. |
Bleeding control did not further improve by levels > 30 µg/mL, potentially enabling lower dosing in a substantial proportion of patients. |
1 Introduction
Hemophilia A is an inherited bleeding disorder caused by an absence or dysfunction of coagulation factor VIII (FVIII) [1]. Without adequate treatment, people with hemophilia A (PwHA) who are severely affected suffer from recurrent bleeds, predominantly in joints, which results in crippling arthropathy, functional limitations and a significantly reduced life expectancy [2, 3]. The primary goal in the management of these PwHA is to prevent bleeds, preferably through regular coagulation factor replacement therapy (prophylaxis) [1, 4]. Prophylaxis with plasma-derived and, later, recombinant FVIII products has effectively reduced episodes of bleeding from an annual average of 20−30 to 1−4 [5,6,7,8]. However, replacement therapy with FVIII products has some disadvantages. This treatment is invasive, requiring intravenous administration every 24–48 h, usually starting before the age of 2 years [9]. Additionally, neutralizing antibodies against FVIII (known as inhibitors) develop in 30% of severely affected PwHA, rendering treatment with FVIII products ineffective [4, 10].
Emicizumab (Hemlibra®) is the first non-factor replacement product and was approved in 2018 by the US FDA and the European Medicines Agency as prophylaxis for PwHA, both with and without FVIII inhibitors. This humanized, immunoglobulin (Ig) G4, bispecific monoclonal antibody (mAb) effectively restores the hemostatic function of missing FVIII by bridging activated factor IX and factor X. Moreover, subcutaneous administration and the less frequent dosing interval of once every 1−4 weeks offer dosing convenience, especially for (pediatric) PwHA with difficult venous access. Emicizumab has limited toxicity, although concomitant use of high doses of activated prothrombin complex concentrate increases the thrombotic risk and should be avoided [11, 12].
A body weight-based standard dosing regimen for emicizumab (1.5 mg/kg per week, 3 mg/kg per 2 weeks, 6 mg/kg per 4 weeks) without the requirement of dose adjustments based on laboratory monitoring has been approved by the regulatory authorities [13]. This dosing recommendation assumes clear dose−concentration [pharmacokinetics (PK)] and concentration−response [pharmacodynamics (PD)] relationships with acceptable variability due to factors other than body weight. Unexpected variability (e.g. resulting from antidrug antibodies or population characteristics) should be absent, as differences in concentration, and even response, require monitoring and individualized dose tailoring [14, 15]. Therefore, the objective of this study was to investigate these basic principles by conducting a systematic review on the PK and associated efficacy of emicizumab in humans.
2 Methods
2.1 Sources
The literature search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16]. The EMBASE, PubMed and Cochrane Central Register of Controlled Trials (CENTRAL) databases were searched from inception through 28 April 2020, and updated on 13 November 2020. The search terms included ‘emicizumab’ and ‘pharmacokinetics’ and their associated synonyms (see electronic supplementary data SD1 for an example of the EMBASE search algorithm. In the EMBASE database, the search was conducted using the corresponding Emtree terms, and for the PubMed and CENTRAL databases, the National Library of Medicine Medical Subject Heading (MeSH) search terms were used. Finally, the included studies were manually searched for relevant references, and the European Public Assessment Report (EPAR) for emicizumab was used as a cross-reference for possible missed studies [12].
2.2 Study Selection
The search results from the three databases were merged in Endnote X9 version 19.3.3.13966 (Clarivate Analytics, Philadelphia, PA, USA), and duplicate records (title, abstract, or both) were removed automatically. Hereafter, the records were imported into the web-based tool Rayyan (https://rayyan.qcri.org/ [17]) and were screened and categorized. First, the records and then the full-text articles were screened and categorized in duplicate by two reviewers (AD, LB). The following inclusion criteria were applied: emicizumab studies providing (1) data on humans, (2) original PK data or modeled PK data or PK/PD relationships, and (3) access to the abstract and the full text in English. In the event of doubt regarding eligibility, the records or articles were included. Disagreements were discussed until consensus was reached, and, when necessary, a third reviewer (TE) was consulted.
2.3 Data Extraction
The following data were extracted from the included studies: (1) study characteristics (authors, year of publication, number of subjects, phase, design, dosing regimen, follow-up, funding); (2) population characteristics (population [volunteer/PwHA], anti-FVIII antibodies, age group, hemophilia A severity, ethnicity); and (3) evaluated PK and efficacy data. Relevant primary PK parameters included the absorption rate constant (ka), apparent clearance (CL/F), and apparent volume of distribution (Vd/F), while secondary (exposure) PK parameters included terminal half-life (t½), area under the plasma concentration−time curve extrapolated to infinity (AUC∞), maximum plasma concentration (Cmax), trough plasma concentration in steady-state conditions (Ctrough,ss) and time to reach Cmax (tmax). The relevant efficacy parameter was expressed as the annualized bleeding rate (ABR) of different bleeding types (i.e. treated bleeds or treated joint bleeds). When relevant data were missing from the text, a data request was sent to the corresponding authors or sponsors.
