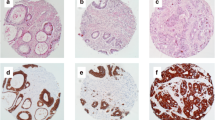
Abstract
Background
Extramural vascular invasion (EMVI) is a known poor prognostic factor in colorectal carcinoma; however, its molecular basis has not been defined. This study aimed to assess the expression of molecular markers in EMVI positive colorectal carcinoma to understand their tumor microenvironment.
Methods
Immunohistochemistry was performed on tissue microarrays of surgically resected colorectal cancer specimens for immunological markers, and BRAFV600E mutation (and on the tissue blocks for mismatch repair proteins). Automated quantification was used for CD8, LAG3, FOXP3, PU1, and CD163, and manual quantification was used for PDL1, HLA I markers (beta-2 microglobulin, HC10), and HLA II. The Wilcoxon rank-sum test was used to compare EMVI positive and negative tumors. A logistic regression model was fitted to assess the predictive effect of biomarkers on EMVI.
Results
There were 340 EMVI positive and 678 EMVI negative chemo naïve tumors. PDL1 was barely expressed on tumor cells (median 0) in the entire cohort. We found a significantly lower expression of CD8, LAG3, FOXP3, PU1 cells, PDL1 positive macrophages, and beta-2 microglobulin on tumor cells in the EMVI positive subset (p ≤ 0.001). There was no association of BRAFV600E or deficient mismatch repair proteins (dMMR) with EMVI. PU1 (OR 0.8, 0.7–0.9) and low PDL1 (OR 1.6, 1.1–2.3) independently predicted EMVI on multivariate logistic regression among all biomarkers examined.
Conclusion
There is a generalized blunting of immune response in EMVI positive colorectal carcinoma, which may contribute to a worse prognosis. Tumor-associated macrophages seem to play the most significant role in determining EMVI.




Similar content being viewed by others
References
Harrison JC, Dean PJ, El-Zeky F, Vander Zwaag R. From Dukes through Jass: pathological prognostic indicators in rectal cancer. Hum Pathol. 1994;25(5):498–505. https://doi.org/10.1016/0046-8177(94)90122-8.
Ale Ali H, Kirsch R, Razaz S, et al. Extramural venous invasion in rectal cancer: overview of imaging, histopathology, and clinical implications. Abdom Radiol (NY). 2019;44(1):1–10. https://doi.org/10.1007/s00261-018-1673-2.
Freedman LS, Macaskill P, Smith AN. Multivariate analysis of prognostic factors for operable rectal cancer. Lancet. 1984;2(8405):733–6. https://doi.org/10.1016/s0140-6736(84)92636-9.
Siddiqui MRS, Simillis C, Hunter C, et al. A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases. Br J Cancer. 2017;116(12):1513–9. https://doi.org/10.1038/bjc.2017.99.
Qwaider YZ, Sell NM, Stafford CE, et al. Adjuvant chemotherapy benefits on patients with extramural vascular invasion in stages II and III colon cancer. J Gastrointest Surg. 2021;25(8):2019–25. https://doi.org/10.1007/s11605-020-04810-4.
Gunal A, Hui P, Kilic S, et al. KRAS mutations are associated with specific morphologic features in colon cancer. J Clin Gastroenterol. 2013;47(6):509–14. https://doi.org/10.1097/MCG.0b013e3182703030.
Williamson JS, Jones HG, Williams N, et al. Extramural vascular invasion and response to neoadjuvant chemoradiotherapy in rectal cancer: Influence of the CpG island methylator phenotype. World J Gastrointest Oncol. 2017;9(5):209–17. https://doi.org/10.4251/wjgo.v9.i5.209.
Bae JM, Kim JH, Cho NY, Kim TY, Kang GH. Prognostic implication of the CpG island methylator phenotype in colorectal cancers depends on tumour location. Br J Cancer. 2013;109(4):1004–12. https://doi.org/10.1038/bjc.2013.430.
Kokelaar RF, Jones HG, Williamson J, et al. DNA hypermethylation as a predictor of extramural vascular invasion (EMVI) in rectal cancer. Cancer Biol Ther. 2018;19(3):214–21. https://doi.org/10.1080/15384047.2017.1416933.
Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–91. https://doi.org/10.1016/S1470-2045(17)30422-9.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. https://doi.org/10.1056/NEJMoa1500596.
Talbot IC, Ritchie S, Leighton MH, Hughes AO, Richard Bussey HJ, Morson BC. Spread of rectal cancer within veins. Am J Surg. 1981;141(1):15–7. https://doi.org/10.1016/0002-9610(81)90004-0.
