Abstract
Background
Patients suffering from high risk stage II colon cancer (CC) may benefit from adjuvant onco-therapy, but additional prognostic markers are needed for better treatment stratification. We investigated the prognostic value of Programmed Death Ligand-1 (PD-L1) in a true population-based cohort of patients with stage II CC.
Methods
PD-L1 expression on tumour cells was evaluated by immunohistochemistry in 572 colon cancers. Whole sections from tumour blocks representing the deepest invasive front of the primary tumour were used for analysis. A cut-off of 5% positivity was used for dichotomizing the data. The prognostic value was investigated in Cox proportional hazard models for recurrence-free survival (RFS) and overall survival (OS).
Results
Overall, 6% of the tumours were classified as high PD-L1. High PD-L1 was related to female gender (p = 0.028), high malignancy grade (< 0.001), right side localization (p < 0.001) and microsatellite instability (MSI) (p < 0.001). Thirty-one (18%) of the MSI and 4 (1%) of the microsatellite stable tumours were classified as high PD-L1, respectively. PD-L1 expression provided no prognostic value as a single marker. In patients with MSI tumours, high PD-L1 expression had no significant impact regarding OS or RFS.
Conclusions
PD-L1 expression in tumour cells of stage II CC did not provide any prognostic impact, neither in the entire population-based cohort nor in the group of MSI patients. Additional investigations of the immunogenic microenvironment are needed for evaluating the prognostic information in CC.
Similar content being viewed by others
Background
Colon cancer is one of the most common cancers in the Western world. About 1/3 have stage II disease, and this group of patients are in general having a good prognosis with a 5-year overall survival (OS) of approximately 70–80% after surgery alone [1]. Current international guidelines (ASMO and ESMO) do not recommend routine adjuvant chemotherapy in stage II CC, but rather that this treatment be limited to patients having a high risk of recurrence, based on an individual evaluation including high-risk markers [2, 3]. However, the currenct high risk factors are questionable [4], and there is a need for additional prognostic markers for better treatment stratification.
In recent years, the tumour microenvironment has been investigated, and the role of the interaction between cancer cells and the immune system in cancer surveillance has been emphasized [5]. Tumour-infiltrating lymphocytes (TILs) are considered as the host’s immune response against solid tumours, and infiltration by activated CD8+ cytotoxic T-lymphocytes is correlated with better survival of colorectal cancer (CRC) patients [6]. Activated lymphocytes expressing Programmed Death Receptor-1 (PD-1) can bind specific to the ligand Programmed Death Ligand-1 (PD-L1), which is expressed on the cell membrane in malignant epithelial tumours, including colorectal adenocarcinomas [7]. This immune-checkpoint is up-regulated in many tumours, and the interaction of PD-L1 on tumour cells with its receptor PD-1 on the activated T-cells induces a down-regulation of the antigen-stimulated lymphocyte proliferation and cytokine production, resulting in an inhibition of the host-immune response [8].
Current results of the prognostic value of PD-L1 in CRC are controversial. Some studies only report on trends towards worse prognosis for high PD-L1 expression [9, 10], while others identify high PD-L1 to be independently associated with worse recurrence free survival (RFS) [11, 12]. In contrast, other studies report no prognostic impact [13, 14], or even a tendency of high expression of PD-L1 to be related to a better prognosis [15]. However, studies vary greatly in methods and the study populations are highly heterogeneous, including different stages of disease, and no studies have previously investigated the expression of PD-L1 in a cohort exclusively of patients with stage II CC.
With this motivation, the aim of the present study was to evaluate the prognostic impact of PD-L1 in a nationwide, population-based cohort of stage II CC.
Methods
This study is reported in accordance with the REMARK guidelines [16].
Patient population
The population and sources of data has previously been descreibed in detail [17]. In brief all patients surgically treated for stage II CC in 2002 in Denmark were identified by a search in the nationwide registry administrated by the Danish Colorectal Cancer Group (DCCG) (N = 746). Exclusion criteria were as follows: missing tumour block (N = 11), insufficient tissue for analyses (N = 2), incorrectly staged patients (N = 25), treatment with adjuvant chemotherapy (N = 26)/radiotherapy (N = 1) and death within 90 days after the operation (N = 75). Furthermore patients with loco-advanced disease (N = 8) and patients diagnosed with another malignancy prior to CC were excluded from the study (N = 26), and the final study population comprised 572 patients.