Trough concentrations with corresponding errors [standard deviation (SD) and 95% confidence interval (CI)] from steady-state conditions per study subgroup were primarily extracted from the text; however, when data were presented in graphs only and the data requests remained unanswered, the data were extracted from the graphs [18,19,20]. To minimize bias, two independent and blinded reviewers (AD, LB) visually scored the data with magnification and a set square. The data were then scored using WebPlotDigitizer, a reliable and validated web-based tool for extracting underlying numerical data from plots (https://automeris.io/WebPlotDigitizer [21]) [22]. Both the visually and digitally extracted values were compared and, if necessary, reassessed. The data were entered (LB/AD) into a database for systematic data extraction and were double-checked by a second reviewer (AD/LB).
2.4 Data Synthesis
Extracted data relating to study and population characteristics were categorized and summarized. The PK data (Ctrough,ss) were plotted according to doses of the multiple-injection regimens in PwHA and for the single-injection regimens in volunteers (primary and secondary parameters). The percentage coefficient of variation (%CV, or the variability) of trough concentrations was calculated as (SD/mean) × 100%. If unavailable, the SD was derived from the 95% CI using the formula √n × ((upper limit − lower limit)/3.92), or from the interquartile range (IQR) using (IQRmax − IQRmin)/1.35 [23].
Exposure effects modeling was conducted with weighted (study size) non-linear least squares regression using a standard inhibitory maximum effect (Emax) model. The ABRs of treated bleeds and treated joint bleeds were used as efficacy parameters if calculated by means of negative binominal regression (model-based). As data for the extremes of the curve were lacking, values for baseline ABR and Emax, derived from other sources, were assumed and the Hill coefficient was fixed to 1. The baseline ABRs for treated bleeds and treated joint bleeds were assumed at values of 28.0 and 21.6, respectively, as reported for severe PwHA treated with on-demand therapy in a real-world setting [6]. These values were in line with the reported baseline ABRs for treated bleeds of 21.9, 23.3 and 38.2, and for treated joint bleeds of 6.7 and 26.5 in severe PwHA without prophylactic treatment [18, 19, 24]. The Emax for treated bleeds was set at a value of 0.96 (i.e. 96% drug effect at infinite exposure, corresponding to an ABR of 1), and for treated joint bleeds, the Emax was set at 0.98 (corresponding to an ABR of 0.5). This was done to account for the occurrence of traumatic bleeding (based on clinical experience) and the lack of complete of coagulation by emicizumab (based on mouse and primate models). In addition, the FVIII-equivalent (hemostatic) activity of emicizumab was hypothesized at only 10−20% in humans [24,25,26]. An ABR of 1 was therefore perceived as more realistic than an ABR of 0, and is also in line with the reported ABR range of 0.2−5.1 (electronic supplementary Table ST2). The ABR baseline values were also used to calculate the relative risk reduction (RRR). Sensitivity analysis was performed to assess the validity of the ABR baseline and Emax assumptions.
Summary statistics and graphics were performed using GraphPad Prism version 8.3.0 (GraphPad Software LLC, San Diego, CA, USA), and modeling was performed using R version 4.0.2 (R Core Team, Vienna, Austria).
3 Results
A total of 368 records were identified with the computerized search. After the removal of duplicates (n = 83), 285 titles and abstracts and 77 full-text articles were assessed for eligibility. The updated search in November 2020 yielded 15 studies meeting the inclusion criteria (electronic supplementary Fig. SF1).
3.1 Study Characteristics
The 15 included studies were published between 2016 and 2020 (Table 1). During the drugs’ development, phase I (n = 4), simultaneous phase I/II (n = 1) and phase III (n = 6) clinical studies were conducted in combination with two pharmacometric analyses reporting on the dose−response relationship, with data from the previously published studies. Two studies were conducted after market approval. The majority of the included studies were global multicenter (n = 8), open-label (n = 12), non-randomized (n = 8), non-controlled (n = 9), industry-funded (n = 13) clinical studies with a follow-up period of at least 16 weeks (n = 11). Eight studies had a parallel comparative design, including six reporting on an intra-individual comparison of emicizumab with previous treatment. The multiple-dose injection studies most often included the weekly dosing interval (QW; 75%), followed by the once every 4 weeks regimen (Q4W; 14%) and once every 2 weeks regimen (Q2W; 11%).
3.2 Population Characteristics
Eleven studies [18,19,20, 27,28,29,30,31,32,33,34] provided original PK data of emicizumab in humans and yielded 607 subjects (Table 2) after excluding four studies [24, 35,36,37] with double-reported subjects. These 607 unique subjects were either volunteers (n = 140) or PwHA (n = 467). The PwHA included adults and adolescents (n = 328) and children younger than 12 years (n = 139). The PwHA with and without FVIII inhibitors were similarly represented across studies. Severe hemophilia A and the Asian and Caucasian races were predominant across the studies.
