Abstract
In biomedical diagnostics, surface plasmon resonance (SPR) sensors have emerged as one of the most valuable instruments, particularly for identifying diverse biomarkers such as cancer. Here, we propose a novel layered SPR design that makes use of the optoelectronic properties of ferric oxide (Fe2O3), carbon nanotubes (CNTs), and bi-metallic (silver and platinum) materials to enhance the sensitivity and other performance metrics of SPR-based cancer detection. Because of its increased sensitivity to even minute variations in biomarker concentrations, the suggested sensor may prove helpful in the early identification and monitoring of cancer. The RI for different types of malignant cells varied from 1.392 to 1.401. The acquired numerical data indicate that the MCF-7 cell has the highest sensitivity of 320.571 deg/RIU. The highest values calculated for the characteristic’s parameters are 0.54 deg−1 detection accuracy, 174.129 RIU−1 figure of merit, and the lowest full width half maximum value of 1.841 deg, all for MCF-7 cells.
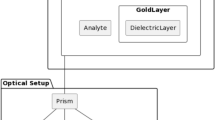
Graphical abstract
Surface plasmon resonance (SPR) sensors have become one of the most important tools in biomedical diagnostics, especially for the identification of various indicators like cancer. Here, we present a novel layered SPR design that improves the sensitivity and other performance metrics of SPR-based cancer detection by using the optoelectronic capabilities of carbon nanotubes (CNTs), ferric oxide (Fe2O3), and bi-metallic (silver and platinum) materials. The proposed sensor may be useful for early cancer detection and monitoring due to its enhanced sensitivity to even small changes in biomarker concentrations. The refractive index (RI) alterations of cancer cells are measured using the angle interrogation technique. Six distinct malignant cell types had RIs ranging from 1.392 to 1.401. According to the obtained numerical data, the MCF-7 cell has the maximum sensitivity, measuring 320.571 deg/RIU. The characteristic's parameters for MCF-7 cells have been computed to yield the greatest values: 0.54 deg-1 detection accuracy, 174.129 RIU-1 figure of merit, and the lowest full width at half maximum value of 1.841 deg.









Similar content being viewed by others
Data availability
No data available.
Code availability
Not applicable.
References
R.L. Siegel, K.D. Miller, H.E. Fuchs, A. Jemal, Cancer statistics, 2022. CA Cancer J. Clin. 72(1), 7–33 (2022). https://doi.org/10.3322/caac.21708
R. Ramer, B. Hinz, Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J. Natl. Cancer Inst. 100(1), 59–69 (2008). https://doi.org/10.1093/jnci/djm268
R. Cailleau, R. Young, M. Olivé, W.J. Reeves, Breast tumor cell lines from pleural effusions2. JNCI. J. Natl. Cancer Inst. 53(3), 661–674 (1974). https://doi.org/10.1093/jnci/53.3.661
M.H. Sani, S. Khosroabadi, A novel design and analysis of high-sensitivity biosensor based on nano-cavity for detection of blood component, diabetes, cancer and glucose concentration. IEEE Sens. J. 20(13), 7161–7168 (2020). https://doi.org/10.1109/JSEN.2020.2964114
V.C. Diculescu, A.M.O. Brett, DNA-electrochemical biosensors and oxidative damage to DNA: application to cancer. Biosens. Cancer (2012). https://doi.org/10.1201/b12737-15
R. Peltomaa, B. Glahn-Martínez, E. Benito-Peña, M.C. Moreno-Bondi, Optical biosensors for label-free detection of small molecules. Sensors (Basel) 18(12), 4126 (2018). https://doi.org/10.3390/s18124126
B. Hossain, A.K. Paul, A. Islam, M. Rahman, A.K. Sarkar, L.F. Abdulrazak, A highly sensitive surface plasmon resonance biosensor using snse allotrope and heterostructure of BlueP/MoS2 for cancerous cell detection. Optik (Stuttg) (2021). https://doi.org/10.1016/j.ijleo.2021.168506
A.N. Yaroslavsky, X. Feng, S.H. Yu, P.R. Jermain, T.W. Iorizzo, V.A. Neel, Dual-wavelength optical polarization imaging for detecting skin cancer margins. J. Invest. Dermatol. 140(10), 1994-2000.e1 (2020). https://doi.org/10.1016/j.jid.2020.03.947
