Abstract
3D printing has emerged as an advanced manufacturing technology in the field of pharmaceutical sciences. Despite much focus on enteral applications, there has been a lack of research focused on potential benefits of 3D printing for parenteral applications such as wound dressings, biomedical devices, and regenerative medicines. 3D printing technologies, including fused deposition modeling, vat polymerization, and powder bed printing, allow for rapid prototyping of personalized medications, capable of producing dosage forms with flexible dimensions based on patient anatomy as well as dosage form properties such as porosity. Considerations such as printing properties and material selection play a key role in determining overall printability of the constructs. These parameters also impact drug release kinetics, and mechanical properties of final printed constructs, which play a role in modulating immune response upon insertion in the body. Despite challenges in sterilization of printed constructs, additional post-printing processing procedures, and lack of regulatory guidance, 3D printing will continue to evolve to meet the needs of developing effective, personalized medicines for parenteral applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
3D printing has revolutionized the way researchers approach developing treatments for patients in recent years. 3D printing is an additive manufacturing technology in which objects are constructed in a layer-by-layer fashion. Layer adhesion can be achieved via heat fusion, ultraviolet light (UV), and through chemical bonding depending upon the type of 3D printing technology used. Common techniques include fused deposition modeling (FDM), vat polymerization (VP), and powder bed printing. Although its history can be traced back to the 1980s, 3D printing was not well studied for pharmaceutical applications until the mid-2000s. In 2015, the US Food and Drug Administration (FDA) approved the first 3D printed drug product Spritam®, a fast disintegrating orodispersible tablet containing levetiracetam for epilepsy treatment [1]. Spritam® is produced using ZipDose® technology, which is a proprietary powder bed-based 3D printing technology capable of producing highly porous tablets. The FDA approval of this product was pivotal as it demonstrated the commercial success of 3D printed drug products. There have been numerous research articles and reviews highlighting applications of 3D printing technology in oral dosage forms. However, there is a lack of literature and research centered on 3D printing for parenteral applications.
3D printing allows for quick and flexible design and production of patient-personalized parenteral medicines, with precise control over size and shape, porosity, and mechanical properties of printed constructs [2]. For example, 3D printing technology enables researchers to produce scaffolds with tunable drug release kinetics by modulating pore size/architecture as well as shape of printed constructs [3]. Through optimization of materials and printing parameters, 3D printed constructs can be fabricated exhibiting porous architecture with mechanical properties that more closely mimic native tissue, resulting in more biocompatible constructs favoring cell adhesion and proliferation, suitable for regenerative applications [4]. In addition, 3D printing technology has evolved to allow for multi-material printing, which allows scientists to harness the benefits of each material in a single dosage form. This enhanced design flexibility has paved the way for the development of complex constructs, such as fabricating prints with a core/shell structure to enhance either stent patency or improve vascularization for bone regeneration [5,6].
The present review highlights recent parenteral applications aided by 3D printing, current challenges, and future perspectives of this emerging manufacturing technology.
KEY ASPECTS OF FABRICATING PARENTERAL DOSAGE FORMS VIA 3D PRINTING
Types of 3D Printing Technology
Extrusion-Based 3D Printing
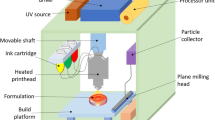
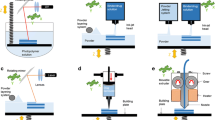
FDM is a popular type of extrusion-based 3D printing technology used to produce parenteral dosage forms. As shown in Fig. 1a, FDM works by feeding a filament, typically consisted of a thermoplastic material (or blend of materials) into a high temperature nozzle to melt the material before being extruded onto the lower temperature build plate, where it is then cooled and solidified [7]. It allows for the production of 3D printed constructs in a quick and efficient manner, ideal for rapid prototyping [8]. Aside from material properties, final product quality is governed by FDM process parameters, including extrusion temperature, layer thickness, and nozzle diameter of the printing head [9]. Common filament materials used include biocompatible thermoplastic polymers such as polyvinyl alcohol (PVA), poly(lactic acid) (PLA), and polyvinylpyrrolidone [10]. One disadvantage of FDM technology is that typically, printing material needs to be inserted into a nozzle in the form of a solid filament, which does not exist for many pharmaceutical materials [10]. Thus, companion techniques, such as hot melt extrusion (HME), may be used to transform pharmaceutical grade materials, including active pharmaceutical ingredients (API), into FDM-suitable filaments [8]. Filaments can also be impregnated with an API solution during the production of filaments, usually via HME [11]. However, thermolabile therapeutics are not suitable for extrusion via FDM, due to potential degradation concerns [12]. It is worth mentioning that extrusion-based bioprinting at room or body temperature using bioinks has showed attractive clinical potential in achieving personalized treatment. For example, Long et al. developed a personalized 3D printed wound dressing composed of chitosan and pectin using an extrusion-based bioprinter, with the ability to control dimensional properties such as thickness (e.g., layer height of 0.25 mm) and pore size, while allowing for a facile lidocaine incorporation for immediate pain relief [13].
Vat Polymerization
In the case of VP technology, a build plate moves along the z-axis inside of a vat containing liquid resin, consisted of photopolymerizable monomer(s) and photoinitiator(s) (Fig. 1b). Once exposed to a specific wavelength of light (dependent on resin/printing material), polymerization of the monomer resin occurs [14]. This process continues, layer-by-layer, as unreacted functional groups in the previous layer are polymerized under light exposure, causing adherence to the current layer, resulting in layer formation [15]. API’s and excipients can be blended with a resin, effectively becoming trapped in the polymer matrix upon photopolymerization [16]. The two main VP techniques are “stereolithography” (SLA), which uses a UV laser beam, and “digital light processing” (DLP), which uses UV light from a projector, to cure the resin. Compared to SLA, DLP is more efficient as it can cure an entire layer at one time, reducing overall printing time. However, objects printed via SLA have a better spatial resolution (down to 25 μm) than DLP (35–100 μm), mainly due to the small optical spot size of SLA lasers [17]. VP printed objects typically undergo a post-printing curing process to ensure complete polymerization and further improve mechanical integrity of the prints, while reducing potential toxicity associated with the presence of residual monomers and oligomers [18,19]. SLA parameters (such as laser power and scan speed) and general VP parameters (e.g., resin characteristics and curing duration) contribute to the overall resolution of final prints [20]. VP printing can circumvent issues associated with other 3D printing technologies, such as avoiding thermal degradation of thermolabile therapeutics [21]. More importantly, VP techniques can achieve the highest printing resolution among all 3D printing technologies and hence process great translational potential in personalized implants. VP printing has been successfully used for parenteral applications, such as producing hydrogels for nerve and tissue regeneration [22,23,24,25]. However, the clinical applications of VP printing are still limited due to the lack of photopolymers with suitable mechanical properties and biocompatibility. Another major drawback to VP printing is that most commercial VP printers do not allow for multi-resin printing, thus limiting material selection and print design. To address this challenge, Konasch et al. developed a hybrid additive manufacturing technique combining both SLA and inkjet printing technologies to produce poly(ethylene glycol) diacrylate (PEGDA)-based matrices with multiple drug depots [26]. Essentially, a modified SLA printer was used to build the layers of the matrix system, consisting of PEGDA and lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) as the photoinitiator. Two inkjet printheads were used to deposit two materials layer-by-layer inside of the PEGDA-based matrix and modulated drug release was achieved by changing spatial positioning of such depots. Other researchers have attempted to produce multi-layered constructs by manually pausing prints, swapping resin tanks, and continuing the print, which is inefficient and negates the autonomy inherent to the 3D printing process [27].
Powder Bed Printing
Powder bed printing encompasses inkjet printing and selective laser sintering (SLS) technologies. Typically, powder bed printed constructs exhibit a minimum feature size down to approximately 50 μm [28]. Inkjet printing, illustrated in Fig. 1c, is a technique in which droplets of a binder solution are dispensed through printing heads, driven either by piezoelectric or thermal processes, onto a thin layer of bulk material, positioned in the powder bed [29]. Inkjet printing parameters including nozzle diameter and binder rheological properties have been shown to play a key role in modulating binder droplet size, which in turn impacts printing precision [30]. Huang et al. produced levofloxacin implants with predefined microstructures via inkjet printing. It was observed that more complex drug release (e.g., bimodal and pulsatile) can be achieved via this method in comparison to the traditional compression method, as 3D printing allows for the flexibility to incorporate multiple types of structures such as reservoir and matrix architectures in one dosage form [31]. A disadvantage associated with inkjet printing is caused by the binder hitting the powder bed and displacing powder, leading to sub-surface depletion zones. SLS is very similar to inkjet printing, instead using a laser to bind particles/powder together to form layers, as opposed to depositing a binding solution (Fig. 1d) [32]. Xia et al. developed SLS printed nano-hydroxyapatite/poly-ε-caprolactone (PCL) scaffolds with a highly porous architecture (150 μm in layer thickness and 70–79% porosity) and sustained rhBMP-2 release for improved bone defect repair [33]. One main drawback associated with SLS is potential degradation of payload when exposed to high energy lasers used in the printing process, which has limited its pharmaceutical applications [14].