3.3 Dose–Concentration Relationship
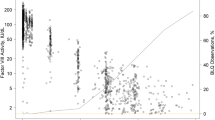
The original PK data were used to investigate the dose−concentration relationship, therefore excluding two pharmacometric analysis studies (reused data), two preliminary studies (shorter follow-up period than their consecutive studies) and a case report (no steady-state conditions). The PK data from the PwHA (n = 469) demonstrated a linear dose–concentration relationship when the Ctrough,ss was plotted according to increasing doses of emicizumab per week (Fig. 1). The longer dose intervals (Q2W and Q4W) were associated with a lower Ctrough,ss.
Linear dose–concentration relationship of emicizumab in PwHA. The mean or median Ctrough,ss according to increasing doses of emicizumab per week (mg/kg/week). Q2W and Q4W intervals shown per week. Data from PwHA (n = 469) receiving multiple dose regimens were included (see footnote ‘a’ for 15 study subgroups, in electronic supplementary Table ST2). PwHA people with hemophilia A, Ctrough,ss trough plasma concentration in steady-state conditions, Q2W 2-weekly dose interval, Q4W 4-weekly dose interval
The PK data from the volunteers (n = 112) in the single-dose injection studies with increasing doses (0.1, 0.3 and 1 mg/kg) demonstrated constant primary PK parameters and a dose-linear increase in the exposure metrics Cmax and AUC∞, with an exponential decay over time. These PK parameters obtained per study subgroup are presented in electronic supplemental Fig. SF2 and electronic supplementary Table ST1 and were similar across ethnicities.
The study size weighted variability (%CV) of the Ctrough,ss was similar across FVIII inhibitor status (present or absent) and the various dosing intervals (QW, Q2W or Q4W), whereas children had slightly less variability than adults and adolescents (Fig. 2). The overall weighted %CV of the Ctrough,ss in PwHA was 32% and ranged from 17 to 44% (electronic supplementary Table ST2).
Trough concentrations of emicizumab with variability across various subgroups in PwHA. The weighted variability (%CV) of the Ctrough,ss of emicizumab was similar across FVIII inhibitor status and across various dosing intervals, whereas children had slightly less variability than adults/adolescents. The overall weighted %CV was 32%. Data from PwHA (n = 469) receiving multiple dose regimens were included (see footnote ‘a’ for 15 study subgroups, in electronic supplementary Table ST2). PwHA people with hemophilia A, %CV percentage coefficient of variation, Ctrough,ss trough plasma concentration in steady-state conditions, Q2W 2-weekly dose interval, Q4W 4-weekly dose interval
3.4 Concentration–Response Relationship
Table 3 shows the relevant parameter estimates from the published population PK models on emicizumab. The model by Yoneyama et al. was based on PK data from the phase I and I/II studies to establish the dosing regimens, and the model by Retout et al. was based on the long-term phase I/II and phase III HAVEN 1−4 studies [24, 36]. The total PK interindividual variability (approximately 60% [36]) could be explained primarily by the covariates body weight, neutralizing antidrug antibodies (ADAs) against emicizumab, age > 30 years, and, to a lesser extent, abnormal albumin (ALB) levels, as well as African race.
Eleven PwHA study subgroups within five studies [18,19,20, 31, 32] provided the model-based mean ABRs of treated bleeds. The Ctrough,ss was the only exposure metric that could be extracted in combination with these ABRs. The ABRs were fitted with corresponding Ctrough,ss values in an Emax model (Fig. 3). The half maximal effective concentration (EC50) was estimated at 1.47 µg/mL [standard error (SE) 0.90], assuming an ABR baseline of 28.0 and Emax of 0.96. The effectiveness plateau of the concentration–response relationship was clearly established, and all ABR observations resided herein. Based on this model, a Ctrough,ss of 30 and 50 µg/mL would result in an ABR of treated bleeds of 2.4 and 1.9, respectively, and with an RRR of 91% and 93%, respectively. A second Emax model was fitted with the ABRs of treated joint bleeds, instead of treated bleeds, with corresponding Ctrough,ss values (electronic supplementary Fig. SF3). The EC50 was estimated at 1.09 µg/mL (SE 0.36), assuming baseline ABR of 21.6 and Emax of 0.98, and Ctrough,ss values of 30 and 50 µg/mL would result in ABRs of treated joint bleeds of 1.1 and 1.0, respectively, and with an RRR of 94.9% and 95.4%, respectively.