S. Firdous, S. Anwar, R. Rafya, Development of surface plasmon resonance (SPR) biosensors for use in the diagnostics of malignant and infectious diseases. Laser Phys. Lett. 15(6), 065602 (2018). https://doi.org/10.1088/1612-202X/aab43f
M.A. Islam et al., Design and analysis of go coated high sensitive tunable SPR sensor for OATR spectroscopic biosensing applications. IEEE Access 10, 103496–103508 (2022). https://doi.org/10.1109/ACCESS.2022.3211099
R. Kumar, S. Pal, Y.K. Prajapati, J.P. Saini, Sensitivity enhancement of MXene based SPR sensor using silicon: theoretical analysis. SILICON (2021). https://doi.org/10.1007/s12633-020-00558-3/Published
L. He et al., Label-free femtomolar cancer biomarker detection in human serum using graphene-coated surface plasmon resonance chips. Biosens. Bioelectron. 89, 606–611 (2017). https://doi.org/10.1016/j.bios.2016.01.076
G. AlaguVibisha et al., Sensitivity enhancement of surface plasmon resonance sensor using hybrid configuration of 2D materials over bimetallic layer of Cu–Ni. Opt. Commun. 463, 125337 (2020). https://doi.org/10.1016/j.optcom.2020.125337
M. El-assar, T.E. Taha, F.E.A. El-Samie, H.A. Fayed, M.H. Aly, ZnSe-based highly-sensitive SPR biosensor for detection of different cancer cells and urine glucose levels. Opt. Quantum Electron. 55(1), 76 (2022). https://doi.org/10.1007/s11082-022-04326-y
O. Lazcka, F.J. Del Campo, F.X. Muñoz, Pathogen detection: A perspective of traditional methods and biosensors. Biosens. Bioelectron. 22(7), 1205–1217 (2007). https://doi.org/10.1016/j.bios.2006.06.036
F.-R. Li, Q. Li, H.-X. Zhou, H. Qi, C.-Y. Deng, “Detection of circulating tumor cells in breast cancer with a refined immunomagnetic nanoparticle enriched assay and nested-RT-PCR. Nanomed. Nanotechnol. Biol. Med. 9(7), 1106–1113 (2013). https://doi.org/10.1016/j.nano.2013.03.002
L. Hajba, A. Guttman, Circulating tumor-cell detection and capture using microfluidic devices. TrAC Trends. Anal. Chem. 59, 9–16 (2014). https://doi.org/10.1016/j.trac.2014.02.017
G. Verma, in Introducing Nanoscale: Its Realm and Attributes, ed. by G. Verma. Micro and Nano Technologies, Nanostructures (Elsevier, 2023), pp. 1–24. https://doi.org/10.1016/B978-0-12-820048-3.00013-9
M.A. Mollah, M. Yousufali, I.M. Ankan, M.M. Rahman, H. Sarker, K. Chakrabarti, Twin core photonic crystal fiber refractive index sensor for early detection of blood cancer. Sens. Bio-Sens. Res. 29, 100344 (2020). https://doi.org/10.1016/j.sbsr.2020.100344
A. Yasli, Cancer detection with surface plasmon resonance-based photonic crystal fiber biosensor. Plasmonics 16(5), 1605–1612 (2021). https://doi.org/10.1007/s11468-021-01425-6
D. Sun, Y. Ran, G. Wang, Label-free detection of cancer biomarkers using an in-line taper fiber-optic interferometer and a fiber bragg grating. Sensors 17(11), 2556 (2017). https://doi.org/10.3390/s17112559
A. Uniyal, B. Chauhan, A. Pal, Y. Singh, Surface plasmon biosensor based on Bi2Te3 antimonene heterostructure for the detection of cancer cells. Appl. Opt. 61(13), 3711–3719 (2022). https://doi.org/10.1364/AO.454789
M. Moznuzzaman, M.R. Islam, I. Khan, Effect of layer thickness variation on sensitivity: an SPR based sensor for formalin detection. Sens. Bio-Sens. Res. 32, 100419 (2021). https://doi.org/10.1016/j.sbsr.2021.100419
A. Farmani, A. Mir, Graphene sensor based on surface plasmon resonance for optical scanning. IEEE Photonics Technol. Lett. 31(8), 643–646 (2019). https://doi.org/10.1109/LPT.2019.2904618
V. Chabot, Y. Miron, M. Grandbois, P.G. Charette, Long range surface plasmon resonance for increased sensitivity in living cell biosensing through greater probing depth. Sens. Actuators, B Chem. 174, 94–101 (2012). https://doi.org/10.1016/j.snb.2012.08.028
M. Pandaram, S. Santhanakumar, R. Veeran, R.K. Balasundaram, R. Jha, Z. Jaroszewicz, Platinum layers sandwiched between black phosphorous and graphene for enhanced SPR sensor performance. Plasmonics 17(1), 213–222 (2022). https://doi.org/10.1007/s11468-021-01507-5