Histopathological data were obtained by microscopic examination and from the national Patobank containing all pathology reports in Denmark. The term “not assessed” was used if the pathological feature was not described. Clinical data were obtained from The National Patient Registry.
Recurrence primarily occurred within the first five years of follow-up and to encompass the majority of recurrences a follow-up period of seven years was selected.
Samples
Formalin-fixed, paraffin-embedded tissue blocks were collected from the departments of pathology in Denmark. The tissue blocks were stored and transported at room temperature. One tumour block representing the deepest invasive margin, was selected from each patient. Prior to inclusion, all histological slides from each tumour were evaluated by first a trainee and afterwards an experienced pathologist.
Immunohistochemistry
From the selected tumour blocks serial 4 μm thick tissue sections were cut and mounted on FLEX IHC Microscope Slides (K8020, DAKO, Glostrup, Denmark). One whole tumour section per patient was used for the evaluation of PD-L1 expression. Staining was performed using a Ventana BenchMark ULTRA (Ventana Medical Systems, Tucson, Arizona, USA) automated immunohistochemistry (IHC) slide staining system. Tissue sections were heated and deparaffinised in EZprep (no.950–102, Ventana). Pre-treatment and demasking were carried out using ULTRA CC1 (no. 950–224) and ULTRA CC2 (no. 950–223), and endogenous peroxidase activity was blocked by Optiview Peroxidase Inhibitor (no. 760–700 Ventana). The slides were incubated with a rabbit monoclonal anti-PD-L1 (clone SP263A, no. 790–4905/741–4905 Ventana) for 16 min. This clone was chosen based on a pilot study. For amplification Optiview HQ Universal Linker (no. 760–700, Ventana) and Optiview HRP Multimer (no. 760–700, Ventana) was each used for 8 min.
The primary antibody was visualized using Optiview H2O2 and DAB (no. 760–700, Ventana), followed by Optiview Copper (no. 760–700, Ventana). Counterstain was done using Hematoxylin II (no. 790–2208, Ventana) and bluing Reagent (no. 760–2037, Ventana). Finally the histological slides were cover slipped with Tissue-Tek PERTEX (Histolab Products AB, Göteborg, Sweden).
Evaluation of microsattellite instability (MSI) was performed using IHC. Tumours displaying loss of one or more of the 4 mismatch repair proteins (MLH1, MSH2, MSH6 and PMS2) were considered as MSI, whereas tumours with intact mismatch repair proteins were considered as microsatellite stable (MSS). Staining of mismatch repair proteins was performed using a DAKO Autostainer Link 48 (DAKO) with monoclonal mouse antibody against MLH1 (Novocastra, Leica, Germany, clone ES05, dilution 1:100, product code NCL-L-MLH1), MSH2 (Novocastra, Leica, clone 25D12, dilution 1:100, product code NCL-L-MSH2), MSH6 (BD Transduction Laboratories, clone 44/MSH6, dilution 1:200, material number 610919), and PMS2 (BD Pharmingen, clone A16–4, dilution 1:500, material number 556415). Tissue sections were incubated for 30 min at room temperature with the primary antibodies diluted in Envision Flex antibody diluent (code S2022 DAKO). The antibody signal was amplified using Envision Flex+ Mouse(Linker) (DAKO) for 20 min. Bound antibodies were detected using Envision FLEX/HRP (DAKO) and visualized by Envision FLEX DAB (DAKO) and chromogene diluted in Envision Flex Substrate Buffer (DAKO). The sections were incubated in 0.5% CuSO4 in TBS buffer pH 7.6 for 10 min to enhance the immunohistochemical staining. Sections were counterstained with Meyer’s hematoxylin (Merck, Damstadt, Germany).
Scoring of PD-L1 expression
Tumour PD-L1 expression was evaluated based on immunostaining of the cell membrane of the epithelial tumour cells. The immunostaining of the stromal cells were not evaluated. Tumour cells were considered positive when any cell membrane staining (partial of complete) was present. Staining intensity was not evaluated and cytoplasmatic immunoreaction was not considered.