Emax model with concentration–response relationship of emicizumab in PwHA. Fit of an Emax model of ABRs of treated bleeds according to the Ctrough,ss of emicizumab in PwHA (n = 349), described by \({\text{ABR}} = {\text{ABR}}_{\text{baseline}} \times \left( {1 - (\frac{{(C_{{{\text{trough}},{\text{ss}}}} \times E_{{\max }} )}}{{({\text{C}}_{{{\text{trough}},{\text{ss}}}} ~ + {\text{EC}}_{{50}} )}}} \right)\). Included ABRs were model-based, estimated using negative binominal regression. The EC50 was estimated at 1.47 µg/mL (SE 0.90) and the effectiveness plateau was established. The dashed line is the 95% confidence interval, and symbol size equals study size. Data from PwHA receiving maintenance were included (see footnote ‘b’ for 11 study subgroups, in electronic supplementary Table ST2). Emax maximum effect, PwHA people with hemophilia A, ABRs annualized bleeding rates, Ctrough,ss trough plasma concentration in steady-state conditions, EC50 half maximal effective concentration, SE standard error
For Ctrough,ss of 30 and 50 µg/mL, sensitivity analyses showed ABRs of treated bleeds of 2.9 and 1.9, respectively, with a conservative setting (baseline ABR 21.9 and Emax 1 [24]), and ABRs of 1.9 and 1.9, respectively, with a more liberal setting (baseline ABR 38.2 and Emax 0.90 [18]) [see electronic supplementary Table ST3]. In addition, the Ctrough,ss of 30 and 50 µg/mL showed in sensitivity analyses ABRs of treated joint bleeds of 1.4 and 0.9, respectively, with a conservative setting (baseline ABR 6.7 and Emax 1 [18]), and ABRs of 1.0 and 0.9, respectively, with a more liberal setting (baseline ABR 26.5 and Emax 0.97 [19]).
4 Discussion
For emicizumab in humans, this systematic review demonstrated a linear dose–concentration relationship with moderate interindividual variability, with all ABR observations residing in the effectiveness plateau of this concentration–response relationship.
4.1 Dose–Concentration Relationship
With increasing doses of emicizumab, the primary PK parameters remained constant, while the exposure metrics exhibited a linear dose increase. These findings are in line with the results of the model by Retout et al., who showed a dose-proportional AUC increase due to constant clearance [36]. Compared with the primary PK parameters of other therapeutic IgG4-based mAbs, the clearance of emicizumab was comparable (within 0.2−0.5 L/day), but distribution volumes were increased (10 vs. 6 L), and, consequently, the reported half-life of emicizumab is longer [38]. This higher volume of distribution is notable, indicating distribution to other compartments or binding of emicizumab, possibly to its targets in the blood circulation. In general, therapeutic mAbs demonstrate linear PK at high doses when the target-saturated concentration is achieved [39, 40], and this was already observed at doses of ≥ 0.1 mg/kg for emicizumab (electronic supplementary Fig. SF2).
The sources for variability of the PK parameters were explored in the two published population PK modeling studies, in which body weight and neutralizing ADAs against emicizumab were identified as influential covariates [24, 36]. A body weight-based dosing regimen was justified because including body weight in the model reduced the interindividual variability in CL/F from 56.4 to 30.0%, and in V/F from 60.7 to 28.1% [36]. Additionally, Retout et al. reported that age and ALB concentration were significantly correlated to primary PK parameters in their model. For age > 30 years, bioavailability gradually decreased, and for age > 65 years, bioavailability strongly decreased (e.g. a 31% lower exposure for a PwHA aged 77 years than for a PwHA aged 30 years), which is uncommon for therapeutic mAbs [41]. Although the authors did not report a relationship with clinical response, PwHA older than 65 years may be more susceptible to lower emicizumab concentrations, potentially even reducing bleeding control in a small proportion of PwHA. A low ALB concentration of 33 g/L was associated with a 16% decrease in exposure [36].
The PK variability across several studies has been previously described in modeling studies, but this review is the first to investigate variability across seven studies. We have reported slightly higher %CV for adults and adolescents compared with children. This finding is in line with the considerable influence of the covariate ‘age’ on exposure identified by Retout et al. [36]. Therefore, we recommend to investigate the bioavailability and exposure in this patient subgroup (i.e. aged > 30 years, especially > 65 years) in future studies.
4.2 Concentration–Response Relationship
Our search did not yield individual patient data, making it unfeasible to use a complex model, such as repeated time-to-event. However, the estimated EC50 of 1.47 µg/mL for treated bleeds was close to the previously reported values of 1.19 μg/mL [24] and 3.58 µg/mL [42] obtained by more sophisticated models, endorsing our model. The Ctrough,ss of 51.1 µg/mL obtained by the approved standard dosing regimens is 35-fold this EC50, which is abundant [13]. Our Emax model visualized the concentration–response relationship and revealed a clear effectiveness plateau from 30 µg/mL, beyond which no further increase in beneficial drug effect was achieved. All the bleeding rate observations of the included studies were well within this plateau, potentially enabling lower dosing for a substantial proportion of the PwHA.
