Abstract
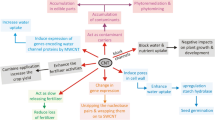
Oxidized multi-walled carbon nanotubes (MWCNTs) having a diameter of 14–30 nm and length of 200–300 nm were used to the prime rice seeds with different concentrations of MWCNT (70, 80 and 90 μg/mL). The effects on germination, growth, anatomy, physiology, yield, quantitative seed components and toxicity (using human cell lines) were evaluated. The treatments, when extended to realistic field environments, resulted in significantly better yield and productivity of rice. The MWCNT-treated plants had denser stomata and larger root length, which resulted in faster growth and facilitated both water and mineral uptake, thus boosting the crop yield. Increased vascular tissues enhanced the chlorophyll content and photosynthetic activity. No toxic effects of MWCNT were observed in the DNA of the CNT-treated plants, and in the human cell lines, treated with harvested grain extract of MWCNT-primed plants. This study provides some new insights into the use of nanomaterials in plants and their potential benefits in agriculture thus ushering in a new organic-inorganic interface.







Similar content being viewed by others
References
Iijima, S., & Ichihashi, T. (1993). Single-shell carbon nanotubes of 1-nm diameter. Nature, 363(6430), 603–605.
Giraldo, J. P., Landry, M. P., Faltermeier, S. M., McNicholas, T. P., Iverson, N. M., Boghossian, A. A., & Strano, M. S. (2014). Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nature Materials, 13(4), 400–408.
Wong, M. H., Giraldo, J. P., Kwak, S. Y., Koman, V. B., Sinclair, R., Lew, T. T. S., & Strano, M. S. (2017). Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics. Nature Materials, 16(2), 264–272.
Khodakovskaya, M. V., de Silva, K., Nedosekin, D. A., Dervishi, E., Biris, A. S., Shashkov, E. V., & Zharov, V. P. (2011). Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proceedings of the National Academy of Sciences, 108(3), 1028–1033.
Lahiani, M. H., Dervishi, E., Chen, J., Nima, Z., Gaume, A., Biris, A. S., & Khodakovskaya, M. V. (2013). Impact of carbon nanotube exposure to seeds of valuable crops. ACS Applied Materials & Interfaces, 5(16), 7965–7973.
Husen, A., & Siddiqi, K. S. (2014). Carbon and fullerene nanomaterials in plant system. Journal of Nanobiotechnology, 12, 16–27.
Joshi, A., Kaur, S., Dharamvir, K., Nayyar, H., & Verma, G. (2018). Multi-walled carbon nanotubes applied through seed-priming influence early germination, root hair, growth and yield of bread wheat (Triticum aestivum L.). Journal of the Science of Food and Agriculture, 98(8), 3148–3160.
Joshi, A., Kaur, S., Singh, P., Dharamvir, K., Nayyar, H., & Verma, G. (2018). Tracking multi-walled carbon nanotubes inside oat (Avena sativa L.) plants and assessing their effect on growth, yield, and mammalian (human) cell viability. Applied Nanoscience, 8(6), 1399–1414.
Khodakovskaya, M. V., Kim, B. S., Kim, J. N., Alimohammadi, M., Dervishi, E., Mustafa, T., & Cernigla, C. E. (2013). Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small, 9(1), 115–123.
Lahiani, M. H., Dervishi, E., Ivanov, I., Chen, J., & Khodakovskaya, M. (2016). Comparative study of plant responses to carbon-based nanomaterials with different morphologies. Nanotechnology, 27(26), 265102–265116.
Zhang, H., Yue, M., Zheng, X., Xie, C., Zhou, H., & Li, L. (2017). Physiological effects of single-and multi-walled carbon nanotubes on rice seedlings. IEEE Transactions on Nanobioscience, 16(7), 563–570.
Rico, C. M., Hong, J., Morales, M. I., Zhao, L., Barrios, A. C., Zhang, J. Y., & Gardea-Torresdey, J. L. (2013). Effect of cerium oxide nanoparticles on rice: A study involving the antioxidant defense system and in vivo fluorescence imaging. Environmental Science & Technology, 47(11), 5635–5642.
Nair, P. M. G., & Chung, I. M. (2014). Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere, 112, 105–113.