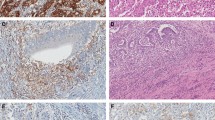
The percentage of positive tumour cells were scored semi-quantitatively as 0 (no positive tumour cells), 1 (≤1% positive), 2 (> 1 and ≤ 5% positive), 3 (> 5 and ≤ 20% positive), 4 (> 20 and ≤ 50% positive) and 5 (> 50% positive) (Fig. 1). A subset of 50 randomly selected tumours was examined by a second pathologist in order to assess inter-observer variation. For prognostic evaluation data were dichotomized, using 5% PD-L1 expression as cut-off. In absence of a standardized scoring system the cut-off was based on previously studies [9, 12, 14].
Statistics
The inter-observer reproducibility of PD-L1 scoring was evaluated by kappa statistics. Simple and weighted kappa (κ) values were calculated, and agreement was described according to Landis et al [18] as moderate, substantial, and almost perfect for κ values of 0.41–0.60, 0.61–0.80, and 0.81–1, respectively.
The endpoint OS was defined as time from operation to death of any cause or last follow-up. RFS was defined as time from operation to death of any cause or recurrence of CC. Patients later diagnosed with another cancer were censored at the date of diagnosis (N = 102). The median age was used as cut-off to dichotomize the parameter age. Survival curves were generated according to the Kaplan-Meier method and the log-rank test was used to test for differences between groups. The multivariable Cox-regression model was used to test for independent prognostic value with hazard ratio (HR) of 1.0 as reference and a 95% confidence interval (CI). A cut-off significance level of 0.10 was pre-specified for a variable to be included in the multivariable Cox regression model.
Chi2-statistics were used to test associations between clinicopathological variables. A p-value < 0.05 was considered significant. The statistical analyses were performed using STATA software version 14.0 (StataCorp, Texas, USA), and all statistical tests were two-sided.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. In the follow-up period of seven years, 266 (46.5%) patients died; 110 (19.2%) patients experienced disease recurrence and 78 (13.6%) patients were diagnosed with another cancer. The median age at time of surgery was 73 years (range 29–95), and the mean follow-up time was 6.9 years (range 3–84 months).
PD-L1 expression
The IHC staining of PD-L1 often had a highly heterogeneous expression both between the central part of the tumour and the invasive margin and along the invasive tumour front (Fig. 2).
Results regarding PD-L1 expression are displayed in Table 2. Nearly half of the population (46%) had no PD-L1 expression in tumour cells. After dichotomization, using 5% PD-L1 expression as cut-off, 35 (6%) of the tumours were classified as high PD-L1. In the group of MSI 31 (18%) of the tumours were classified as high PD-L1 and in the subgroup of MSS 4 (1%) were classified as high PD-L1. High PD-L1 was related to female gender (p = 0.028), high malignancy grade (< 0.001), right side localization (p < 0.001) and MSI (p < 0.001).
The inter-observer agreement for the semi-quantitative evaluation of PD-L1 expression was moderate with κ = 0.418 and weighted κ = 0.573. The agreement improved to substantial, when categorizing the data into high PD-L1 expression (> 5%) or low PD-L1 expression (≤5%), κ = 0.691.
Survival analysis
The 5-year RFS for the population with low PD-L1 was 69.2% versus 67.7% in the group with high PD-L1 expression, and OS was 74.7% versus 70.5%, respectively. When considering patients with MSI tumours the 5 year RFS for low PD-L1 was 77.4% versus 67.5% in the group of high PD-L1, and OS was 79.4% versus 70.7%. No significant differences in survival rates were observed, considering the entire cohort (Fig. 3). In the group of patients with MSI tumours the Kaplan Meier curves were separated for RFS, but results were insignificant, p = 0.256 (Fig. 4). This also accounted the group of patients with MSI T3 tumours (N = 155), p = 0.149.
Outcomes from the corresponding univariable Cox regression analyses are shown in Table 3. Patients with MSI tumours and high PD-L1 expression did not have a significant worse OS or RFS, HR = 1.104 (0.604–2.016), p = 0.748 and HR = 1.429 (0.769–2.653), p = 0.258, respectively. Age ≥ 73 years, T4 tumour and perforation were significantly related to an adverse outcome of both OS and RFS. Patients with MSI T3 tumours and high PD-L1 expression did neither have a significant worse OS or RFS, HR = 1.531 (0.757–3.100), p = 0.236 and HR = 1.637 (0.833–3.216), p = 0.153, respectively.
Multivariable Cox regression analyses were not performed, as PD-L1 had p-value > 0.10 in the univariable Cox-regression analysis.