Unquestionably, setting other values for Emax and baseline ABRs would result in other predictions. A sensitivity analyses was performed (see electronic supplementary Table ST3) to show the impact of the ABR baseline and Emax assumptions on the estimates of EC50 and the ABRs for Ctrough,ss of 30 μg/mL and 50 μg/mL. In this regard the estimates remained approximately the same, indicating robustness. Furthermore, the EC50 decreased fivefold when the ABRs (outliers) from HAVEN 1 were excluded from the model. We hypothesized that many subjects from this study had long-term inhibitors, and, by definition, had severe arthropathy, which is something to keep in mind for physicians. The ABR of treated joint bleeds was also explored because treated joint bleeds are generally better defined, reducing misclassification [43,44,45,46]. In the Emax model of treated joint bleeds, the effectiveness plateau was reached at even lower concentrations of 20 µg/mL.
4.3 Dosing and Monitoring Considerations
The question that remains is to what extent are we overdosing our PwHA? Based on our Emax models, a Ctrough,ss of 30 and 50 µg/mL would result in ABRs of treated bleeds of 2.4 and 1.9, respectively (RRRs of 91% and 93%, respectively) and ABRs of treated joint bleeds of 1.1 and 1.0, respectively (RRRs of 94.9% and 95.4%, respectively). Are the theoretic ABR differences of 0.5 and 0.1 clinically relevant while the RRRs remain essentially equal? Understandably, manufacturers design dosing regimens without laboratory monitoring because this is user friendly and robust. Moderate interindividual variability in PK without monitoring necessitates higher dosing to guarantee efficacy for all users. In combination with an absence of toxicity at high drug concentrations, which is the case for most therapeutic mAbs, this makes overdosing clinically not problematic. Nonetheless, concerns were expressed by Hooimeijer et al., who reported joint pain episodes in a PwHA at high emicizumab concentrations of 90 µg/mL, which resolved after dose reduction to concentrations of approximately 30 µg/mL [33]. Furthermore, when drugs are costly (the wholesale acquisition cost of emicizumab is approximately US$482,000 for the first year of treatment and US$448,000 for the following years), monitoring and subsequent dose reduction may lead to substantial savings in health care [47]. The weighted overall variability of the Ctrough,ss was 32%, which is common for therapeutic mAbs. Notably, individual Ctrough,ss ranged widely from 2.8 to 148 μg/mL [18]. No specific loss of efficacy or adverse effects for these extreme individual cases were described. As more studies presented subjects with plasma concentrations < 20 μg/mL (electronic supplementary Table ST2), we propose a beneficial role for Therapeutic Drug Monioting (TDM) to increase the dose to an efficacious concentration. In addition, we recommend conducting a prospective, individual PK-guided dosing study to target an efficacious trough concentration of 30 μg/mL, and to collect data on both interindividual and interoccasion variability in the real-world setting to optimize efficacy, safety, and cost effectiveness of emicizumab treatment.
Laboratory monitoring with PD biomarkers has been unsuccessful, and functional assays should be interpreted with caution and as relative indications of the procoagulant potential [34, 48]. Until proper functional tests become available, the emicizumab concentration appears to be the best predictor for bleeding risk [24]. The concentration is not routinely monitored in clinical practice, although monitoring may be useful for research purposes, to check adherence, or in case of suspected neutralizing ADA against emicizumab [49]. The emicizumab concentration was measured in PK samples during phase I−III clinical studies using an enzyme-linked immunosorbent assay (ELISA), and during phase IV studies using a modified, calibrated one-stage clotting assay (OSA). Unfortunately, Roche has not provided access to the ELISA, while the OSA is only available at specialized laboratories, as it needs to be calibrated by a standardized kit of two reference values [50]. An assay capable of measuring emicizumab concentrations in human plasma on routinely available platforms, such as liquid chromatography–tandem mass spectrometry (LC–MS/MS), would be valuable. An LC–MS/MS method for the quantification of emicizumab has been developed and validated at the University Medical Center Utrecht [51].
4.4 Limitations and Strengths
This systematic review was limited by the number of available studies, the inclusion of funded studies, a heterogeneous population, limited study sizes, and lack of studies with a blinded, placebo-controlled, head-to-head design owing to the rarity and severity of hemophilia A. There is some uncertainty in our findings, particularly in the estimated ABRs at Ctrough,ss of 30 μg/mL and 50 μg/mL, as a consequence of limited availability of data at lower and higher emicizumab concentrations. A search in the ClinicalTrials.gov database in January 2021 identified at least three relevant ongoing studies on the PK of emicizumab (i.e. HAVEN 5−7), of which two are still recruiting PwHA. Therefore, it is certain that the present review will require an update in the near future. Another difficulty was the risk-of-bias assessment in the included studies due to a lack of standardized tools for such PK studies, in contrast to the quality assessment tools used for (randomized) clinical studies. However, the data used were considered methodologically sound as PK parameters are objective measurements, studies were included using a systematic PRISMA search approach, and we only used, for the Emax model, negative binomial mean ABRs (excluding the median ABRs [28, 34, 35]) and excluded the studies with baseline imbalances [28, 35].
