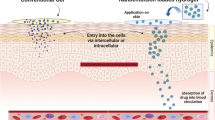
Abstract
In the present study, a novel transdermal delivery system was developed and its advantages were demonstrated. Ibuprofen is a commonly used anti-inflammatory, antipyretic, and analgesic drug; however, because of its short biological half-life, it must be frequently administered orally and is highly irritating to the digestive tract. To prepare a novel transdermal delivery system for ibuprofen, a microemulsion was used as a drug carrier and dispersed in a hyaluronic acid-based hydrogel (ME/Gel) to increase percutaneous drug absorption while avoiding gastrointestinal tract irritation. The prepared microemulsion had a droplet size of ~ 90 nm, and the microemulsion had good stability in the hydrogel. Rheological tests revealed that the ME/Gel is a pseudoplastic fluid with decreased viscosity and increased shear rate. It displayed a certain viscoelasticity, and the microemulsion distribution displayed minor effects on the rheological characteristics of the hydrogel system. There was no significant difference in the rheology of the ME/Gel at 25°C and 32°C (normal skin surface temperature), which is beneficial for clinical application. Drug transdermal flux was significantly higher than that of the hydrogel and commercial cream groups (p < 0.01). The 24-h cumulative drug permeation amount was 1.42-fold and 2.52-fold higher than that of the hydrogel and cream groups, respectively. By loading into the ME/Gel, the cytotoxicity of the drug to HaCaT cells was reduced. These results indicate that the prepared ME/Gel can effectively improve transdermal ibuprofen delivery and the biosafety of the drug and could therefore have applicability as a drug delivery system.






Similar content being viewed by others
Abbreviations
- IBU:
-
Ibuprofen
- HA:
-
Hyaluronic acid
- ME:
-
Ibuprofen-loaded microemulsion
- ME/Gel:
-
Ibuprofen-loaded microemulsion-based hyaluronic acid hydrogel
- Gel:
-
Ibuprofen-loaded hyaluronic acid hydrogel
- NS:
-
Normal saline
References
Potthast H, Dressman JB, Junginger HE, Midha KK, Oeser H, Shah VP, et al. Biowaiver monographs for immediate release solid oral dosage forms: ibuprofen. J Pharm Sci. 2005;94:2121–31.
Radman M, Babic A, Runjic E, JelicicKadic A, Jeric M, Moja L, et al. Revisiting established medicines: an overview of systematic reviews about ibuprofen and paracetamol for treating pain in children. Eur J Pain. 2019;23:1071–82.
Hussain MD, Saxena V, Brausch JF. Talukder RM Ibuprofen-phospholipid solid dispersions: improved dissolution and gastric tolerance. Int J Pharm. 2012;422:290–4.
Zhang J, Michniak-Kohn BB. Investigation of microemulsion and microemulsion gel formulations for dermal delivery of clotrimazole. Int J Pharm. 2018;536:345–52.
Mistraletti G, Paroni R, Umbrello M, Moro Salihovic B, Coppola S, Froio S, et al. Different routes and formulations of melatonin in critically ill patients. A pharmacokinetic randomized study. Clin Endocrinol (Oxf). 2019;91:209–18.
Ferreira P, Noronha L, Teixeira R, Vieira Í, Borba-Santos L, Viçosa A, de Moraes M, Calil-Elias S, de Freitas Z, da Silva F, Rozental S, Futuro D, Ferreira V. Investigation of a microemulsion containing clotrimazole and itraconazole for transdermal delivery for the treatment of sporotrichosis. J Pharm Sci. 2019.
Talaat SM, Elnaggar YSR, Abdalla OY. Lecithin microemulsion lipogels versus conventional gels for skin targeting of terconazole: in vitro, ex vivo, and in vivo investigation. AAPS PharmSciTech. 2019;20:161.
Wu JY, Li YJ, Liu TT, Ou G, Hu XB, Tang TT, et al. Microemulsions vs chitosan derivative-coated microemulsions for dermal delivery of 8-methoxypsoralen. Int J Nanomedicine. 2019;14:2327–40.
Ghorbanzadeh M, Farhadian N, Golmohammadzadeh S, Karimi M, Ebrahimi M. Formulation, clinical and histopathological assessment of microemulsion based hydrogel for UV protection of skin. Colloids Surf B Biointerfaces. 2019;179:393–404.