Leijssen LGJ, Dinaux AM, Amri R, et al. Impact of intramural and extramural vascular invasion on stage II-III colon cancer outcomes. J Surg Oncol. 2019;119(6):749–57. https://doi.org/10.1002/jso.25367.
Quirke P, Morris E. Reporting colorectal cancer. Histopathology. 2007;50(1):103–12. https://doi.org/10.1111/j.1365-2559.2006.02543.x.
Morris M, Platell C, de Boer B, McCaul K, Iacopetta B. Population-based study of prognostic factors in stage II colonic cancer. Br J Surg. 2006;93(7):866–71. https://doi.org/10.1002/bjs.5345.
Washington MK, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133(10):1539–51. https://doi.org/10.1043/1543-2165-133.10.1539.
Williams GT, Quirke P, Shepherd NA. Dataset for colorectal cancer.
Loughrey MB, Webster F, Arends MJ, et al. Dataset for pathology reporting of colorectal cancer: recommendations from the international collaboration on cancer reporting (ICCR). Ann Surg. 2022;275(3):e549–61. https://doi.org/10.1097/SLA.0000000000005051.
De Salins AGD, Tachon G, Cohen R, et al. Discordance between immunochemistry of mismatch repair proteins and molecular testing of microsatellite instability in colorectal cancer. ESMO Open. 2021;6(3):100120. https://doi.org/10.1016/j.esmoop.2021.100120.
Talbot IC, Ritchie S, Leighton M, Hughes AO, Bussey HJ, Morson BC. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology. 1981;5(2):141–63. https://doi.org/10.1111/j.1365-2559.1981.tb01774.x.
Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6. https://doi.org/10.1038/nm.3967.
Wielandt AM, Villarroel C, Hurtado C, et al. Characterization of patients with sporadic colorectal cancer following the new consensus molecular subtypes (CMS). Rev Med Chil. 2017;145(4):419–30. https://doi.org/10.4067/S0034-98872017000400001.
Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. https://doi.org/10.1126/science.1129139.
Hu G, Li Z, Wang S. Tumor-infiltrating FoxP3+ Tregs predict favorable outcome in colorectal cancer patients: a meta-analysis. Oncotarget. 2017;8(43):75361–71. https://doi.org/10.18632/oncotarget.17722.
Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–34. https://doi.org/10.1158/1078-0432.CCR-06-0369.
Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13(3):902–11. https://doi.org/10.1158/1078-0432.CCR-06-2363.
Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60(7):909–18. https://doi.org/10.1007/s00262-011-1046-y.
Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–52. https://doi.org/10.1016/j.immuni.2018.03.014.
Li Y, Liang L, Dai W, et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15(1):55. https://doi.org/10.1186/s12943-016-0539-x.
Kim JH, Park HE, Cho N-Y, Lee HS, Kang GH. Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br J Cancer. 2016;115(4):490–6. https://doi.org/10.1038/bjc.2016.211.
Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29(9):1104–12. https://doi.org/10.1038/modpathol.2016.95.
Lee LH, Cavalcanti MS, Segal NH, et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29(11):1433–42. https://doi.org/10.1038/modpathol.2016.139.
Masugi Y, Nishihara R, Yang J, et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017;66(8):1463–73. https://doi.org/10.1136/gutjnl-2016-311421.
Yomoda T, Sudo T, Kawahara A, et al. The immunoscore is a superior prognostic tool in stages II and III colorectal cancer and is significantly correlated with programmed death-ligand 1 (PD-L1) expression on tumor-infiltrating mononuclear cells. Ann Surg Oncol. 2019;26(2):415–24. https://doi.org/10.1245/s10434-018-07110-z.
Eriksen AC, Sørensen FB, Lindebjerg J, et al. Programmed death Ligand-1 expression in stage II colon cancer—experiences from a nationwide population-based cohort. BMC Cancer. 2019;19(1):142. https://doi.org/10.1186/s12885-019-5345-6.
Liu S, Gönen M, Stadler ZK, et al. Cellular localization of PD-L1 expression in mismatch-repair-deficient and proficient colorectal carcinomas. Mod Pathol. 2019;32(1):110–21. https://doi.org/10.1038/s41379-018-0114-7.
Lee SJ, Jun S-Y, Lee IH, et al. CD274, LAG3, and IDO1 expressions in tumor-infiltrating immune cells as prognostic biomarker for patients with MSI-high colon cancer. J Cancer Res Clin Oncol. 2018;144(6):1005–14. https://doi.org/10.1007/s00432-018-2620-x.
Miller TJ, Anyaegbu CC, Lee-Pullen TF, Spalding LJ, Platell CF, McCoy MJ. PD-L1+ dendritic cells in the tumor microenvironment correlate with good prognosis and CD8+ T cell infiltration in colon cancer. Cancer Sci. 2021;112(3):1173–83. https://doi.org/10.1111/cas.14781.