The ABR is not an entirely objective outcome parameter. Misinterpretation of bleeds might have occurred, as verification by the physician and complementary imaging were often missing. Subjective assessments, combined with follow-up periods of < 12 months and small study sizes, may have affected the calculated ABRs. Moreover, clinically unstable disease leads to numerous (spontaneous) bleeds, especially in the first weeks of emicizumab treatment, leading to overestimation of ABRs in shorter studies [43,44,45,46]. Recently, the analysis of pooled bleeding data from HAVEN 1−4 reported ABRs maintaining < 1 in 24-week intervals and an increase in the proportion of PwHA without treated bleeds from 70.8% in the first 6 months to 80.2% after 1 year of emicizumab treatment [52]. Consequently, predicted ABRs may be overestimated in our model.
The strength of this review was the large amount of information that has been summarized in tables and graphs. The novelty of this review was the critical appraisal by an independent research group, the dosing and monitoring considerations, and the proposed role for TDM in relation to low concentrations and cost effectiveness. This information may offer guidance in clinical decision making and in future study designs assessing (cost) effectiveness, safety, and PK/PD modeling studies [53].
5 Conclusion
This systematic review provided a comprehensive overview of PK and associated efficacy data for emicizumab in humans. Emicizumab demonstrated a clear linear dose−concentration profile with moderate interindividual variability. The control of bleeds did not further improve above emicizumab concentrations of 30 µg/mL, potentially enabling lower dosing in a substantial proportion of PwHA. In conclusion, this review supports body weight-based dosing, although individualized monitoring of emicizumab concentrations may allow for more cost-effective dosing.
References
Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935–9.
Darby SC, Sau WK, Spooner RJ, Giangrande PLF, Hill FGH, Hay CRM, Lee CA, Ludlam CA, Williams M. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110:815–25.
van Vulpen LFD, Holstein K, Martinoli C. Joint disease in haemophilia: pathophysiology, pain and imaging. Haemophilia. 2018;24(Suppl 6):44–9. https://doi.org/10.1111/hae.13449.
Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158. https://doi.org/10.1111/hae.14046.
Mannucci PM, Tuddenham EGD. The hemophilias—from royal genes to gene therapy. N Engl J Med. 2002;344:1773–9.
Ay C, Perschy L, Rejtö J, Kaider A, Pabinger I. Treatment patterns and bleeding outcomes in persons with severe hemophilia A and B in a real-world setting. Ann Hematol. 2020;99(12):2763–71. https://doi.org/10.1007/s00277-020-04250-9.
Rayment R, Chalmers E, Forsyth K, Gooding R, Kelly AM, Shapiro S, Talks K, Tunstall O, Biss T. Guidelines on the use of prophylactic factor replacement for children and adults with Haemophilia A and B. Br J Haematol. 2020;190(5):684–95. https://doi.org/10.1111/bjh.16704.
Berntorp E, Dolan G, Hay C, et al. European retrospective study of real-life haemophilia treatment. Haemophilia. 2017;23(1):105–14. https://doi.org/10.1111/hae.13111.
Beeton K, Neal D, Watson T, Lee CA. Parents of children with haemophilia - A transforming experience. Haemophilia. 2007;13(5):570–9. https://doi.org/10.1111/j.1365-2516.2007.01494.x.
Van Den Berg HM, Fischer K, Carcao M, Chambost H, Kenet G, Kurnik K, Königs C, Male C, Santagostino E, Ljung R. Timing of inhibitor development in more than 1000 previously untreated patients with severe hemophilia A. Blood. 2019;134(3):317–20. https://doi.org/10.1182/blood.2019000658.
Rodriguez-Merchan EC, Valentino LA. Emicizumab: Review of the literature and critical appraisal. Haemophilia. 2019;25(1):11–20. https://doi.org/10.1111/hae.13641.
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Assessment Report—Emicizumab (Hemlibra). EMA/125963/2019. European Medicines Agency; 31 Jan 2019
Hemlibra (emicizumab) summary of product characteristics. Basel; F. Hoffmann-La Roche; 2018
Powell JR, Cook J, Wang Y, Peck R, Weiner D. Drug dosing recommendations for all patients: a roadmap for change. Clin Pharmacol Ther. 2021;109(1):65–72. https://doi.org/10.1002/cpt.1923.
Atkinson AJ, Lalonde RL. Introduction of quantitative methods in pharmacology and clinical pharmacology: a historical overview. Clin Pharmacol Ther. 2007;82(1):3–6. https://doi.org/10.1038/sj.clpt.6100248.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4.
Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–18. https://doi.org/10.1056/nejmoa1703068.
Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811–22. https://doi.org/10.1056/nejmoa1803550.
Shima M, Nogami K, Nagami S, Yoshida S, Yoneyama K, Ishiguro A, Suzuki T, Taki M. A multicentre, open-label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors. Haemophilia. 2019;25(6):979–87. https://doi.org/10.1111/hae.13848.
Rohatgi A. Webplotdigitizer: Version 4.4. 2020.
Moeyaert M, Maggin D, Verkuilen J. Reliability, validity, and usability of data extraction programs for single-case research designs. Behav Modif. 2016;40(6):874–900. https://doi.org/10.1177/0145445516645763.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. 2nd ed. New York: Wiley; 2019. https://doi.org/10.1002/9781119536604.
Yoneyama K, Schmitt C, Kotani N, Levy GG, Kasai R, Iida S, Shima M, Kawanishi T. A pharmacometric approach to substitute for a conventional dose-finding study in rare diseases: example of phase III dose selection for emicizumab in hemophilia A. Clin Pharmacokinet. 2018;57(9):1123–34. https://doi.org/10.1007/s40262-017-0616-3.
Ferrière S, Peyron I, Christophe OD, Kawecki C, Casari C, Muczynski V, Nathwani A, Kauskot A, Lenting PJ, Denis CV. A hemophilia A mouse model for the in vivo assessment of emicizumab function. Blood. 2020;136(6):740–8. https://doi.org/10.1182/blood.2019004334.
Lenting PJ. Laboratory monitoring of hemophilia A treatments: new challenges. Blood Adv. 2020;4(9):2111–8. https://doi.org/10.1182/bloodadvances.2019000849.
Uchida N, Sambe T, Yoneyama K, Fukazawa N, Kawanishi T, Kobayashi S, Shima M. A first-in-human phase 1 study of ACE910, a novel factor VIII-mimetic bispecific antibody, in healthy subjects. Blood. 2016;127(13):1633–41. https://doi.org/10.1182/blood-2015-06-650226.
Shima M, Hanabusa H, Taki M, Matsushita T, Sato T, Fukutake K, Kasai R, Yoneyama K, Yoshida H, Nogami K. Long-term safety and efficacy of emicizumab in a phase 1/2 study in patients with hemophilia A with or without inhibitors. Blood Adv. 2017;1(22):1891–9. https://doi.org/10.1182/bloodadvances.2017006684.
Kotani N, Yoneyama K, Kawakami N, Shimuta T, Fukase H, Kawanishi T. Relative and absolute bioavailability study of emicizumab to bridge drug products and subcutaneous injection sites in healthy volunteers. Clin Pharmacol Drug Dev. 2019;8(6):702–12. https://doi.org/10.1002/cpdd.617.
Li H, Zhang W, Petry C, et al. Evaluation of the pharmacokinetics, pharmacodynamics, and safety of a single dose of emicizumab in healthy Chinese subjects. Clin Pharmacol Drug Dev. 2021;10(1):30–8. https://doi.org/10.1002/cpdd.805.
Young G, Liesner R, Chang T, et al. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134(24):2127–38. https://doi.org/10.1182/blood.2019001869.
Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019;6(6):e295–305. https://doi.org/10.1016/S2352-3026(19)30054-7.
Hooimeijer HL, Lukens MV, Verhagen MV, Meijer K, Stein-Wit MA, Tamminga RYJ. A boy with joint pain associated with emicizumab treatment: the importance of plasma level measurement. Haemophilia. 2020;26(3):e138–40. https://doi.org/10.1111/hae.13965.
Barg AA, Livnat T, Budnik I, Avishai E, Brutman-Barazani T, Tamarin I, Bashari D, Misgav M, Kenet G. Emicizumab treatment and monitoring in a paediatric cohort: real-world data. Br J Haematol. 2020;191(2):282–90. https://doi.org/10.1111/bjh.16964.
Shima M, Hanabusa H, Taki M, Matsushita T, Sato T, Fukutake K, Fukazawa N, Yoneyama K, Yoshida H, Nogami K. Factor VIII–mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016. https://doi.org/10.1056/nejmoa1511769.
Retout S, Schmitt C, Petry C, Mercier F, Frey N. Population pharmacokinetic analysis and exploratory exposure-bleeding rate relationship of emicizumab in adult and pediatric persons with hemophilia A. Clin Pharmacokinet. 2020;59(12):1611–25. https://doi.org/10.1007/s40262-020-00904-z.
Schmitt C, Adamkewicz JI, Xu J, Petry C, Catalani O, Young G, Negrier C, Callaghan MU, Levy GG. Pharmacokinetics and pharmacodynamics of emicizumab in persons with hemophilia A with factor VIII inhibitors: HAVEN 1 study. Thromb Haemost. 2020;121(3):351–60. https://doi.org/10.1055/s-0040-1717114.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633–59. https://doi.org/10.2165/11535960-000000000-00000.