Carvalho ALM, Silva JA, Lira AAM, Almeida EDP, Nunes RS, Sarmento VHV, et al. Third-generation transdermal delivery systems containing zidovudine: effect of the combination of different chemical enhancers and a microemulsion system. AAPS PharmSciTech. 2018;19:3219–27.
Tabosa MAM. de Andrade ARB, Lira AAM, SarmentoVHV, de Santana DP, Leal LB. Microemulsion formulations for the transdermal delivery of lapachol. AAPS PharmSciTech. 2018;19:1837–46.
Jagdale SC, Deore GK, Chabukswar AR. Development of microemulsion based nabumetone transdermal delivery for treatment of arthritis. Recent Pat Drug Deliv Formul. 2018;12:130–49.
Kim KT, Kim MH, Park JH, Lee JY, Cho HJ, Yoon IS, et al. Microemulsion-based hydrogels for enhancing epidermal/dermal deposition of topically administered 20(S)-protopanaxadiol: in vitro and in vivo evaluation studies. J Ginseng Res. 2018;42:512–23.
Na YG, Byeon JJ, Wang M, Huh HW, Son GH, Jeon SH, et al. Strategic approach to developing a self-microemulsifying drug delivery system to enhance antiplatelet activity and bioavailability of ticagrelor. Int J Nanomedicine. 2019;14:1193–212.
Zeng J, Chen J, Chen L, Zheng W, Cao Y, Huang T. Enhanced oral bioavailability of chlormadinone acetate through a self-microemulsifying drug delivery system for a potential dose reduction. AAPS PharmSciTech. 2018;19:3850–8.
Haque T, Rahman KM, Thurston DE, Hadgraft J, Lane ME. Topical delivery of anthramycin I. Influence of neat solvents. Eur J Pharm Sci. 2017;104:188–95.
Pescina S, Garrastazu G, Del Favero E, Rondelli V, Cantù L, Padula C, et al. Microemulsions based on TPGS and isostearic acid for imiquimod formulation and skin delivery. Eur J Pharm Sci. 2018;125:223–31.
Sudha PN, Rose MH. Beneficial effects of hyaluronic acid. Adv Food Nutr Res. 2014;72:137–76.
Salwowska NM, Bebenek KA, Żądło DA, Wcisło-Dziadecka DL. Physiochemical properties and application of hyaluronic acid: a systematic review. J Cosmet Dermatol. 2016;15:520–6.
Witting M, Boreham A, Brodwolf R, Vávrová K, Alexiev U, Friess W, et al. Interactions of hyaluronic Acid with the skin and implications for the dermal delivery of biomacromolecules. Mol Pharm. 2015;12:1391–401.
Jung HS, Kim KS, Yun SH, Hahn SK. Enhancing the transdermal penetration of nanoconstructs: could hyaluronic acid be the key? Nanomedicine (Lond). 2014;9:743–5.
ÜstündağOkur N, Filippousi M, Okur ME, Ayla Ş. ÇağlarEŞ, Yoltaş A, Siafaka PI, A novel approach for skin infections: controlled release topical mats of poly(lactic acid)/poly(ethylene succinate) blends containing Voriconazole. J Drug Deliv Sci Technol. 2018;46:74–86.
ÜstündağOkur N, Hökenek N, Okur ME, Ayla Ş, Yoltaş A, Siafaka PI, et al. An alternative approach to wound healing field; new composite films from natural polymers for mupirocin dermal delivery. Saudi Pharm J. 2019;27:738–52.
Ukeda H, Kawana D, Maeda S, Sawamura M. Spectrophotometric assay for superoxide dismutase based on the reduction of highly water-soluble tetrazolium salts by xanthine-xanthine oxidase. Biosci Biotechnol Biochem. 1999;63:485–8.
Ren Q, Deng C, Meng L, Chen Y, Chen L, Sha X, et al. In vitro, ex vivo, and in vivo evaluation of the effect of saturated fat acid chain length on the transdermal behavior of ibuprofen-loaded microemulsions. J Pharm Sci. 2014;103:1680–91.
Ghosh PK, Murthy RS. Microemulsions: a potential drug delivery system. Curr Drug Deliv. 2006;3:167–80.