Design and Personalization
Additive manufacturing techniques enable the design and production of patient-centric dosage forms with precise control over dimension and microstructure, a feat not achieved through traditional manufacturing techniques, like compression. These factors ultimately play a key role in modulating drug release kinetics [34]. Computer-aided design (CAD), preparation, and evaluation have shown great application prospects in the field of 3D printing. Firstly, 3D scanning combined with digital modeling can greatly improve the accuracy of models and printlets, promoting personalized clinical use. Dosage forms (e.g., implants, wound dressing) can be efficiently designed in a CAD software to precisely match patient anatomy, typically through the use of medical imaging data such as computer tomography (CT) or magnetic resonance imaging (MRI) scans (as illustrated in Fig. 2). Then, a software such as Slicer can be used to open these files, typically in a digital imaging and communications in medicine (DICOM) format. Next, segmentation is used to partition the image into different sections of interest (i.e., tissue, organs), and the image is saved as an STL file (3D readable format) and only the region of interest that has been sectioned off will be saved. A slicing software such as Chitubox can then be used to open up STL files and slice them to determine the number of layers in the final 3D printed object, before finally being printed. For example, customized molars with carious cavities were obtained via 3D scanning and FDM printing, and personalized dental fillers with high mechanical strength and “on-demand” drug release characteristics were fabricated [35]. In another study, CT images were used to create a CAD model of orbital floor implants, and a bio-compatible polycarbonate ISO (PC-ISO) material was used to print implants for the treatment of orbital fractures [36].
Workflow diagram depicting process of fabricating a therapeutic loaded (indicated by green triangles) and personalized 3D printed wound dressing, including a 3D design of construct via computer aided design software, such as Tinkercad; b Export design as stereolithography/standard triangle language (STL) file into slicing software, such as Chitubox, and slice into layers; c Upload the STL file into 3D printer and execute printing; and d Apply wound dressing to affected area to release payload as desired
Computer-aided methods have also been used to visualize and optimize the printing process. For example, finite element method (FEM) was used to elucidate the mechanism of FDM process by simulating the stress-strain behavior of filament during extrusion (Fig. 3) [37]. Computational fluid dynamics (CFD) was used to understand the melt flow field in the printing head during extrusion and provide useful information for further formulation and process optimization [38]. CFD has also been used to predict the flow velocity within different nozzle geometries during bioprinting and to establish a viability-stress-time-viscosity mathematical relationship [39]. Furthermore, computer-aided methods have been used for in vitro and in vivo evaluation of printlets. FEM was used to mimic the biomechanical properties of implants by simulating the Von Mises stress and strain distribution, while CFD was used to visualize the two-liquid mixing process and predict the perfusion process [40].
Radial stress-stain simulation of filament: a a 2D mesh model; b typical nonlinear material properties; c von Mises stress distribution, MPa; and d von Mises strain distribution, % (37)
Printability
In general, the term “printability” relates to the deformation resistance of material(s) during and after printing, which is influenced by factors including mechanical properties, thermal properties, and/or gelation mechanism of the material(s) [41]. Adequate mechanical properties are important to enable successful deposition of multiple layers during the printing process [42]. With respect to bioprinting, in which biological materials (e.g., cells) are blended with traditional scaffolding materials, cell survival rate post-printing is also a key parameter that contributes to the overall printability of the printing ink [43].
Printability of extrusion-based systems can be impacted by a variety of factors, including material properties (e.g., swelling, mechanical, and rheological) as well as printing parameters such as nozzle size, air pressure, and printing speed [44]. Yang et al. investigated the printability of a gelatin-based thermosensitive extrudable paste and found that addition of 25% microcrystalline cellulose resulted in filament with enhanced mechanical properties, and thus improved deformation resistance [38]. It has been previously reported that a shear thinning material with a viscosity ranging from 400 to 3000 mm2 s−1 exhibits a rapid gelling time, allowing for successful deposition and layering and hence, high fidelity prints [45,46]. Printing fidelity of VP processes is influenced by parameters such as photopolymer concentration, addition of plasticizers, and printing parameters (e.g., layer height and exposure time) [40]. Light attenuating additives (i.e., tartrazine, coccine) are commonly used in VP resins to absorb excess light, allowing for controllable photopolymerization, resulting in formation of layers with desired thickness [47]. In addition, post-printing curing procedures involving exposure to UV light and elevated temperature may lead to shape deformations, thus reducing overall printability [48]. Powder bed printability can be attributed to parameters including powder particle size and binder over-spreading. In general, binder over-spreading can lead to prints with reduced dimensional accuracy [49]. Specifically, inkjet printability is largely dependent upon properties of ink, nozzle, and actuator. Ink properties such as viscosity and surface tension impact the resolution and uniformity of printed constructs. Nozzle properties (e.g., nozzle diameter and nozzle-substrate distance) impact Reynolds, Weber, and Ohnesorge numbers which are dimensionless numbers used to describe printability and droplet formation from inkjet printers [50]. In addition, inkjet printers rely on actuators, which can include piezoelectric actuation, electromagnetic forces, thermal actuation, and pneumatic pumps to eject ink droplets from the nozzle by overcoming ink surface tension [51]. Actuator type and its parameters can impact droplet size and overall print quality of constructs. Similarly, powder composition and properties such as particle size and polymer molecular weight (MW) impact printability and drug release behavior of SLS printed constructs [52]. Finer powder results in structures exhibiting enhanced green strength, smoother surface, quicker drug release, and reduced porosity, as well as an overall improvement in mechanical properties [53,54,55]. Laser properties (e.g., laser energy density) can also have an impact on SLS printability by altering powder bed temperature [32]. Lastly, high laser scan speeds have resulted in constructs exhibiting increased porosity, leading to rapid drug release and reduced mechanical properties due to shortened contact time between laser and powder [56,57].
Printing Materials
Materials used in 3D printed parenteral constructs need to be biocompatible to minimize immune response in the body, in addition to demonstrating suitable mechanical properties to ensure sufficient printability. Considerations such as ability to promote cell adhesion and proliferation should also be taken into account for parenteral applications including bone and tissue scaffolds [58]. Some materials commonly used in 3D printed parenteral constructs are listed in Tables I and II.
Synthetic Materials
Synthetic polymers such as polyesters, PVA, and polyurethane (PU) typically have more reproducible polymer characteristics and desirable mechanical properties (e.g., tensile strength and elastic modulus) compared to natural materials, which makes them more suitable for 3D printing applications [61].
Polyesters
Biodegradable polyester-based synthetic polymers, such as PLA, poly(lactic-co-glycolic acid) (PLGA), and PCL, are relatively hydrophobic and inherently biologically inert. Owing to their excellent biocompatibility and tunable mechanical properties, PLA and PLGA, a class of aliphatic polyesters, are suitable for 3D printing applications [62,63]. Tappa et al. developed FDM printed PLA-based osseous fixation devices, including surgical screws, pins, and bone plates [64]. The 3D printed PLA devices exhibited compressive strengths between 20 and 500 MPa, demonstrating feasibility in orthopedic applications. In another study, Wang et al. developed a 3D printed bilayer membrane, consisting of a PLGA nanofiber outer layer (layer height: 0.05 μm) and alginate hydrogel inner layer (layer height: 100 μm), designed to mimic the epidermal and dermal layers of the skin for use as a wound dressing with demonstrated accelerated wound healing ability in vivo [65]. Combining polyester materials with other polymers such as PU has been used to further enhance mechanical properties of polyesters [66]. However, the hydrophobicity of polyesters results in inadequate cell adhesion and poor osteogenesis, as well as potential bacterial adhesion and biofilm formation [67]. Thus, 3D printed polyester-based constructs have been functionalized with biomolecules such as collagen, minocycline, and hydroxyapatite to improve cell adhesion and promote bone regeneration [68,69] In addition, chemical structure modifications have been used to improve cell binding and hydrophilicity of polyesters [70].