Kamath AV. Translational pharmacokinetics and pharmacodynamics of monoclonal antibodies. Drug Discov Today Technol. 2016;21–22:75–83. https://doi.org/10.1016/j.ddtec.2016.09.004.
Viola M, Sequeira J, Seiça R, Veiga F, Serra J, Santos AC, Ribeiro AJ. Subcutaneous delivery of monoclonal antibodies: how do we get there? J Control Release. 2018;286:301–14. https://doi.org/10.1016/j.jconrel.2018.08.001.
Gill KL, Machavaram KK, Rose RH, Chetty M. Potential sources of inter-subject variability in monoclonal antibody pharmacokinetics. Clin Pharmacokinet. 2016;55(7):789–805. https://doi.org/10.1007/s40262-015-0361-4.
Jonsson F, Schmitt C, Petry C, Mercier F, Frey N, Retout S. Exposure-bleeding count modeling of emicizumab for the prophylaxis of bleeding in persons with hemophilia A with/without inhibitors against factor VIII. Clin Pharmacokinet. 2021. https://doi.org/10.1007/s40262-021-01006-0.
Keipert C, Müller-Olling M, Gauly F, Arras-Reiter C, Hilger A. Annual bleeding rates: pitfalls of clinical trial outcomes in hemophilia patients. Clin Transl Sci. 2020;13(6):1127–36. https://doi.org/10.1111/cts.12794.
Ceponis A, Wong-Sefidan I, Glass CS, von Drygalski A. Rapid musculoskeletal ultrasound for painful episodes in adult haemophilia patients. Haemophilia. 2013;19(5):790–8. https://doi.org/10.1111/hae.12175.
Kidder W, Nguyen S, Larios J, Bergstrom J, Ceponis A, von Drygalski A. Point-of-care musculoskeletal ultrasound is critical for the diagnosis of hemarthroses, inflammation and soft tissue abnormalities in adult patients with painful haemophilic arthropathy. Haemophilia. 2015;21(4):530–7. https://doi.org/10.1111/hae.12637.
Berro M, Elichiry M, Wasen K, Insagaray J, Rodríguez I. Use of ultrasound for evaluation of painful joint episodes perceived as haemarthrosis in adult patients with severe haemophilia. Haemophilia. 2018;24(3):e124–5. https://doi.org/10.1111/hae.13439.
Rind D. Emicizumab for hemophilia A with inhibitors: effectiveness and value. Boston: ICER; 2018.
Nardi MA. Emicizumab and the clinical laboratory. Am Soc Clin Lab Sci. 2020. https://doi.org/10.29074/ascls.119.002204.
Coppola A, Castaman G, Santoro RC, et al. Management of patients with severe haemophilia a without inhibitors on prophylaxis with emicizumab: AICE recommendations with focus on emergency in collaboration with SIBioC, SIMEU, SIMEUP, SIPMeL and SISET. Haemophilia. 2020;26(6):937–45. https://doi.org/10.1111/hae.14172.
Bowyer A, Kitchen S, Maclean R. Effects of emicizumab on APTT, one-stage and chromogenic assays of factor VIII in artificially spiked plasma and in samples from haemophilia A patients with inhibitors. Haemophilia. 2020;26:536–42.
Donners A, Gerencsér L, van der Elst K, Fischer K, Urbanus R, El Amrani M. Mass spectrometry for the quantification of emicizumab in plasma of haemophilia A patients. Manuscr. Prep.
Callaghan MU, Negrier CG, Paz-Priel I, et al. Long-term outcomes with emicizumab prophylaxis for hemophilia A with/without FVIII inhibitors from the HAVEN 1–4 studies. Blood. 2021;137(16):2231–42. https://doi.org/10.1182/blood.2020009217.
Siddaway AP, Wood AM, Hedges LV. How to do a systematic review: a best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annu Rev Psychol. 2019;70:747–70. https://doi.org/10.1146/annurev-psych-010418-102803.
Acknowledgements
The authors would like to acknowledge Rolf Urbanus for his thoughtful involvement, and Erik van Maarseveen for his important contribution to this review in an early phase.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no funding for this review.
Conflict of interest/Competing interest
AD, CR, LB, AH, RS, TE, KF declare no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Original data are available upon request.
Code availability
Not applicable.
Author contributions
TE conceived the topic of the manuscript; AD and LB performed the literature search and data analysis; AH and AD performed exposure-effects modeling; AD drafted the manuscript, and KF, AH, LB, RS, CR, and TE critically revised the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Donners, A.A.M.T., Rademaker, C.M.A., Bevers, L.A.H. et al. Pharmacokinetics and Associated Efficacy of Emicizumab in Humans: A Systematic Review. Clin Pharmacokinet 60, 1395–1406 (2021). https://doi.org/10.1007/s40262-021-01042-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01042-w