Burnell SEA, Capitani L, MacLachlan BJ, Mason GH, Gallimore AM, Godkin A. Seven mysteries of LAG-3: a multi-faceted immune receptor of increasing complexity. Immunother Adv. 2022;2(1):ltab025. https://doi.org/10.1093/immadv/ltab025.
Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34. https://doi.org/10.1056/NEJMoa2109970.
Rhyner Agocs G, Assarzadegan N, Kirsch R, et al. LAG-3 expression predicts outcome in stage II colon cancer. J Pers Med. 2021. https://doi.org/10.3390/jpm11080749.
Zhou G, Noordam L, Sprengers D, et al. Blockade of LAG3 enhances responses of tumor-infiltrating T cells in mismatch repair-proficient liver metastases of colorectal cancer. Oncoimmunology. 2018;7(7):e1448332. https://doi.org/10.1080/2162402X.2018.1448332.
Chen J, Chen Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med Oncol. 2014;31(8):82. https://doi.org/10.1007/s12032-014-0082-9.
Singhal S, Stadanlick J, Annunziata MJ, et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci Transl Med. 2019. https://doi.org/10.1126/scitranslmed.aat1500.
Lu D, Ni Z, Liu X, et al. Beyond T cells: understanding the role of PD-1/PD-L1 in tumor-associated macrophages. J Immunol Res. 2019;2019:1919082. https://doi.org/10.1155/2019/1919082.
Shabo I, Stål O, Olsson H, Doré S, Svanvik J. Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Int J Cancer. 2008;123(4):780–6. https://doi.org/10.1002/ijc.23527.
Shabo I, Olsson H, Elkarim R, Sun X-F, Svanvik J. Macrophage infiltration in tumor stroma is related to tumor cell expression of CD163 in colorectal cancer. Cancer Microenviron. 2014;7(1–2):61–9. https://doi.org/10.1007/s12307-014-0145-7.
Shabo I, Olsson H, Sun X-F, Svanvik J. Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. Int J Cancer. 2009;125(8):1826–31. https://doi.org/10.1002/ijc.24506.
Anderson P, Aptsiauri N, Ruiz-Cabello F, Garrido F. HLA class I loss in colorectal cancer: implications for immune escape and immunotherapy. Cell Mol Immunol. 2021;18(3):556–65. https://doi.org/10.1038/s41423-021-00634-7.
Løvig T, Andersen SN, Thorstensen L, et al. Strong HLA-DR expression in microsatellite stable carcinomas of the large bowel is associated with good prognosis. Br J Cancer. 2002;87(7):756–62. https://doi.org/10.1038/sj.bjc.6600507.
Sconocchia G, Eppenberger-Castori S, Zlobec I, et al. HLA class II antigen expression in colorectal carcinoma tumors as a favorable prognostic marker. Neoplasia. 2014;16(1):31–42. https://doi.org/10.1593/neo.131568.
Diederichsen ACP, Hjelmborg JVB, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52(7):423–8. https://doi.org/10.1007/s00262-003-0388-5.
Möller P, Momburg F, Koretz K, et al. Influence of major histocompatibility complex class I and II antigens on survival in colorectal carcinoma. Cancer Res. 1991;51(2):729–36.
Acknowledgement
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
D.T.T. has received consulting fees from ROME Therapeutics, Tekla Capital, Ikena Oncology, Foundation Medicine, Inc., NanoString Technologies, EMD Millipore Sigma, and Pfizer that are not related to this work. D.T.T. is a founder and has equity in ROME Therapeutics, PanTher Therapeutics and TellBio, Inc., which is not related to this work. D.T.T. receives research support from ACD-Biotechne, PureTech Health LLC, and Ribon Therapeutics, which was not used in this work. D.T.T.’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies. A.P.—Equity in C2i genomics and, in the last 36 months, has served as an advisor/consultant for Eli Lilly, Pfizer, Inivata, Biofidelity, Natera, Checkmate Pharmaceuticals and Guardant. She has been on the DSMC for a Roche study and has received research funding to the Institution from PureTech, PMV Pharmaceuticals, Plexxicon, Takeda, BMS, Mirati, Novartis, Genentech, Natera, and Daiichi Sankyo. M.T.—ROME Therapeutics, consulting fees and equity.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Authors have declared disclosures as necessary.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sonal, S., Deshpande, V., Ting, D.T. et al. Molecular Basis of Extramural Vascular Invasion (EMVI) in Colorectal Carcinoma. Ann Surg Oncol 29, 7372–7382 (2022). https://doi.org/10.1245/s10434-022-12212-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12212-w