Discussion
In this study, we investigated the prognostic value of PD-L1 expression on tumour cells in an unbiased, nationwide and population-based cohort of patients with stage II CC, treated exclusively with surgery. PD-L1 expression as a single marker did not provide any significant prognostic value regarding OS or RFS, neither in the the entire cohort nor in the subgroup of patients with MSI tumours.
In the entire cohort we found 6% of the tumours to have a high expression of PD-L1 on tumour cells, which is in accordance with other studies of CRC, reporting 5% positivity [11]. Likewise we found high PD-L1 expression associated to female gender, high malignancy grade, right sided localisation, and MSI, which also has been found by Lee et al, who investigated all stages of CRC [11]. Regarding MSI and MSS tumour subgroups, we found 18% of the MSI tumours to have high PD-L1 expression and 1% of the MSS tumours to have high PD-L1 expression. The difference in PD-L1 expression between MSI and MSS tumours has previously been descriebed in studies using a different scoring system [11, 19], although a recent study reported no differences in PD-L1 positivity among MSI and MSS tumours [20].
The association between MSI and high PD-L1 expression may be explained by the abundant infiltration of TILs in these tumours. Deficiency of the mismatch repair proteins results in a number of mutations. Therefore MSI tumours have a high load of tumour specific neo-antigens, which can induce an immunological response with recruitment and activation of T-cells [21]. One way to stimulate PD-L1 upregulation is afforded by the pro-inflammatory cytokine interferon-gamma (IFN-γ), which is produced by activated T-cells and Natural Killer cells [22]. The high expression of PD-L1 in MSI tumours with abundant infiltration of TILs is in accordance with the consensus molecular subtype (CMS) classification. The molecular group CMS1 is characterized by hypermutation, MSI and intense immune reaction [23], and this immunogenic group has been documented with a high PD-L1 expression [24].
In the group of patients with MSI tumours, the Kaplan-Meier curves were clearly separated regarding RFS, with a worse RFS related to a high PD-L1 expression, but statistical significance was not reached. This is in accordance with studies of tumour expression of PD-L1 in MSI stage I-IV CRC. Kim et al [9] reported a tendency towards a worse prognosis for tumours with high PD-L1 expression; however results were non-significant. Rosenbaum et al [10] reported no prognostic value for dichotomized data, but the group with the highest expression of PD-L1 (≥50%) had a markedly reduced disease-specific survival. We only found 14 patients to have PD-L1 expression ≥50%, and using this cut-off in our cohort did not enhance the prognostic impact (data not shown). Rosenbaum et al investigated all stages of CRC and found PD-L1 expression related to stage, which might explain the difference.
In contrast to our data, Koganemaru et al [12] reported high PD-L1 expression being an independent prognostic marker. They used the same cut-off (5%) in their evaluation of PD-L1, but they exclusively investigated stage III CRC. They found an association between PD-L1 and N status with high PD-L1 expression being related to higher N status. This may be part of the explanation for the inconsistency, as we only investigated stage II CC. Unfortunately, Koganemaru et al did not report any data on MSI status.
Lee et al [11] documented PD-L1 expression as an independent prognostic marker in patients with MSI tumours. In the present populationbased study 172 patients had a MSI tumour and only 31 (18%) of these tumours were classified as high PD-L1, resulting in a small group and thus low statistical power in the Cox regression analysis. This may be part of the explanation for the non-significant results.
Furthermore, previous studies reporting independent prognostic impact of PD-L1, differ in investigated cohorts and evaluation methods. Lee et al [11] included all stages of both colon and rectal cancers. Also their evaluation method differed in a number of ways from our technical approach. They used tissue microarrays (TMAs), as do most other studies [9,10,11,12,13,14,15]. In constrast we evaluated the PD-L1 expression in whole sections and observed a lot of heterogeneity both between the central part of the tumour and the invasive margin, and along the invasive tumour front. The use of TMAs may lead to selection bias, although several studies try to avoid this by using several representative TMAs from each tumour [9, 11, 13]. Lee et al [14] descreibe intra-tumoral heterogeneity of PD-L1 expression on tumour cells in 13% of the investigated tumours based on evaluation of TMAs from the central and invasive tumour compartments. They included all stages of CRC. In contrast we evaluated PD-L1 expression in a highly homogenous cohort of stage II CC patients and found a considerable intra-tumoral heterogeneity.