Sun L, Liu Z, Wang L, Cun D, Tong HHY, Yan R, et al. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J Control Release. 2017;254:44–54.
Zhao X, Liu JP, Zhang X, Li Y. Enhancement of transdermal delivery of theophylline using microemulsion vehicle. Int J Pharm. 2006;327(1-2):58–64.
Furubayashi T, Inoue D, Kamaguchi A, Higashi Y, Sakane T. Influence of formulation viscosity on drug absorption following nasal application in rats. Drug Metab Pharmacokinet. 2007;22:206–11.
Shmidov Y, Zhou M, Yosefi G, Bitton R, Matson JB. Hydrogels composed of hyaluronic acid and dendritic ELPs: hierarchical structure and physical properties. Soft Matter. 2019;15:917–25.
Morsi N, Ibrahim M, Refai H, El Sorogy H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur J Pharm Sci. 2017;104:302–14.
Zhang YT, Li Z, Zhang K, Zhang HY, He ZH, Xia Q, et al. Co-delivery of evodiamine and rutaecarpine in a microemulsion-based hyaluronic acid hydrogel for enhanced analgesic effects on mouse pain models. Int J Pharm. 2017;528:100–6.
Kelchen MN, Brogden NK. In vitro skin retention and drug permeation through intact and microneedle pretreated skin after application of propranolol loaded microemulsions. Pharm Res. 2018;35:228.
Lu IJ, Fu YS, Chang WY, Wu PC. Using microemulsion as carrier for drug transdermal delivery: the effect of surfactants and cosurfactants. Curr Pharm Des. 2019. https://doi.org/10.2174/1381612825666190527091528.
Björklund S, Pham QD, Jensen LB, Knudsen NØ, Nielsen LD, Ekelund K, et al. The effects of polar excipients transcutol and dexpanthenol on molecular mobility, permeability, and electrical impedance of the skin barrier. J Colloid Interface Sci. 2016;479:207–20.
Osborne DW, Musakhanian J. Skin penetration and permeation properties of Transcutol®-neat or diluted mixtures. AAPS PharmSciTech. 2018;19:3512–33.
Cao M, Ren L, Chen G. Formulation optimization and ex vivo and in vivo evaluation of celecoxib microemulsion-based gel for transdermal delivery. AAPS PharmSciTech. 2017;18:1960–71.
Hu XB, Kang RR, Tang TT, Li YJ, Wu JY, Wang JM, et al. Topical delivery of 3,5,4′-trimethoxy-trans-stilbene-loaded microemulsion-based hydrogel for the treatment of osteoarthritis in a rabbit model. Drug Deliv Transl Res. 2019;9:357–65.
Tung NT, Vu VD, Nguyen PL. DoE-based development, physicochemical characterization, and pharmacological evaluation of a topical hydrogel containing betamethasone dipropionate microemulsion. Colloids Surf B Biointerfaces. 2019;181:480–8.
Mastrangelo R, Montis C, Bonelli N, Tempesti P, Baglioni P. Surface cleaning of artworks: structure and dynamics of nanostructured fluids confined in polymeric hydrogel networks. Phys Chem Chem Phys. 2017;19:23762–72.
International Organization for Standardization. Biological evaluation of medical devices—Part 5: tests for in vitro cytotoxicity. ISO10993-5:2009.
Yilmaz E, Borchert HH. Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity and erythema--an in vivo study. Int J Pharm. 2006;307:232–8.
Contributions
All authors contributed to the experimental studies, data collection and statistical analysis, and the structuring and writing of the article.
Availability of Data and Materials
The data used to derive Fig. 3 and Fig. 5b in this work can be obtained upon reasonable request from the corresponding author.
Funding
This work was financially supported by the National Natural Science Foundation of China (81673612, 81573619).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Informed Consent
The study protocol was approved by the Experimental Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine (License No. SYXK (HU) 2014-0008).
Consent for Publication
All authors accept the submission conditions.
Competing Interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhang, K., Wang, Z. et al. Transcutol® P/Cremophor® EL/Ethyl Oleate–Formulated Microemulsion Loaded into Hyaluronic Acid–Based Hydrogel for Improved Transdermal Delivery and Biosafety of Ibuprofen. AAPS PharmSciTech 21, 22 (2020). https://doi.org/10.1208/s12249-019-1584-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1584-8