Photopolymers
Biocompatible photopolymers such as PEGDA, PEG dimethacrylate (PEGDMA), and gelatin methacrylate (GelMA) are commonly used in VP technology (Table II). PEGDMA hydrogels exhibit similar compressive modulus to musculoskeletal tissue, making them a suitable choice for bone regeneration applications. A bioprinting setup, consisting of a Hewlett-Packard (HP) Deskjet thermal inkjet printer modified with an overhead UV lamp, was used to produce 3D printed PEGDMA bone constructs with Irgacure I-2959 as the photoinitiator [71]. In another study, Zhou et al. developed a GelMA-based bioink suitable for DLP printing containing LAP photoinitiator and a hyaluronic acid (HA) derivative to create functional living skin for skin regeneration applications [59]. Another photopolymer, poly(propylene fumarate) (PPF), has been used in various biomedical applications including bone tissue engineering due to its similar compressive modulus values as human trabecular bone [72]. Buyuksungur et al. developed PCL-based nanohydroxyapatite (HAp) and PPF-modified implants (PCL/HAp/PPF) produced via FDM printing for bone defect treatment [60]. The 3D printed PCL/HAp/PPF implants demonstrated improved compressive tension stiffness values (394 and 463 N/mm) when compared to healthy rabbit femur (316 and 392 N/mm) after 8 weeks of implantation.
Other Synthetic Materials
PVA is a highly water-soluble synthetic polymer produced via the hydrolysis of polyvinyl acetate. It is an attractive biomaterial due to its biocompatibility and unique ability to resist protein adsorption, as well as favorable mechanical properties (e.g., high tensile strength and elongation before breaking) [73]. Recently, Qamar et al. developed FDM printed PVA-based hernial meshes using a MakerBot FDM printer. It was observed that tensile strength of the meshes increased with an increase in thread diameter and decrease in pore size [74]. Boyer et al. developed FDM printed, cross-linked PVA-based porous hepatobiliary stents for the treatment of biliary obstruction [75].
PU is generally non-biodegradable but biocompatible and has been extensively used in various medical applications including vascular grafts, catheters, heart valves, and wound dressings. Recently, researchers have synthesized biodegradable PU to expand their biomedical applications [76,77]. Interestingly, PU are comprised of alternating hard and soft segments, the former owing to long-chain diols, which leads to enhanced elasticity, and the latter responsible for overall material strength due to the presence of crystalline regions [78]. Jung et al. developed 3D printed thermoplastic PU-based tracheal prostheses with higher tensile strength and enhanced flexibility compared to native trachea tissue. The microporous architecture of the 3D printed prostheses promoted biological interactions by allowing for cellular infiltration and facilitating ingrowth of connective tissue [79].
Natural Polymers
Natural polymers (e.g., chitosan, collagen, and gelatin) possess better biocompatibility and biological activity, including supporting cell attachment and differentiation, compared to synthetic polymers, making them an ideal choice for use in bioprinting applications [80]. However, natural polymers typically exhibit weaker mechanical properties than synthetic polymers, which may lead to a reduction in printability and hence unsuccessful prints. To overcome this hurdle, strategies such as forming composites with “stronger” materials (e.g., tricalcium phosphate, graphene), increasing crystallinity, and optimizing cross-linking conditions have enabled successful printing of constructs with enhanced mechanical properties [81].
Polysaccharide-Based Materials
Chitosan (CS), a cationic linear polysaccharide, has been shown to accelerate wound repair by promoting tissue growth and differentiation [82]. CS has also been shown to accelerate the formation of osteoblasts, leading to enhanced bone regeneration, and promote connective tissue regeneration [83]. Physicochemical properties (such as solubility, crystallinity, and degradation) of CS can be modulated by altering CS MW and degree of deacetylation [84]. CS has been used in 3D printing of both soft tissue (e.g., wound dressing) and hard tissue applications such as bone regeneration [85]. However, CS alone has relatively weak mechanical properties and poor printability. Thus, blending other materials (such as gelatin) with CS has been shown to improve mechanical properties, resulting in a higher fidelity print [86]. In a recent study, Intini et al. developed a FDM printed CS-raffinose scaffold for diabetes-related wound healing [87]. Addition of raffinose has been shown to enhance the mechanical properties, wettability, and hydrophilicity of CS films, thus promoting tissue regeneration in a rat model [88].
Alginate is a polysaccharide isolated from the cell walls of brown algae. Alginate can be cross-linked with calcium ions (Ca2+) in a facile manner to produce constructs that effectively mimic an extracellular matrix (ECM) structure [89]. The use of alginate in wound dressings has resulted in accelerated wound healing, due to its ability to maintain a moist environment and minimize bacterial infection [65]. Thus, alginate remains an excellent material choice for parenteral applications including wound dressings and tissue regeneration. Composite materials containing alginate and other polymers (e.g., gelatin) have been used to improve mechanical properties of alginate [90]. Li et al. developed graphene oxide (GO)–coated 3D printed alginate/gelatin scaffolds with enhanced mechanical strength as well as improved osteogenic differentiation and cell adhesion for bone regeneration applications [91].
HA, a linear biodegradable polysaccharide, has a ubiquitous presence and serves numerous roles in the human body, such as maintaining the ECM structure by interacting with proteoglycans and link proteins, in addition to acting as a signal molecule by interacting with various cell surface receptors, thereby mediating cellular functions [92]. Therefore, HA is an ideal material for use in parenteral applications such as wound healing and tissue/cartilage engineering. However, similar to other natural polymers, HA exhibits unfavorable mechanical properties, leading to low shape fidelity and poor printability for 3D printing applications. To overcome this limitation, Ouyang et al. developed extrusion-based 3D printed HA scaffolds via dual cross-linking (i.e., supramolecular and UV cross-linking) for use in cartilage and tissue engineering applications [42].
Protein-Based Materials
Over the last decade, silk derived from Bombyx mori (B. mori) silkworms has gained much attention across a host of biomedical applications including drug delivery and tissue regeneration, due to its impressive mechanical properties, biocompatibility, and processability [93]. The two main components of B. mori silk are as follows: (1) sericin (SS), a glue-like outer protein coating that is commonly removed via a degumming process to enhance overall mechanical properties; and (2) fibroin (SF), an insoluble inner core protein that provides mechanical stability [94]. Silk has been shown to exhibit enhanced tensile modulus and strength, compared to traditional well-studied polymers (e.g., collagen and PLA). Silk (without SS) exhibits an over 300-fold increase in modulus and approximately a 100-fold increase in ultimate tensile strength when compared to collagen. It also exhibits a 5-fold increase in modulus and a nearly 14-fold increase in ultimate tensile strength when compared to PLA [93]. In general, SF is an attractive bioink, as it can maintain cell viability and structural integrity of a 3D scaffold. Recently, a stem cell laden SF/gelatin hydrogel was bioprinted using an in-house designed multi-head deposition-based 3D printer [95]. Impressively, the printed SF/gelatin hydrogel maintained cell viability for more than 30 days.
Collagen is the most abundant protein ubiquitous in the human body, exhibiting a rod shaped quaternary structure formed via the entanglement of three left handed helices [96]. Collagen has been used to mimic the ECM structure in vitro, and has been shown to promote cell adhesion, proliferation, and migration of various types of cells, including bone marrow mesenchymal cells for tissue engineering applications [97]. Nocera et al. developed a porous collagen (type I)-based scaffold using an in-house extrusion-based 3D printer equipped with syringes and 21G needles [98]. The 3D printed scaffolds can support cell attachment and proliferation of fibroblast cells without cytotoxicity.
Gelatin, a type of linear peptide (MW: 15 to 250 kDa), is produced via heat and enzymatic denaturation of collagen [99]. Cross-linking of gelatin can be accomplished via chemical and enzymatic reactions, in addition to physical cross-linking which can be accomplished by heating gelatin solution to around 40–50°C, before cooling below 30°C at which point a semi-solid gel is formed [99]. Negrini et al. developed chemically cross-linked, gelatin-based scaffolds for adipose tissue engineering applications using an extrusion-based 3D printer [100]. Results showed that the scaffolds remained stable for 21 days and exhibited similar mechanical properties as native adipose tissue and supported adipogenic differentiation.
3D PRINTING IN PARENTERAL APPLICATIONS
3D printing technology has been successfully utilized to fabricate parenteral constructs such as implants, stents, and wound dressings (Table III).
Long-Acting Implants/Inserts
Long-acting implants/inserts have been widely utilized for various clinical applications, including contraception, cancer treatment, and localized delivery of anesthetics and antibiotics [105,106,107,108]. 3D printed implants can be designed to achieve tunable, sustained drug release through precise control over implant shape, size, and microstructure. In a recent study, Stewart et al. produced 3D printed rod-shaped PVA/PLA implants via FDM, with designed “windows” to modulate drug release [101]. Implants with smaller “windows” and a decreased number of total “windows” resulted in slower payload release. Impressively, these implants, dip-coated with a PCL polymer mixture, can retard payload release for up to 300 days. In order to provide personalized vaginal rings to avoid pelvic inflammatory disease and uterine perforations [109], Fu et al. developed 3D printed PLA/PCL composite vaginal rings with customized shapes (i.e., “O,” “Y,” and “M”) to accurately mimic the structure of female anatomy [104]. All printed vaginal rings exhibited shape-dependent progesterone release for 7 days. Similarly, Tappa et al. developed 3D printed vaginal inserts containing either estrogen or progesterone [110]. The inserts were fabricated to mimic clinically relevant surgical meshes, intrauterine devices, and pessaries, and demonstrated excellent biocompatibility and sustained payload release (Fig. 4).