Yan, S., Zhang, H., Huang, Y., Tan, J., Wang, P., Wang, Y., & Li, L. (2016). Single-wall and multi-wall carbon nanotubes promote rice root growth by eliciting the similar molecular pathways and epigenetic regulation. IET Nanobiotechnology, 10(4), 222–229.
Lin, C., Fugetsu, B., Su, Y., & Watari, F. (2009). Studies on toxicity of multi-walled carbon nanotubes on Arabidopsis T87 suspension cells. Journal of Hazardous Materials, 170(2–3), 578–583.
Deng, Y., Petersen, E. J., Challis, K. E., Rabb, S. A., Holbrook, R. D., Ranville, J. F., Nelson, B. C., & Xing, B. (2017). Multiple method analysis of TiO2 nanoparticle uptake in rice (Oryza sativa L.) plants. Environmental Science & Technology, 51(18), 10615–10623.
Awasthi, R., Kaushal, N., Vadez, V., Turner, N. C., Berger, J., Siddique, K. H., & Nayyar, H. (2014). Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Functional Plant Biology, 41(11), 1148–1167.
Liu, Q., Chen, B., Wang, Q., Shi, X., Xiao, Z., Lin, J., & Fang, X. (2009). Carbon nanotubes as molecular transporters for walled plant cells. Nano Letters, 9(3), 1007–1010.
Barrs, H. D., & Weatherley, P. E. (1962). A re-examination of the relative turgidity technique for estimating water deficits in leaves. Australian Journal of Biological Sciences, 15(3), 413–428.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1), 1–15.
Robinson, D. G., Ehlers, U., Herken, R., Herrmann, B., Mayer, F. & Schürmann, F. W., 2012. Methods of preparation for electron microscopy: An introduction for the biomedical sciences. Springer Science & Business Media.
Tiwari, K. L., Jadhav, S. K., & Gupta, S. (2012). Modified CTAB technique for isolation of DNA from some medicinal plants. Research Journal of Medicinal Plant, 6(1), 65–73.
Lamberts, L., De Bie, E., Vandeputte, G. E., Veraverbeke, W. S., Derycke, V., De Man, W., & Delcour, J. A. (2007). Effect of milling on colour and nutritional properties of rice. Food Chemistry, 100(4), 1496–1503.
Chen, H. H., Chen, Y. K., & Chang, H. C. (2012). Evaluation of physicochemical properties of plasma treated brown rice. Food Chemistry, 135(1), 74–79.
Du, C., Yeh, J., & Pan, N. (2005). High power density supercapacitors using locally aligned carbon nanotube electrodes. Nanotechnology, 16(4), 350–353.
Ruelle, B., Peeterbroeck, S., Gouttebaron, R., Godfroid, T., Monteverde, F., Dauchot, J. P., & Dubois, P. (2007). Functionalization of carbon nanotubes by atomic nitrogen formed in a microwave plasma Ar+ N 2 and subsequent poly (ε-caprolactone) grafting. Journal of Materials Chemistry, 17(2), 157–159.
Tripathi, S., Sonkar, S. K., & Sarkar, S. (2011). Growth stimulation of gram (Cicer arietinum L.) plant by water soluble carbon nanotubes. Nanoscale, 3(3), 1176–1181.
Wild, E., & Jones, K. C. (2009). Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants. Environmental Science & Technology, 43(14), 5290–5294.
Lin, D., & Xing, B. (2007). Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environmental Pollution, 150(2), 243–250.
Lee, W. M., Kwak, J. I., & An, Y. J. (2012). Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: Media effect on phytotoxicity. Chemosphere, 86(5), 491–499.
Zhai, G., Gutowski, S. M., Walters, K. S., Yan, B., & Schnoor, J. L. (2015). Charge, size, and cellular selectivity for multiwall carbon nanotubes by maize and soybean. Environmental Science & Technology, 49(12), 7380–7390.
Ratnikova, T. A., Podila, R., Rao, A. M., & Taylor, A. G. (2015). Tomato seed coat permeability to selected carbon nanomaterials and enhancement of germination and seedling growth. The Scientific World Journal, 2015. https://doi.org/10.1155/2015/419215.
Yuan, H., Hu, S., Huang, P., Song, H., Wang, K., Ruan, J., & Cui, D. (2011). Single walled carbon nanotubes exhibit dual-phase regulation to exposed Arabidopsis mesophyll cells. Nanoscale Research Letters, 6(1), 44–53.
