Abstract
Purpose
Topical beta-blockers are efficacious for treating infantile hemangiomas, but no formulations have been specifically optimized for skin delivery. Our objective was to quantify skin concentrations and drug permeation of propranolol (a nonselective beta-blocker) after application of microemulsions to intact and microneedle pretreated skin.
Methods
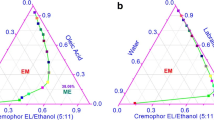
Four propranolol-loaded microemulsions were characterized for droplet size, surface charge, conductivity, pH, drug solubility, and drug release. Skin concentrations and drug permeation through skin were quantified using LC-MS. Skin-to-receiver ratios were used to compare the microemulsion formulations to a drug-in-PBS solution.
Results
Propranolol solubility was significantly greater in microemulsions vs PBS. Cumulative drug release from the microemulsions over 24 h ranged from 13 to 26%. Skin concentrations and drug permeation through intact skin was significantly higher from PBS; however, the skin-to-receiver ratios were significantly higher for water-rich microemulsions compared to PBS or surfactant-rich microemulsions. Microneedle pretreatment significantly increased skin concentrations for all formulations. Skin-to-receiver ratios significantly increased after microneedle pretreatment for surfactant-rich microemulsions.
Conclusions
Microemulsion formulation can be altered to elicit different drug delivery profiles through MN-treated skin. This could be advantageous for maximizing local skin drug concentrations and improving dosing schedules for infantile hemangioma treatment.






Similar content being viewed by others
Abbreviations
- ER:
-
Enhancement ratio
- IH:
-
Infantile hemangioma
- MN:
-
Microneedle
References
Guy RH. Transdermal drug delivery. Handb Exp Pharmacol. 2010;197:399–410.
Flynn G. Cutaneous and transdermal delivery - processes and systems of delivery. 4th ed. In: Banker G, Siepmann J, Rhodes C, editors. Modern Pharmaceutics. Boca Raton: CRC Press; 2002.
Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–68.
Zhang Y, Brown K, Siebenaler K, Determan A, Dohmeier D, Hansen K. Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm Res. 2012;29(1):170–7.
Shin JU, Kim JD, Kim HK, Kang HK, Joo C, Lee JH, et al. The use of biodegradable microneedle patches to increase penetration of topical steroid for prurigo nodularis. Eur J Dermatol. 2018;28(1):71–7.
Choi SY, Kwon HJ, Ahn GR, Ko EJ, Yoo KH, Kim BJ, Lee C, Kim D. Hyaluronic acid microneedle patch for the improvement of crow’s feet wrinkles. Dermatol Ther. 2017;30(6). https://doi.org/10.1111/dth.12546
Milewski M, Stinchcomb AL. Vehicle composition influence on the microneedle-enhanced transdermal flux of naltrexone hydrochloride. Pharm Res. 2011;28(1):124–34.
Gujjar M, Banga AK. Vehicle influence on permeation through intact and compromised skin. Int J Pharm. 2014;472(1–2):362–8.
Pappinen S, Urtti A. Microemulsions. In: Dragicevic N, Maibach HI, editors. Percutaneous penetration enhancers chemical methods in penetration enhancement: Nanocarriers. Berlin, Heidelberg: Springer Berlin Heidelberg; 2016. p. 253–62.
Heuschkel S, Goebel A, Neubert RH. Microemulsions--modern colloidal carrier for dermal and transdermal drug delivery. J Pharm Sci. 2008;97(2):603–31.
Songkro S, Lo NL, Tanmanee N, Maneenuan D, Boonme P. In vitro release, skin permeation and retention of benzophenone-3 from microemulsions (o/w and w/o). J Drug Deliv Sci Technol. 2014;24(6):703–11.
Amarji B, Garg NK, Singh B, Katare OP. Microemulsions mediated effective delivery of methotrexate hydrogel: more than a tour de force in psoriasis therapeutics. J Drug Target. 2016;24(2):147–60.
Ren Q, Deng C, Meng L, Chen Y, Chen L, Sha X, et al. In vitro, ex vivo, and in vivo evaluation of the effect of saturated fat acid chain length on the transdermal behavior of ibuprofen-loaded microemulsions. J Pharm Sci. 2014;103(6):1680–91.