3D printed constructs: a Control donut-shaped pessary; b Control Gellhorn-shaped pessary; c Control intrauterine device (IUD); d Pessary printed combinations of filaments (red—poly(lactic acid) and white—polycaprolactone (PCL)-estradiol (E2)); e PCL-estrone(E1) IUD; and f PCL-E2 IUD (110)
3D printing technology also allows for seamless integration of multiple API’s in a single dosage form for combination therapy. Qiao et al. developed 3D printed PLGA scaffolds for the combination therapy of doxorubicin and cisplatin against breast cancer [111]. The 3D printed scaffolds (pore sizes > 200 μm) were produced using a customized E-jet printer and were capable of delivering both drugs in a controlled release manner for up to 30 days, demonstrating synergistic antitumor effect of the combination therapy. Won et al. developed a 3D printed core (alginate and dexamethasone)/shell (PCL and bevacizumab) structured rod using a multi-head bioprinter for the treatment of retinal vascular diseases [112]. The rods exhibited sustained bevacizumab release over 60 days and dexamethasone release over 7 days, leading to suppressed angiogenesis over a 4-week period in a rat model.
Regenerative Applications
3D printing technology has shown promising in regenerative applications, particularly for treating bone defect, as it can accurately and quickly produce customizable scaffolds with defined microstructures and precise control over factors (such as shape, porosity, and mechanical properties), all of which impact magnitude of osteogenesis and angiogenesis [5,113,114,115,116]. For example, Zhang et al. developed a multifunctional bioceramic scaffold capable of promoting vascularized bone regeneration to treat large segmental bone defects [5]. Hollow-pipe-packed silicate bioceramic (BRT-H) scaffolds with a core/shell structure were produced via a modified extrusion-based 3D printer. The synergistic effect of the hollow channel structures produced via 3D printing and ionic components (e.g., silicon, magnesium, and calcium) of the alginate-based scaffold led to enhanced tissue growth and vascularization [117]. Similarly, Martin et al. engineered a multifunctional 3D printed PLA scaffold via FDM for bone regeneration [103]. The printed PLA scaffold exhibited a lattice-shaped structure with a controllable pore size of 1000 μm and a porosity around 55%. Multifunctionalization via a combination of collagen, minocycline, and hydroxyapatite, aided by scaffold porosity, resulted in improved antibacterial/antibiofilm properties while promoting osteogenesis. Calcium phosphate scaffolds (CPS) containing antibiotics (i.e., rifampin and vancomycin) have been developed via inkjet 3D printing for osteomyelitis therapy [102]. 3D printed porous CPS implants allowed for a 6-fold increase in vancomycin release compared to manually molded poly(methyl methacrylate) spacers, resulting in a reduction in mean bacterial load.
Implantable Biomedical Devices
Stents/Drug-Eluting Stents
Stents have been widely used to widen the affected blood vessels and restore blood flow for treating cardiovascular diseases, a leading cause of death around the world. Factors including stent strut thickness and structure (i.e., shape, geometry) have been shown to have a substantial impact on overall mechanical properties (e.g., radial force, radial recoil, and flexibility) of stents and hence stent effectiveness [118]. 3D printing technology allows for precise control over stent shape and dimensions using either a single material or combinations of multiple materials, to achieve desired mechanical and physical properties depending on application site, which is crucial to stent effectiveness [119]. Moreover, conventional methods (e.g., laser cutting) to produce metallic and polymeric stents can negatively impact overall stent microstructure, leading to microcracks [120]. 3D printing can minimize damage to stent microstructure by avoiding the use of high temperatures inherent to conventional laser cutting manufacturing. Recently, 3D printing technology has been implemented to produce biodegradable polymer-based stents with structure flexibility, ideal for ease of insertion, while maintaining a rigid structure to support the blood vessel. Guerra et al. developed biodegradable stents consisting of either PLA filament, PCL filament, or a combination of both via FDM printing [6]. PLA and PCL exhibit vastly different mechanical properties and degradation profiles, which when used alone, are insufficient for use in stent applications. However, when used together, composite stents can achieve more desirable mechanical properties. Composite stents composed of a PLA core and PCL shell exhibited a Young’s modulus around 1400 MPa and about 3% degradation over the span of 6 weeks, suitable for stent applications.
Drug-eluting stents (DES) can not only physically provide structure to keep the blood vessel open, but also release multiple therapeutics designed to treat post-surgical side effects such as inflammation. Therapeutics can be blended with polymers to create a drug-loaded filament for 3D printing, or coated on the surface of printed stents [121]. Kim et al developed a 3D printed PCL DES, using a deposition-based 3D printer, to treat recurrent obstructive salivary gland disease, commonly caused by the buildup of salivary stones [121]. The stent shape was derived from CT images to mimic salivary ducts after removal of salivary stones. The DES was designed and printed within 7 min, and showed sustained amoxicillin release (up to 28 days) to treat S. aureus and a relatively faster cefotaxime release (up to 3 days) to treat E. coli, resulting in enhanced antimicrobial activity via the combination therapy [122].
Wound Dressings
Additive manufacturing has shown great promise in wound healing applications. Wound healing is a complex process, involving hemostasis, inflammation, proliferation, and remodeling [123]. Ideally, tissue-engineered constructs should facilitate the biological function (e.g., suitable angiogenesis and re-epithelialization) and closely resemble the structural organization of the native tissue, which is a feat that can be accomplished through 3D printing technology [124]. 3D printing allows for precise control over the spatial distribution of biological components, biomaterials, and therapeutics, to enhance cell migration and proliferation, and accelerating the overall wound healing process while reducing inflammation and scarring [125,126]. The 3D printed wound dressing demonstrated excellent flexibility and similar adhesive strength when compared to marketed wound dressings. Similarly, Hung et al. developed a PU scaffold using a self-modified, low temperature FDM printer. Either sustained co-delivery or sequential delivery of combination therapeutics can be achieved by modulating scaffold designs and printing parameters [127]. 3D printing can also produce patient-specific wound dressings that match anatomically complex architecture. For example, Muwaffak et al. developed a patient-specific 3D printed antimicrobial wound dressing made of PCL incorporated with metal ions (e.g., copper, zinc, or silver) [128]. A 3D scanner was used to obtain images of a patient’s ear and nose, which were then uploaded and edited in a 3D printing software. Results showed that 3D printed personalized dressings can match the anatomical complexity of a patient while providing sustained release of metal ions for 72 h, resulting in effective inhibition against S. aureus.
Traditional Medical Devices
Additive manufacturing has also been utilized to produce prototype traditional medical devices such as autoinjectors and ophthalmic devices. EpiPen®, one of the most well-known examples of autoinjectors, used to treat acute anaphylaxis by delivering epinephrine via IM route [129]. Typically, EpiPen® is only capable of administering a single dose of epinephrine, which may be inadequate for patients requiring an additional dose to alleviate their symptoms [130,131]. Thus, researchers have developed 3D printed prototypes designed to meet the need of administering multiple doses of epinephrine, manufactured using 3D printers such as the Stratasys OBJET CONNEX 500, the Stratasys Dimension, and the MakerBot Replicator [132]. Researchers have also developed more sustainable and eco-friendly alternatives to traditional disposable autoinjectors by designing 3D printed reloadable autoinjectors, containing replaceable epinephrine-loaded cartridges [133]. The use of 3D printing in the field of ophthalmology has also been on the rise, with the emergence of more biocompatible materials which can reduce the risk of rejection and irritation [134]. For example, 3D printed corneas have been produced from biocompatible materials such as alginate, collagen, and human stem cells, and have been designed to match patient specific corneal geometrical and thickness specifications [135]. Researchers are continuing to make strides in other ophthalmic applications, such as producing 3D printed intraocular lenses (IOL), which require careful consideration of other parameters, such as refractive index (RI) of 3D printed layers, and 3D printed retinas, the success of which depends on the ability to successfully print multiple retinal cell types [134].