S. Shukla, M. Rani, N.K. Sharma, V. Sajal, Sensitivity enhancement of a surface plasmon resonance based fiber optic sensor utilizing platinum layer. Optik (Stuttg) 126(23), 4636–4639 (2015)
Z. Lin, S. Chen, C. Lin, Sensitivity improvement of a surface plasmon resonance sensor based on two-dimensional materials hybrid structure in visible region: A theoretical study. Sensors (Switzerland) 20(9), 2445 (2020). https://doi.org/10.3390/s20092445
N.K. Sharma, S. Shukla, V. Sajal, Surface plasmon resonance based fiber optic sensor using an additional layer of platinum: A theoretical study. Optik (Stuttg) 133, 43–50 (2017). https://doi.org/10.1016/j.ijleo.2017.01.004
A.M. Ahmed, M. Shaban, Highly sensitive Au–Fe2O3–Au and Fe2O3–Au–Fe2O3 biosensors utilizing strong surface plasmon resonance. Appl. Phys. B 126(4), 57 (2020). https://doi.org/10.1007/s00340-020-7405-7
S. Knight et al., In-situ terahertz optical Hall effect measurements of ambient effects on free charge carrier properties of epitaxial graphene. Sci. Rep. 7(1), 5151 (2017). https://doi.org/10.1038/s41598-017-05333-w
Y.J. Park, K.M.A. Sobahan, C.K. Hwangbo, Optical and structural properties of Fe2O3 thin films prepared by ion-beam assisted deposition. Surf. Coatings Technol. 203(17), 2646–2650 (2009). https://doi.org/10.1016/j.surfcoat.2009.02.085
L. Dghoughi et al., Physico-chemical, optical and electrochemical properties of iron oxide thin films prepared by spray pyrolysis. Appl. Surf. Sci. 253(4), 1823–1829 (2006). https://doi.org/10.1016/j.apsusc.2006.03.021
E.L. Miller, D. Paluselli, B. Marsen, R.E. Rocheleau, Low-temperature reactively sputtered iron oxide for thin film devices. Thin Solid Films 466(1), 307–313 (2004). https://doi.org/10.1016/j.tsf.2004.02.093
N. Beermann, L. Vayssieres, S. Lindquist, A. Hagfeldt, Photoelectrochemical studies of oriented nanorod thin films of hematite. J. Electrochem. Soc. 147(7), 2456 (2000). https://doi.org/10.1149/1.1393553
A. Joshi, L. Sharma, S. Kaur, K. Dharamvir, H. Nayyar, G. Verma, Plant nanobionic effect of multi-walled carbon nanotubes on growth, anatomy, yield and grain composition of rice. Bionanoscience 10(2), 430–445 (2020). https://doi.org/10.1007/s12668-020-00725-1
C.-M. Tîlmaciu, M.C. Morris, Carbon nanotube biosensors. Front. Chem. (2015). https://doi.org/10.3389/fchem.2015.00059
P. Kaur, S. Jaiswal, D. Manhas, V. Kaur, V. Babar, G. Verma, An experimental and theoretical approach to organic functionalization of carbon nanofibers using fresh neem leaves. Int. J. Hydrogen Energy 48(70), 27242–27258 (2023). https://doi.org/10.1016/j.ijhydene.2023.03.432
C. Li, G. Shi, Carbon nanotube-based fluorescence sensors. J. Photochem. Photobiol. C Photochem. Rev. 19, 20–34 (2014). https://doi.org/10.1016/j.jphotochemrev.2013.10.005
D.A. Heller et al., Peptide secondary structure modulates single-walled carbon nanotube fluorescence as a chaperone sensor for nitroaromatics. Proc. Natl. Acad. Sci. U.S.A. 108(21), 8544–8549 (2011). https://doi.org/10.1073/pnas.1005512108
A. Pal, A. Jha, A theoretical analysis on sensitivity improvement of an SPR refractive index sensor with graphene and barium titanate nanosheets. Optik (Stuttg). 231, 166378 (2021). https://doi.org/10.1016/j.ijleo.2021.166378
G. Ansari, A. Pal, A.K. Srivastava, G. Verma, Detection of hemoglobin concentration in human blood samples using a zinc oxide nanowire and graphene layer heterostructure based refractive index biosensor. Opt. Laser Technol. 164(2), 111 (2023). https://doi.org/10.1016/0030-3992(80)90045-6
K. Shah, N.K. Sharma, V. Sajal, Analysis of fiber optic SPR sensor utilizing platinum based nanocomposites. Opt. Quantum Electron. (2018). https://doi.org/10.1007/s11082-018-1533-x
G. Ansari, A. Pal, A.K. Srivastava, G. Verma, Machine learning approach to surface plasmon resonance bio-chemical sensor based on nanocarbon allotropes for formalin detection in water. Sens. Bio-Sens. Res. 42, 100605 (2023). https://doi.org/10.1016/j.sbsr.2023.100605