Tripathi, S., & Sarkar, S. (2015). Influence of water soluble carbon dots on the growth of wheat plant. Applied Nanoscience, 5(5), 609–616.
Begum, P., & Fugetsu, B. (2012). Phytotoxicity of multi-walled carbon nanotubes on red spinach (Amaranthus tricolor L.) and the role of ascorbic acid as an antioxidant. Journal of Hazardous Materials, 243, 212–222.
Larue, C., Laurette, J., Herlin-Boime, N., Khodja, H., Fayard, B., Flank, A. M., & Carriere, M. (2012). Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): Influence of diameter and crystal phase. Science of the Total Environment, 431, 197–208.
Chen, G., Qiu, J., Liu, Y., Jiang, R., Cai, S., Liu, Y., & Ouyang, G. (2015). Carbon nanotubes act as contaminant carriers and translocate within plants. Scientific Reports, 5, 15682–15690.
Khodakovskaya, M., Dervishi, E., Mahmood, M., Xu, Y., Li, Z., Watanabe, F., & Biris, A. S. (2009). Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano, 3(10), 3221–3227.
Joshi, A., Sharma, A., Nayyar, H., Verma, G., & Dharamvir, K. (2015). Carbon nanofibers suppress fungal inhibition of seed germination of maize (Zea mays) and barley (Hordeum vulgare L.) crop. AIP Conference Proceedings, 1675(1), 030034–030038.
Wang, X., Han, H., Liu, X., Gu, X., Chen, K., & Lu, D. (2012). Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum L.) plants. Journal of Nanoparticle Research, 14(6), 841–850.
Scholes, G. D., & Sargent, E. H. (2014). Bioinspired materials: Boosting plant biology. Nature Materials, 13(4), 329–331.
Khodakovskaya, M. V., De Silva, K., Biris, A. S., Dervishi, E., & Villagarcia, H. (2012). Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano, 6(3), 2128–2135.
Tiwari, D. K., Dasgupta-Schubert, N., Cendejas, L. V., Villegas, J., Montoya, L. C., & García, S. B. (2014). Interfacing carbon nanotubes (CNT) with plants: Enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nanoagriculture. Applied Nanoscience, 4(5), 577–591.
Siddiqi, K. S., & Husen, A. (2016). Engineered gold nanoparticles and plant adaptation potential. Nanoscale Research Letters, 11(1), 400. https://doi.org/10.1186/s11671-016-1607-2.
Karlsson, H. L., Cronholm, P., Gustafsson, J., & Moller, L. (2008). Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chemical Research in Toxicology, 21(9), 1726–1732.
Liu, Y., Zhao, Y., Sun, B., & Chen, C. (2012). Understanding the toxicity of carbon nanotubes. Accounts of Chemical Research, 46(3), 702–713.
Zhou, Q., & Hu, X. (2017). Systemic stress and recovery patterns of rice roots in response to graphene oxide nanosheets. Environmental Science & Technology, 51(4), 2022–2030.
Acknowledgments
HN is thankful to DST (PURSE grant), India, for instrumental facilities. The authors also want to express sincere gratitude toward Dr. Nidarshana Chaturvedi, Department of Biochemistry, Panjab University, Chandigarh, India, for DNA isolation process, Dr. Dhirendra Pratap Singh, Scientist, National Institute of Occupational Health, Occupational Medicine Division, Ahmedabad, India, for cell line studies performed at National Agri-Food Biotechnology Institute, Nutrition Science & Technology, Mohali, India and Neha Thakur, Dr. SSB, UICET, Chandigarh, India for their extensive help during quantitative seed analysis experimentation.
Funding
AJ received financial support from Department of Science and Technology, Ministry of Science and Technology (Grant no. IF130393 INSPIRE FELLOWSHIP). GV received funding from agencies SAP (UGC, New Delhi), TEQIP-III, and PURSE (DST, New Delhi) Grants.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. AJ, GV, HN, KD have contributed equally, while LS and SK helped in plant experiments.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joshi, A., Sharma, L., Kaur, S. et al. Plant Nanobionic Effect of Multi-walled Carbon Nanotubes on Growth, Anatomy, Yield and Grain Composition of Rice. BioNanoSci. 10, 430–445 (2020). https://doi.org/10.1007/s12668-020-00725-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-020-00725-1