A standardized scoring system for PD-L1 expression in CC is missing and several unvalidated methods are in use. In the present study, the proportion of PD-L1 positive tumour cells was evaluated considering only membranous staining as positive. Intensity of the staining and cytoplasmic immunoreaction were not considered. Whether to take cytoplasmic staining of PD-L1 into account differs among studies. Some investigations use a combination of membranous staining and staining intensity [11] while others do not consider cytoplasmic staining at all [10]. PD-L1 expressed on the cell surface is essential for the interaction with the PD-1 receptor on the T-cells, indicating that only PD-L1 expressed on the membrane is of clinical importance. Furthermore, in the evaluation of lung cancer, only membrane staining is applied when evaluating the indication for immunotherapy with a PD-L1 inhibitor [25].
As mentioned above, we did not consider intensity of the staining. Intensity of IHC may be difficult to interpret. Poor reproducibility of IHC staining intensities of various proteins has been documented, while excellent inter-observer reproducibility was found estimating the fraction of positive tumour cells [26]. PD-L1 was not included in that study, but the same most likely applies to this protein. Furthermore, the evaluation of staining intensity is not only influenced by subjectivity. Various other factors may affect the staining intensity, encompassing both pre-analytical and analytical factors such as fixation (time and type), storage, and IHC protocols. Also section thickness affects staining intensity, and even modern state of the art microtomes produce sections with varying thicknesses.
The lack of a standardized IHC method challenges the assessment of PD-L1 expression, and moreover, different trials use different antibodies and assays. In melanoma, diverse assays have been found to variate in staining sensitivity of tumour cells [27]. For future studies, standardized techniques for evaluating PD-L1 in CC are required regarding antibodies, assays, interpretation, and threshold cut-off in scoring the immunostain. Also, focus should be directed on how to handle the marked heterogeneity, as this might hinder reproducibility of IHC scoring systems.
The present study is limited by the retrospective design, as we had no influence on the pre-analytical phase of the IHC. However, we used a validated antibody on a fully automatic platform, and only considered membranous staining according to the manufactures recommendation. The PD-L1 antibody used in this study stained both the malignant epithelial cells and immune cells in the stroma, which made it difficult to discriminate these cellular populations when the density of immune cells in the tumour stroma interface was high.
The prognostic value of PD-L1 expression on tumour cells is controversial. We investigated the expression of PD-L1 exclusively in stage II CC in a well-defined and unbiased population, and did not find any prognostic value of PD-L1 as a single biomarker. The PD-L1 expression in tumour cells should be seen in the context of the entire immune tumour microenvironment. The expression of PD-L1 is influenced by TILs, which is related to MSI. We did not prove any prognostic value of PD-L1 in patients with MSI tumours, however this interaction should be taken into consideration in future studies.
Conclusions
In this nationwide population-based cohort of stage II colon cancer, we found membranous PD-L1 expression (cut-off 5%) in tumour cells of stage II colon cancer to be associated with female gender, high malignancy grade, right side localisation and MSI. The expression of PD-L1 was often highly heterogenous. No prognostic information was detected of PD-L1 as a single biomarker in this cohort of stage II colon cancer.
Abbreviations
- CC:
-
Colon cancer
- CRC:
-
Colorectal cancer
- DCCG:
-
Danish Colorectal Cancer Group
- HR:
-
Hazard ratio; IHC: Immunohistochemical
- MSI:
-
Microsatellite instability
- MSS:
-
Microsatellite stability
- NOS:
-
Not otherwise specified
- OS:
-
Overall survival
- PD-1:
-
Programmed death receptor 1
- PD-L1:
-
Programmed death ligand 1
- RFS:
-
Recurrence-free survival
- TILs:
-
Tumour infiltrating lymphocytes
- TMA:
-
Tissue microarray
References
Morris M, Platell C, McCaul K, Millward M, van Hazel G, Bayliss E, et al. Survival rates for stage II colon cancer patients treated with or without chemotherapy in a population-based setting. Int J Color Dis. 2007;22(8):887–95.
Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, et al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(6):64–72.
Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408–19.
O'Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29(25):3381–8.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800.
Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19(3):309–14.
Kim JH, Park HE, Cho NY, Lee HS, Kang GH. Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br J Cancer. 2016;115(4):490–6.
Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29(9):1104–12.
Lee LH, Cavalcanti MS, Segal NH, Hechtman JF, Weiser MR, Smith JJ, et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29(11):1433–42.