FUTURE PERSPECTIVES AND CHALLENGES
3D printing has tremendous potential in personalized medicines via parenteral routes. It has been successfully utilized to print cells in specific and predetermined spatial arrangements, which closely mimic the cellular organization of native tissue for tissue regeneration applications. Researchers have now turned their attention to using this additive manufacturing technology to print entire organs to solve organ donor shortage and immune rejection issues [136]. In the future, this technology may become more popular in hospital and emergency room settings, as 3D printing allows for rapid fabrication of clinically relevant and on-demand constructs. Another intriguing avenue that researchers have begun exploring is the combination of 3D printed constructs and biomedical electronics. 3D printed implants/inserts offer an array of advantages over implants fabricated via traditional molding/extrusion methods, such as the ability to achieve patient-specific characteristics, load multiple therapeutics in one dosage form while avoiding incompatibilities between drugs, in addition to maintaining precise control over microstructure and mechanical properties, and drug release kinetics [137]. On the other hand, biomedical electronics have been used to achieve externally controlled drug delivery, including hormone-releasing microchips and miniaturized neural drug delivery systems [138,139]. Recently, Kong et al. developed an FDM printed, Bluetooth-enabled gastric resident electronic device capable of on-demand release of antimicrobial and hormonal agents [140]. In this application, 3D printing allows for the fabrication of an insert with precise dimensions and the seamless integration of multiple materials, including PLA and PU to amplify the adhesion strength between the materials, to achieve gastric retention over 36 days.
Despite the tremendous potential that 3D printing technology offers, there are some challenges that need to be addressed before this technology becomes mainstream in manufacturing of parenteral constructs. General 3D printing considerations, such as material selection, printing parameters, post-printing treatment, and material toxicity concerns, need to be addressed prior to achieving a successful print [141]. The development of new materials or printing inks is the key to the success in propelling 3D printing-based parenteral applications. Scientists often need to modify commercially available materials to satisfy certain printing requirements, such as improving mechanical properties for adequate printability [38]. In addition to impacting printability, mechanical properties of 3D printed constructs play a key role in modulating cell-scaffold interactions, especially in terms of cell adhesion ability and stent patency [142]. Products manufactured via certain 3D printing techniques, such as VP, require an additional post-printing curing step to enhance mechanical integrity of the product, adding to the complexity of the overall 3D printing process [18]. This step may also have a negative impact on loaded therapeutics, which can lead to compromised biocompatibility. Another important factor to consider is the sterilization of parenteral prints. Traditional sterilization techniques include exposure to gamma-irradiation, ethylene oxide gas, UV irradiation, ethanol washing, and autoclaving [143]. A recent study investigated the efficacy of sterilization techniques, including plasma irradiation and autoclave steam sterilization (121°C and 134°C), for surgical guides and implants [144]. It was concluded that both plasma irradiation and autoclave steam sterilization are suitable sterilization methods, and that high temperature steam sterilization caused no significant deformation of 3D printed implants. While these sterilization methods are promising, they are not suitable for all 3D printed constructs, as high temperature sterilization methods can potentially compromise the integrity and efficacy of prints containing temperature sensitive polymers and/or thermolabile therapeutics. In addition, while personalized constructs can be produced, the time it takes to 3D print individual prints far exceeds the time it takes to commercially manufacture them as a result of the layer-by-layer addition process inherent to 3D printing. Furthermore, there is a need to develop multi-material 3D printers to enable flexible construct design and combination therapy. Last but not the least, toxicological effects of materials used in the 3D printing process remain a paramount concern for researchers. Additives such as photoinitiators and cross-linking agents are typically required in the 3D printing process, to achieve successful prints. However, unreacted resin components found in VP type 3D printing techniques have been shown to present cytotoxicity concerns, and are a key reason many 3D printing procedures involve wash steps to remove unreacted material [145]. Two photoinitiators commonly used in the 3D printing process of parenteral application including LAP and Irgacure I-2959 have demonstrated increased cytotoxicity levels at elevated concentrations [145,146]. Alternative photoinitiators, such as riboflavin, have been shown to exhibit UV cross-linking ability while remaining non-cytotoxic, despite exhibiting lower print resolution due to longer reaction times [147]. Other cross-linking agents used in the development of parenteral delivery systems, such as glutaraldehyde, which has been used in combination with chitosan to produce hydrogels, has been shown to demonstrate mutagenic and neurotoxic properties [148]. Thus, materials used in the 3D printing process of parenteral applications must be carefully selected to ensure that final printed products remain biocompatible and non-toxic.
Despite the tremendous potential 3D printing has to offer for parenteral applications, regulatory guidance on characterization and assessment methods as well as process validation methodology remains scarce. While dozens of 3D printed medical devices have received FDA approval such as dental crowns and bone plates, only one pharmaceutical drug product, Spritam® medication, has been approved by the FDA [149,150]. Clinical trials are underway for other 3D printed medical devices, such as 3D printed patient-specific intramedullary guide and 3D printed denture framework. Most recently, the FDA held a public workshop in 2014 and issued a guidance in 2017 covering technical considerations for additive manufactured medical devices, such as information regarding design and manufacturing considerations for 3D printed medical devices [151]. This guidance recommends material controls, describing specifications for raw materials including particle size, viscosity, and filament dimensions, should be well controlled. The guidance also recommends to understand and document the impact of post-processing steps involved in residue removal and sterilization, including heat or chemical treatments, on final product performance and properties. Furthermore, process validation, including assessments on device dimensions, feature geometry, and material properties, must be performed on final prints to ensure quality is maintained for parts produced in a single build cycle and between multiple build cycles. Lastly, final product mechanical properties such as modulus, yield strength, and creep should be investigated once all post-processing, cleaning, and sterilization steps have been performed. While this guidance provides insightful information, numerous regulatory concerns remain unaddressed, including regulation of 3D printed on-demand personalized products at hospitals and pharmacies and the regulation of printer ink and 3D printer manufacturing [152]. There is a growing interest to use 3D printing to produce parenteral dosage forms, and with this increased appeal, more specific and defined regulations will need to be established.
CONCLUSION
Although still in its infancy, 3D printing has already demonstrated tremendous potential for producing parenteral constructs. The importance of producing on-demand, personalized medications tailored to patient anatomy and disease conditions cannot be overstated. In addition, the ability to progress from design to prototype in a matter of hours allows scientist and physicians to quickly and efficiently test out various designs and therapeutic regimens until a desirable treatment is obtained. Additive manufacturing techniques also allow for the flexibility to combine multiple therapeutics in a single dosage form in a controllable and organized fashion. Biomedical devices and implantable scaffolds can be printed with controllable dimensions and microstructures, leading to tunable degradation and drug release characteristics, in addition to playing a key role in modulating cell proliferation and migration abilities. Thus, despite not being an optimal solution for large-scale manufacturing, the use of 3D printing for parenteral applications will continue to rise, to meet the growing demand for patient-centric medications.
References
CENTER FOR DRUG EVALUATION AND RESEARCH. Approval package for SPRITAM. 2015; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207958Orig1s000Approv.pdf
Afsana JV, Haider N, Jain K. 3D printing in personalized drug delivery. Curr Pharm Des. 2019;24(42):5062–71.
Do A, Worthington K, Tucker B, Salem AK, Therapeutics T, Engineering B, et al. Controlled drug delivery from 3D printed two-photon polymerized poly(ethylene glycol) dimethacrylate devices. Int J Pharm. 2019;552:217–24.
Buj-Corral I, Bagheri A, Petit-Rojo O. 3D printing of porous scaffolds with controlled porosity and pore size values. Materials (Basel). 2018;11(9):1–18.
Zhang W, Feng C, Yang G, Li G, Ding X, Wang S, et al. 3D-printed scaffolds with synergistic effect of hollow-pipe structure and bioactive ions for vascularized bone regeneration. Biomaterials [Internet]. 2017;135:85–95. Available from. https://doi.org/10.1016/j.biomaterials.2017.05.005.
Guerra AJ, Cano P, Rabionet M, Puig T, Ciurana J. 3D-printed PCL/PLA composite stents: towards a new solution to cardiovascular problems. Materials (Basel). 2018;11(9):1–13.
Long J, Gholizadeh H, Lu J, Seyfoddin A. Application of fused deposition modelling (FDM) method of 3D printing in drug delivery. 2017 433–439.
Melocchi A, Parietti F, Maroni A, Foppoli A, Gazzaniga A, Zema L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int J Pharm [Internet]. 2016;509(1–2):255–63. Available from. https://doi.org/10.1016/j.ijpharm.2016.05.036.
Popescu D, Zapciu A, Amza C, Baciu F, Marinescu R. FDM process parameters influence over the mechanical properties of polymer specimens: a review. Polym Test. 2018;69(May):157–66.
Azad MA, Olawuni D, Kimbell G, Badruddoza AZM, Hossain MS, Sultana T. Polymers for extrusion-based 3D printing of pharmaceuticals: a holistic materials–process perspective. Pharmaceutics. 2020;12:1–34.
Algahtani MS, Mohammed AA, Ahmad J. Extrusion-based 3D printing for pharmaceuticals: contemporary research and applications. Curr Pharm Des. 2019;24(42):4991–5008.
Araújo MRP, Sa-Barreto LL, Gratieri T, Gelfuso GM, Cunha-Filho M. The digital pharmacies era: how 3D printing technology using fused deposition modeling can become a reality. Pharmaceutics. 2019;11:3.