Baroli B, Lopez-Quintela MA, Delgado-Charro MB, Fadda AM, Blanco-Mendez J. Microemulsions for topical delivery of 8-methoxsalen. J Control Release. 2000;69(1):209–18.
Mitragotri S. Synergistic effect of enhancers for transdermal drug delivery. Pharm Res. 2000;17(11):1354–9.
Zu Q, Yu Y, Bi X, Zhang R, Di L. Microneedle-assisted percutaneous delivery of a Tetramethylpyrazine-loaded microemulsion. Molecules. 2017;22(11).
Munden A, Butschek R, Tom WL, Marshall JS, Poeltler DM, Krohne SE, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170(4):907–13.
Leaute-Labreze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taieb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649–51.
Leaute-Labreze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390(10089):85–94.
Painter SL, Hildebrand GD. Review of topical beta blockers as treatment for infantile hemangiomas. Surv Ophthalmol. 2016;61(1):51–8.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–8.
Montenegro L, Carbone C, Condorelli G, Drago R, Puglisi G. Effect of oil phase lipophilicity on in vitro drug release from o/w microemulsions with low surfactant content. Drug Dev Ind Pharm. 2006;32(5):539–48.
Ibrahim SA, Li SK. Efficiency of fatty acids as chemical penetration enhancers: mechanisms and structure enhancement relationship. Pharm Res. 2010;27(1):115–25.
Kaur G, Mehta SK. Developments of Polysorbate (tween) based microemulsions: preclinical drug delivery, toxicity and antimicrobial applications. Int J Pharm. 2017;529(1–2):134–60.
Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev. 2002;54(Suppl 1):S77–98.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56(5):603–18.
Rhee YS, Choi JG, Park ES, Chi SC. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 2001;228(1–2):161–70.
Hua L, Weisan P, Jiayu L, Ying Z. Preparation, evaluation, and NMR characterization of vinpocetine microemulsion for transdermal delivery. Drug Dev Ind Pharm. 2004;30(6):657–66.
Kreilgaard M, Pedersen EJ, Jaroszewski JW. NMR characterisation and transdermal drug delivery potential of microemulsion systems. J Control Release. 2000;69(3):421–33.
Delgado-Charro MB, Iglesias-Vilas G, Blanco-Méndez J, López-Quintela MA, Marty J-P, Guy RH. Delivery of a hydrophilic solute through the skin from novel microemulsion systems. Eur J Pharm Biopharm. 1997;43(1):37–42.
Tabosa MAM, de Andrade ARB, Lira AAM, Sarmento VHV, de Santana DP, Leal LB. Microemulsion formulations for the transdermal delivery of Lapachol. AAPS PharmSciTech. 2018;19(4):1837–46.
Lu GW, Gao P. CHAPTER 3 - emulsions and microemulsions for topical and transdermal drug delivery A2 - kulkarni, Vitthal S. Handbook of non-invasive drug delivery systems. Boston: William Andrew Publishing; 2010. p. 59–94.
Changez M, Varshney M, Chander J, Dinda AK. Effect of the composition of lecithin/n-propanol/isopropyl myristate/water microemulsions on barrier properties of mice skin for transdermal permeation of tetracaine hydrochloride: in vitro. Colloids Surf B: Biointerfaces. 2006;50(1):18–25.
Djordjevic L, Primorac M, Stupar M. In vitro release of diclofenac diethylamine from caprylocaproyl macrogolglycerides based microemulsions. Int J Pharm. 2005;296(1–2):73–9.
El Maghraby GM. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: effects of cosurfactants. Int J Pharm. 2008;355(1–2):285–92.
Guy RH, Hadgraft J. Physicochemical aspects of percutaneous penetration and its enhancement. Pharm Res. 1988;5(12):753–8.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by NIH grant R35GM124551.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kelchen, M.N., Brogden, N.K. In Vitro Skin Retention and Drug Permeation through Intact and Microneedle Pretreated Skin after Application of Propranolol Loaded Microemulsions. Pharm Res 35, 228 (2018). https://doi.org/10.1007/s11095-018-2495-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2495-1