S. Wang, N. Liu, Q. Cheng, B. Pang, J. Lv, Surface plasmon resonance on the antimonene–Fe2O3–copper layer for optical attenuated total reflection spectroscopic application. Plasmonics 16(2), 559–566 (2021). https://doi.org/10.1007/s11468-020-01309-1
B. Karki, P. Sarkar, G. Dhiman, G. Srivastava, M. Kumar, Platinum diselenide and graphene-based refractive index sensor for cancer detection. Plasmonics (2023). https://doi.org/10.1007/s11468-023-02051-0
B. Karki, A. Uniyal, A. Pal, V. Srivastava, Advances in surface plasmon resonance-based biosensor technologies for cancer cell detection. Int. J. Opt. 2022, 1476254 (2022). https://doi.org/10.1155/2022/1476254
Y. Xu, Y.S. Ang, L. Wu, L.K. Ang, High sensitivity surface plasmon resonance sensor based on two-dimensional mxene and transition metal dichalcogenide: A theoretical study. Nanomaterials 9(2), 165 (2019). https://doi.org/10.3390/nano9020165
R. Kumar, S. Pal, Y.K. Prajapati, S. Kumar, J.P. Saini, Sensitivity improvement of a MXene- immobilized SPR sensor with Ga-doped-ZnO for biomolecules detection. IEEE Sens. J. 22(7), 6536–6543 (2022). https://doi.org/10.1109/JSEN.2022.3154099
Y. Zhao, S. Gan, G. Zhang, X. Dai, High sensitivity refractive index sensor based on surface plasmon resonance with topological insulator. Results Phys. 14, 102477 (2019). https://doi.org/10.1016/j.rinp.2019.102477
A.H.M. Almawgani et al., Sensitivity enhancement of optical plasmon-based sensor for detection of the hemoglobin and glucose: A numerical approach. Opt. Quantum Electron. 55(11), 963 (2023)
A. Uniyal, G. Srivastava, P. Sarkar, M. Kumar, S. Singh, Fluorinated graphene and CNT-based surface plasmon resonance sensor for detecting the viral particles of SARS-CoV-2. Phys. B Condens. Matter. 669, 415282 (2023). https://doi.org/10.1016/j.physb.2023.415282
A.H.M. Almawgani et al., Magnesium oxide and silicon-assisted surface plasmon resonance sensor for gas detection: A performance analysis. Plasmonics (2024). https://doi.org/10.1007/s11468-023-02174-4
D. Pal et al., A highly sensitive long-range surface plasmon resonance biosensor for the determination of hemoglobin content in human blood. Plasmonics (2024). https://doi.org/10.1007/s11468-024-02243-2
B. Karki, A. Pal, P. Sarkar, A. Uniyal, R.B. Yadav, Gold, MXene, and graphene nanofilm-based surface plasmon resonance sensor for malaria detection. J. Opt. (2024). https://doi.org/10.1007/s12596-024-01661-z
B. Karki, A. Uniyal, P. Sarkar, A. Pal, R.B. Yadav, Sensitivity improvement of surface plasmon resonance sensor for glucose detection in urine samples using heterogeneous layers: an analytical perspective. J. Opt. (2023). https://doi.org/10.1007/s12596-023-01418-0
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Gufranullah Ansari contributed toward conceptualization (equal) and writing—review & editing (equal), Amrindra Pal contributed toward methodology (equal), and writing—review & editing (equal), Alok K. Srivastava contributed toward investigation (equal), methodology (equal), and Supervision (Lead), Gaurav Verma contributed toward investigation (equal), formal analysis (equal), and supervision (Lead).
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
Not applicable. The work presented in this manuscript is mathematical modeling only for the proposed biosensor. No experiment was performed on the human body and living organisms/animals. So, ethical approval from an ethical committee is not required.
Consent to participant
I am willing to participate in the work presented in this manuscript.
Consent for publication
The author has given their consent to publish this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari, G., Pal, A., Srivastava, A.K. et al. Bi-metallic, ferric oxide, and carbon nanotube-assisted SPR sensor for cancer detection. Journal of Materials Research (2024). https://doi.org/10.1557/s43578-024-01358-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/s43578-024-01358-w