Long J, Etxeberria AE, Nand AV, Bunt CR, Ray S, Seyfoddin A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater Sci Eng C [Internet]. 2019;104:109873. Available from. https://doi.org/10.1016/j.msec.2019.109873.
Alhnan MA, Okwuosa TC, Sadia M, Wan KW, Ahmed W, Arafat B. Emergence of 3D printed dosage forms: opportunities and challenges. Pharm Res. 2016;33(8):1817–32.
McMains S. Layered manufacturing technologies. Commun ACM. 2005;48(6):50–6.
Martinez PR, Goyanes A, Basit AW, Gaisford S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int J Pharm [Internet]. 2017;532(1):313–7. Available from. https://doi.org/10.1016/j.ijpharm.2017.09.003.
Skoog SA, Goering PL, Narayan RJ. Stereolithography in tissue engineering. J Mater Sci Mater Med. 2014;25(3):845–56.
Melchels FPW, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31(24):6121–30.
Gittard S, Narayan R. Laser direct writing of micro- and nano-scale medical devices. Expert Rev Med Devices. 2010;7(3):343–56.
Lee ED, Sim JH, Kweon HJ, Paik IH. Determination of process parameters in stereolithography using neural network. KSME Int J. 2004;18(3):443–52.
Kadry H, Wadnap S, Xu C, Ahsan F. Digital light processing (DLP)3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur J Pharm Sci [Internet]. 2019;135:60–7. Available from. https://doi.org/10.1016/j.ejps.2019.05.008.
Lee S-J. Development of 3D printed hydrogel scaffold with core-shell nanoparticles for nerve regeneration. IEEE Trans Biomed Eng. 2015;64(2):408–18.
Kim JH, Lee JW, Yun WS. Fabrication and tissue engineering application of a 3D PPF/DEF scaffold using Blu-ray based 3D printing system. J Mech Sci Technol. 2017;31(5):2581–7.
Arcaute K, Mann BK, Wicker RB. Stereolithography of three-dimensional bioactive poly(ethylene glycol) constructs with encapsulated cells. Ann Biomed Eng. 2006;34(9):1429–41.
Arcaute K, Mann B, Wicker R. Stereolithography of spatially controlled multi-material bioactive poly(ethylene glycol) scaffolds. Acta Biomater. 2010;6(3):1047–54.
Konasch J, Riess A, Mau R, Teske M, Rekowska N, Eickner T, et al. A novel hybrid additive manufacturing process for drug delivery systems with locally incorporated drug depots. Pharmaceutics. 2019;11(12):1–14.
Robles-Martinez P, Xu X, Trenfield SJ, Awad A, Goyanes A, Telford R, et al. 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics. 2019;11:6.
Fayazfar H, Salarian M, Rogalsky A, Sarker D, Russo P, Paserin V, et al. A critical review of powder-based additive manufacturing of ferrous alloys: process parameters, microstructure and mechanical properties. Mater Des [Internet]. 2018;144:98–128. Available from. https://doi.org/10.1016/j.matdes.2018.02.018.
Shirazi SFS, Gharehkhani S, Mehrali M, Yarmand H, Metselaar HSC, Adib Kadri N, et al. A review on powder-based additive manufacturing for tissue engineering: Selective laser sintering and inkjet 3D printing. Sci Technol Adv Mater [Internet]. 2015;16(3):1–20. Available from. https://doi.org/10.1088/1468-6996/16/3/033502.
Rahmati S, Shirazi SF, Baghayeri H. Piezo-electric head application in a new 3D printing design. Rapid Prototyp J. 2009;15(3):187–91.
Huang W, Zheng Q, Sun W, Xu H, Yang X. Levofloxacin implants with predefined microstructure fabricated by three-dimensional printing technique. Int J Pharm. 2007;339(1–2):33–8.
Fina F, Goyanes A, Gaisford S, Basit AW. Selective laser sintering (SLS) 3D printing of medicines. Int J Pharm [Internet]. 2017;529(1–2):285–93. Available from. https://doi.org/10.1016/j.ijpharm.2017.06.082.
Xia Y, Zhou PY, Cheng XS, Xie Y, Liang C, Li C, et al. Selective laser sintering fabrication of nano-hydroxyapatite/poly-ε-caprolactone scaffolds for bone tissue engineering applications. Int J Nanomedicine. 2013;8:4197–213.
Mathew E, Pitzanti G, Larrañeta E, Lamprou DA. Three-dimensional printing of pharmaceuticals and drug delivery devices. Pharmaceutics. 2020;12(3):1–9.
Yang Y, Li H, Xu Y, Dong Y, Shan W, Shen J. Fabrication and evaluation of dental fillers using customized molds via 3D printing technology. Int J Pharm [Internet]. 2019;562:66–75. Available from. https://doi.org/10.1016/j.ijpharm.2019.03.024.
Mohan AM, MH AAR. Manufacturing of customized implants for orbital fractures using 3D printing. Bioprinting [Internet]. 2021;21:e00118. Available from. https://doi.org/10.1016/j.bprint.2020.e00118.
Yang Y. Strategies and mechanisms to improve the printability of pharmaceutical polymers. International Journal of Pharmaceutics Int J Pharm. 2021.
Yang Y, Wang X, Lin X, Xie L, Ivone R, Shen J, et al. A tunable extruded 3D printing platform using thermo-sensitive pastes. Int J Pharm. 2020;583(April).
Lucas L, Aravind A, Emma P, Christophe M, Edwin-Joffrey C. Rheology, simulation and data analysis toward bioprinting cell viability awareness. Bioprinting. 2021;21.
Yang Y, Zhou Y, Lin X, Yang Q, Yang G. Printability of external and internal structures based on digital light processing 3D printing technique. Pharmaceutics. 2020;12(3):1–16.
Godoi FC, Prakash S, Bhandari BR. 3d printing technologies applied for food design: status and prospects. J Food Eng. 2016;179:44–54.
Ouyang L, Highley CB, Rodell CB, Sun W, Burdick JA. 3D printing of shear-thinning hyaluronic acid hydrogels with secondary cross-linking. ACS Biomater Sci Eng. 2016;2(10):1743–51.
Ouyang L, Yao R, Zhao Y, Sun W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication. 2016;8:3.
Naghieh S, Sarker MD, Sharma NK, Barhoumi Z, Chen X. Printability of 3D printed hydrogel scaffolds: influence of hydrogel composition and printing parameters. Appl Sci. 2020;10:1.
Wang S, Lee JM, Yeong WY. Smart hydrogels for 3D bioprinting. Int J Bioprinting. 2015;1(1):3–14.
Zhao Y, Li Y, Mao S, Sun W, Yao R. The influence of printing parameters on cell survival rate and printability in microextrusion-based 3D cell printing technology. Biofabrication. 2015;7:4.
Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;80(3646439):458–64.
Wu D, et al. Mechanics of shape distortion of DLP 3D printed structures during UV post-curing. Soft Matter. 2019;30.
Chin SY, Dikshit V, Priyadarshini BM, Zhang Y. Powder-based 3D printing for the fabrication of device with micro and mesoscale features. Micromachines. 2020;11(7):29–40.
Delrot P, Modestino MA, Gallaire F, Psaltis D, Moser C. Inkjet printing of viscous monodisperse microdroplets by laser-induced flow focusing. Phys Rev Appl. 2016;6(2):1–8.
Azizi Machekposhti S, Movahed S, Narayan RJ. Physicochemical parameters that underlie inkjet printing for medical applications. Biophys Rev [Internet]. 2020;1(1):011301. Available from. https://doi.org/10.1063/5.0011924.
Yang Y, Xu Y, Wei S, Shan W. Oral preparations with tunable dissolution behavior based on selective laser sintering technique. Int J Pharm [Internet]. 2021;(593, November 2020):120127. Available from. https://doi.org/10.1016/j.ijpharm.2020.120127.
Lu K, Hiser M, Wu W. Effect of particle size on three dimensional printed mesh structures. Powder Technol. 2009;192(2):178–83.
Salmoria GV, Klauss P, Zepon KM, Kanis LA. The effects of laser energy density and particle size in the selective laser sintering of polycaprolactone/progesterone specimens: Morphology and drug release. Int J Adv Manuf Technol. 2013;66(5–8):1113–8.
Gayer C, Abert J, Bullemer M, Grom S, Jauer L, Meiners W, et al. Influence of the material properties of a poly(D,L-lactide)/β-tricalcium phosphate composite on the processability by selective laser sintering. J Mech Behav Biomed Mater. 2018;87(July):267–78.
Fina F, Madla CM, Goyanes A, Zhang J, Gaisford S, Basit AW. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int J Pharm [Internet]. 2018;541(1–2):101–7. Available from. https://doi.org/10.1016/j.ijpharm.2018.02.015.