Koganemaru S, Inoshita N, Miura Y, Miyama Y, Fukui Y, Ozaki Y, et al. Prognostic value of programmed death-ligand 1 expression in patients with stage III colorectal cancer. Cancer Sci. 2017;108(5):853–8.
Masugi Y, Nishihara R, Yang J, Mima K, da Silva A, Shi Y, et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017;66(8):1463–73.
Lee KS, Kwak Y, Ahn S, Shin E, Oh HK, Kim DW, et al. Prognostic implication of CD274 (PD-L1) protein expression in tumor-infiltrating immune cells for microsatellite unstable and stable colorectal cancer. Cancer immunology. Immunotheraphy. 2017;66(7):927–39.
Li Y, Liang L, Dai W, Cai G, Xu Y, Li X, et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15(1):55.
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93(4):387–91.
Eriksen AC, Sørensen FB, Lindebjerg J, Hager H, dePont Christensen R, Kjær-Frifeldt S, et al. The prognostic value of tumour infiltrating lymphocytes in stage II colon cancer. A nationwide population-based study. Transl Oncol. 2018;11(4):979–87.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Korehisa S, Oki E, Iimori M, Nakaji Y, Shimokawa M, Saeki H, et al. Clinical significance of programmed cell death-ligand 1 expression and the immune microenvironment at the invasive front of colorectal cancers with high microsatellite instability. Int J Cancer. 2018;142(4):822–32.
Zhang Y, Sun Z, Mao X, Wu H, Luo F, Wu X, et al. Impact of mismatch-repair deficiency on the colorectal cancer immune microenvironment. Oncotarget. 2017;8(49):85526–36.
Boissiere-Michot F, Lazennec G, Frugier H, Jarlier M, Roca L, Duffour J, et al. Characterization of an adaptive immune response in microsatellite-instable colorectal cancer. Oncoimmunology. 2014;3:e29256.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–61.
Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6.
Reilley M, Blando JM, Katkhuda R, Menter D, Sharma P, Allison JP, et al. Immunologic profiling of consensus molecular subtype (CMS) stratified colorectal cancer (CRC) primary and liver metastectomy specimens: implications for immune targeting of proficient mismatch repair CRC. J Clin Oncol. 2016;34(15):3520.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Zlobec I, Terracciano L, Jass JR, Lugli A. Value of staining intensity in the interpretation of immunohistochemistry for tumor markers in colorectal cancer. Virchows Arch. 2007;451(4):763–9.
Sunshine JC, Nguyen PL, Kaunitz GJ, Cottrell TR, Berry S, Esandrio J, et al. PD-L1 expression in melanoma: a quantitative Immunohistochemical antibody comparison. Clin Cancer Res. 2017;23(16):4938–44.
Acknowledgements
We thank Birgit Roed Sørensen and Christina Braad Petersen for excellent technical assistance. We also thank all pathology departments in Denmark for their support and participation in the study, which has made the population-based design possible. Also, we appreciate the help and advice supported by OPEN, Odense Patient data Explorative Network, Odense University Hospital, Odense, Denmark. Finally, we are grateful to Roche Diagnostics for the discount provided, regarding antibody expenses.
Funding
The study was conducted and affiliated under Danish Colorectal Cancer Group and Danish Colorectal Center South, Vejle Hospital, Part of Lillebaelt Hospital, Denmark. Financial support was granted by The Research Council of Lillebaelt Hospital and the Danish Cancer Research Foundation. The commercial company Roche Diagnostics supported the study providing antibodies at a discount. The funding organisations did not play any role in the study design, data collection, analysis or interpretation of the data, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: ACE, FBS, SKF, JL and TFH. Performed the experiments: ACE. Analyzed the data: ACE, RD and TFH. Wrote the paper: ACE, FBS, HH, JL and TFH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Regional Committees on Health Research Ethics for Southern Denmark approved the study and exempted the study from the rule of informed consent in their approvel (S-20140119). The study was also approved by the Danish Data Protection Agency (14/26345). All patients were screened in the Danish Registry of Tissue Utilization before enrollment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Eriksen, A.C., Sørensen, F.B., Lindebjerg, J. et al. Programmed Death Ligand-1 expression in stage II colon cancer - experiences from a nationwide populationbased cohort. BMC Cancer 19, 142 (2019). https://doi.org/10.1186/s12885-019-5345-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-019-5345-6