Barakh Ali SF, Mohamed EM, Ozkan T, Kuttolamadom MA, Khan MA, Asadi A, et al. Understanding the effects of formulation and process variables on the printlets quality manufactured by selective laser sintering 3D printing. Int J Pharm [Internet]. 2019;570(August):118651. Available from. https://doi.org/10.1016/j.ijpharm.2019.118651.
Ahmed KK, Tamer MA, Ghareeb MM, Salem AK. Recent advances in polymeric implants. AAPS PharmSciTech. 2019;20:7.
Zhou F, Hong Y, Liang R, Zhang X, Liao Y, Jiang D, et al. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials [Internet]. 2020;258(July):120287. Available from. https://doi.org/10.1016/j.biomaterials.2020.120287.
Buyuksungur S, Endogan Tanir T, Buyuksungur A, Bektas EI, Torun Kose G, Yucel D, et al. 3D printed poly(ϵ-caprolactone) scaffolds modified with hydroxyapatite and poly(propylene fumarate) and their effects on the healing of rabbit femur defects. Biomater Sci. 2017;5(10):2144–58.
Seal BL, Otero TC, Panitch A. Polymeric biomaterials for tissue and organ regeneration. Mater Sci Eng R Rep. 2001;34(4–5):147–230.
Ritz U, Gerke R, Götz H, Stein S, Rommens PM. A new bone substitute developed from 3D-prints of polylactide (PLA) loaded with collagen i: an in vitro study. Int J Mol Sci. 2017;18:12.
Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications — a comprehensive review. Adv Drug Deliv Rev [Internet]. 2016;107:367–92. Available from. https://doi.org/10.1016/j.addr.2016.06.012.
Tappa K, Jammalamadaka U, Weisman JA, Ballard DH, Wolford DD, Pascual-Garrido C, et al. 3D printing custom bioactive and absorbable surgical screws, pins, and bone plates for localized drug delivery. J Funct Biomater. 2019;10:2.
Wang S, Xiong Y, Chen J, Ghanem A, Wang Y, Yang J, et al. Three dimensional printing bilayer membrane scaffold promotes wound healing. Front Bioeng Biotechnol. 2019;7(November):1–11.
Mi H-Y, Salick MR, Jing X, Jacques BR, Crone WC, Peng X-F, et al. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater Sci Eng C. 2013;33(8):4767–76.
Krasowska A, Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol. 2014;4(AUG):1–7.
Xu HHK, Wang P, Wang L, Bao C, Chen Q, Weir MD, et al. Calcium phosphate cements for bone engineering and their biological properties. Bone Res [Internet]. 2017;5(April):1–19. Available from. https://doi.org/10.1038/boneres.2017.56.
Saniei H, Mousavi S. Surface modification of PLA 3D-printed implants by electrospinning with enhanced bioactivity and cell affinity. Polymer (Guildf) [Internet]. 2020;196(March):122467. Available from. https://doi.org/10.1016/j.polymer.2020.122467.
Manavitehrani I, Fathi A, Badr H, Daly S, Shirazi AN, Dehghani F. Biomedical applications of biodegradable polyesters. Polymers (Basel). 2016;8:1.
Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng - Part A. 2012;18(11–12):1304–12.
Luo Y, Le Fer G, Dean D, Becker ML. 3D printing of poly(propylene fumarate) oligomers: evaluation of resin viscosity, printing characteristics and mechanical properties. Biomacromolecules. 2019;20(4):1699–708.
Baker MI, Walsh SP, Schwartz Z, Boyan BD. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J Biomed Mater Res - Part B Appl Biomater. 2012;100(B5):1451–7.
Qamar N, Abbas N, Irfan M, Hussain A, Arshad MS, Latif S, et al. Personalized 3D printed ciprofloxacin impregnated meshes for the management of hernia. J Drug Deliv Sci Technol [Internet]. 2019;53(July):101164. Available from. https://doi.org/10.1016/j.jddst.2019.101164.
Boyer CJ, Boktor M, Samant H, White LA, Wang Y, Ballard DH, et al. 3D printing for bio-synthetic biliary stents. Bioengineering. 2019;6:1.
Wang C, Xie J, Xiao X, Chen S, Wang Y. Development of nontoxic biodegradable polyurethanes based on polyhydroxyalkanoate and l-lysine diisocyanate with improved mechanical properties as new elastomers scaffolds. Polymers (Basel). 2019;11:12.
Zdrahala R. Biomedical applications of polyurethanes: a review of past promises, present realities, and a vibrant future. Polyurethanes as Specialty Chemicals. 1999:67–90.
Joseph J, Patel RM, Wenham A, Smith JR. Biomedical applications of polyurethane materials and coatings. Trans Inst Met Finish. 2018;96(3):121–9.
Jung SY, Lee SJ, Kim HY, Park HS, Wang Z, Kim HJ, et al. 3D printed polyurethane prosthesis for partial tracheal reconstruction: a pilot animal study. Biofabrication. 2016;8:4.
Liu F, Chen Q, Liu C, Ao Q, Tian X, Fan J, et al. Natural polymers for organ 3D bioprinting. Polymers (Basel). 2018;10(11):1–26.
Balani K, Verma V, Agarwal A, Narayan R. Physical, thermal, and mechanical properties of polymers. Biosurfaces. 2015:329–44.
Ramya R, Venkatesan J, Kim SK, Sudha PN. Biomedical applications of chitosan: an overview. J Biomater Tissue Eng. 2012;2(2):100–11.
Vunain E, Mishra AK, Mamba BB. Fundamentals of chitosan for biomedical applications [Internet] 1 Chitosan based biomaterials. Elsevier. 2017:3–30 Available from. https://doi.org/10.1016/B978-0-08-100230-8.00001-7.
Zhou HY. Effect of molecular weight and degree of chitosan deacetylation on the preparation and characteristics of chitosan thermosensitive hydrogel as a delivery system. Carbohydr Polym. 2008;73(2):265–73.
Pahlevanzadeh F, Emadi R, Valiani A, Kharaziha M, Poursamar SA, Bakhsheshi-Rad HR, et al. Three-dimensional printing constructs based on the chitosan for tissue regeneration: State of the art, developing directions and prospect trends. Materials. 2020;13.
Fischetti T, Celikkin N, Contessi Negrini N, Farè S, Swieszkowski W. Tripolyphosphate-crosslinked chitosan/gelatin biocomposite ink for 3D printing of uniaxial scaffolds. Front Bioeng Biotechnol. 2020;8(April):1–15.
Intini C, Elviri L, Cabral J, Mros S, Bergonzi C, Bianchera A, et al. 3D-printed chitosan-based scaffolds: an in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr Polym [Internet]. 2018;199(July):593–602. Available from. https://doi.org/10.1016/j.carbpol.2018.07.057.
Bettini R, Romani AA, Morganti MM, Borghetti AF. Physicochemical and cell adhesion properties of chitosan films prepared from sugar and phosphate-containing solutions. Eur J Pharm Biopharm. 2008;68(1):74–81.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–26.
He Y, Yang F, Zhao H, Gao Q, Xia B, Fu J. Research on the printability of hydrogels in 3D bioprinting. Sci Rep [Internet]. 2016;6:1–13. Available from. https://doi.org/10.1038/srep29977.
Li J, Liu X, Crook JM, Wallace GG. 3D printing of cytocompatible graphene/alginate scaffolds for mimetic tissue constructs. Front Bioeng Biotechnol. 2020;8(July):1–11.
Dicker K. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater [Internet]. 2014;10(4):1558–70 Available from: 10.1016/j.earlhumdev.2015.09.003%5Cn 10.1016/j.earlhumdev.2014.01.002%5Cn 10.1016/S0378-3782(12)70006-3%5Cnhttp://www.sciencedirect.com/science/article/pii/S2341287914000763%5Cn 10.1016/.
Vepari C, Kaplan DL. Silk as biomaterial. Prog Polym Sci. 2007;100(2):130–4.
Wang Q, Han G, Yan S, Zhang Q. 3D printing of silk fibroin for biomedical applications. Materials (Basel). 2019;12:3.
Das S, Pati F, Choi YJ, Rijal G, Shim JH, Kim SW, et al. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater [Internet]. 2015;11(1):233–46. Available from. https://doi.org/10.1016/j.actbio.2014.09.023.
Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–58.
Somaiah C, Kumar A, Mawrie D, Sharma A. Collagen promotes higher adhesion. Survival and proliferation of mesenchymal stem cells. 2015:1–15.
Nocera AD, Comín R, Salvatierra NA, Cid MP. Development of 3D printed fibrillar collagen scaffold for tissue engineering. Biomed Microdevices. 2018;20(2):1–13.
Bello AB, Kim D, Kim D, Park H, Lee S. Engineering and functionalization of gelatin biomaterials. From cell culture to medical applications. 2020;26(2):164–80.
Negrini N. 3D printing of chemically crosslinked gelatin hydrogels for adipose tissue engineering. Biofabrication. 2019.
Stewart SA, Domínguez-Robles J, McIlorum VJ, Mancuso E, Lamprou DA, Donnelly RF, et al. Development of a biodegradable subcutaneous implant for prolonged drug delivery using 3D printing. Pharmaceutics. 2020;12:2.
Inzana JA, Trombetta RP, Schwarz EM, Kates SL, Awad HA. 3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection. Eur Cells Mater. 2015;30:232–47.
Martin V, Ribeiro IA, Alves MM, Gonçalves L, Claudio RA, Grenho L, et al. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanohydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater Sci Eng C [Internet]. 2019;101(March):15–26. Available from. https://doi.org/10.1016/j.msec.2019.03.056.
Fu J, Yu X, Jin Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int J Pharm [Internet]. 2018;539(1–2):75–82. Available from https://doi.org/10.1016/j.ijpharm.2018.01.036, 2018
Bagshaw KR, Hanenbaum CL, Carbone EJ, Lo KWH, Laurencin CT, Walker J, et al. Pain management via local anesthetics and responsive hydrogels. Ther Deliv. 2015;6(2):165–76.
Dash AK, Cudworth GC. Therapeutic applications of implantable drug delivery systems. J Pharmacol Toxicol Methods. 1998;40(1):1–12.
Gimeno M, Pinczowski P, Pérez M, Giorello A, Martínez MÁ, Santamaría J, et al. A controlled antibiotic release system to prevent orthopedic-implant associated infections: an in vitro study. Eur J Pharm Biopharm. 2015;96:264–71.
Stewart SA, Domínguez-Robles J, Donnelly RF, Larrañeta E. Implantable polymeric drug delivery devices: classification, manufacture, materials, and clinical applications. Polymers (Basel). 2018;10:12.
Espey E, Ogburn T. Long-acting reversible contraceptives: intrauterine devices and the contraceptive implant. Obstet Gynecol. 2011;117(3):705–19.
Tappa K, Jammalamadaka U, Ballard DH, Bruno T, Israel MR, Vemula H, et al. Medication eluting devices for the field of OBGYN (MEDOBGYN): 3D printed biodegradable hormone eluting constructs, a proof of concept study. PLoS One. 2017;12(8):1–17.
Qiao X, Yang Y, Huang R, Shi X, Chen H, Wang J, et al. E-jet 3D-printed scaffolds as sustained multi-drug delivery vehicles in breast cancer therapy. Pharm Res. 2019;36:12.
Won JY, Kim J, Gao G, Kim J, Jang J, Park YH, et al. 3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone: a synergetic therapy for retinal vascular diseases. Acta Biomater [Internet]. 2020;116:174–85. Available from. https://doi.org/10.1016/j.actbio.2020.09.015.
Lim SH, Chia SMY, Kang L, Yap KYL. Three-dimensional printing of carbamazepine sustained-release scaffold. J Pharm Sci [Internet]. 2016;105(7):2155–63. Available from. https://doi.org/10.1016/j.xphs.2016.04.031.
Wang M, Favi P, Cheng X, Golshan NH, Ziemer KS, Keidar M, et al. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomater [Internet]. 2016;46:256–65. Available from. https://doi.org/10.1016/j.actbio.2016.09.030.
Roh HS, Lee CM, Hwang YH, Kook MS, Yang SW, Lee D, et al. Addition of MgO nanoparticles and plasma surface treatment of three-dimensional printed polycaprolactone/hydroxyapatite scaffolds for improving bone regeneration. Mater Sci Eng C [Internet]. 2017;74:525–35. Available from. https://doi.org/10.1016/j.msec.2016.12.054.
Yang S, Leong KF. The design of scaffolds for use in tissue engineering. Part II Rapid Prototyping Techniques. 2002;8:1.
He D, Zhuang C, Chen C, Xu S, Yang X, Yao C, et al. Rational design and fabrication of porous calcium-magnesium silicate constructs that enhance angiogenesis and improve orbital implantation. ACS Biomater Sci Eng. 2016;2(9):1519–27.
Ho MY, Chen CC, Wang CY, Chang SH, Hsieh MJ, Lee CH, et al. The development of coronary artery stents: from bare-metal to bio-resorbable types. Metals (Basel). 2016;6:7.
Ang HY, Huang YY, Lim ST, Wong P, Joner M, Foin N. Mechanical behavior of polymer-based vs. metallic-based bioresorbable stents. J Thorac Dis. 2017;9(Suppl 9):S923–34.
J Guerra A. 3D-printed bioabsordable polycaprolactone stent: the effect of process parameters on its physical features. Mater Des. 2018;137:430–7.
Kim TH, Lee JH, Ahn CB, Hong JH, Son KH, Lee JW. Development of a 3D-printed drug-eluting stent for treating obstructive salivary gland disease. ACS Biomater Sci Eng. 2019;5(7):3572–81.
Wilson K, Meier J, Ward D. Salivary gland disorders - American family physician. Am Acad Dermatology. 2014;89(11):882–8.
Guo S, DiPietro LA. Critical review in oral biology & medicine: factors affecting wound healing. J Dent Res. 2010;89(3):219–29.
Malda J. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater. 2013;25(36):5011–28.
Van Kogelenberg S, Yue Z, Dinoro JN, Baker CS, Wallace GG. Three-dimensional printing and cell therapy for wound repair. Adv Wound Care. 2018;7(5):145–55.
Mironov V, Trusk T, Kasyanov V, Little S, Swaja R, Markwald R. Biofabrication: a 21st century manufacturing paradigm. Biofabrication. 2009;1:–2.
Wen YT, Dai NT, Hui HS. Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater [Internet]. 2019;88:301–13. Available from. https://doi.org/10.1016/j.actbio.2019.02.044.
Muwaffak Z, Goyanes A, Clark V, Basit AW, Hilton ST, Gaisford S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int J Pharm [Internet]. 2017;527(1–2):161–70. Available from. https://doi.org/10.1016/j.ijpharm.2017.04.077.
Worm M, Nguyen D, Rackley R, Muraro A, Du Toit G, Lawrence T, et al. Epinephrine delivery via EpiPen®Auto-Injector or manual syringe across participants with a wide range of skin-to-muscle distances. Clin Transl Allergy [Internet]. 2020;10(1):1–13. Available from. https://doi.org/10.1186/s13601-020-00326-x.
Uguz A, Lack G, Pumphrey R, Ewan P, Warner J, Dick J, et al. Allergic reactions in the community: a questionnaire survey of members of the anaphylaxis campaign. Clin Exp Allergy. 2005;35(6):746–50.
Korenblat P, Lundie MJ, Dankner RE, Day JH. A retrospective study of epinephrine administration for anaphylaxis: how many doses are needed? Allergy Asthma Proc. 1999;20(6):383–6.
Sheehan TC. Design of a double-dose epinephrine auto- injector using 3D-printing. 2015;
Nair G, Levin M, Sivarasu S. Design and verification of a reloadable adrenaline auto-injector for intramuscular injections. Front Biomed Devices, BIOMED Des Med Devices Conf DMD 2018 2–4.
Sommer AC, Blumenthal EZ. Implementations of 3D printing in ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1815–22.
Isaacson A, Swioklo S, Connon CJ. 3D bioprinting of a corneal stroma equivalent. Exp Eye Res [Internet]. 2018;173(March):188–93. Available from. https://doi.org/10.1016/j.exer.2018.05.010.
Moniaux N, Faivre J. A reengineered liver for transplantation. J Hepatol. 2011;54(2):386–7.
Srinivas L, Jaswitha M, Manikanta V, Bhavya B, Himavant BD. 3D printing in pharmaceutical technology: a review. Int Res J Pharm. 2019;10(2):8–17.
Farra R, Sheppard NF, McCabe L, Neer RM, Anderson JM, Santini JT, et al. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci Transl Med. 2012;4:122.
Dagdeviren C, Ramadi KB, Joe P, Spencer K, Helen N, Shimazu H, et al. Miniaturized neural system for chronic, local intracerebral drug delivery. 2019;10(425).
Kong YL, Zou X, McCandler CA, Kirtane AR, Ning S, Zhou J, et al. 3D-printed gastric resident electronics. Adv Mater Technol. 2019;4:3.
Gujrati A, Sharma A, Mahajan SC. Review on applications of 3D printing in pharmaceuticals. Int J Pharm Sci Rev Res [Internet]. 2019;59(1):148–54 Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2003257894.
Mandrycky C, Wang Z, Kim K, Kim DH. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34(4):422–34.
Stoppel WL, White JC, Horava SD, Henry AC, Roberts SC, Bhatia SR. Terminal sterilization of alginate hydrogels: efficacy and impact on mechanical properties. J Biomed Mater Res - Part B Appl Biomater. 2014;102(4):877–84.
Török G, Gombocz P, Bognár E, Nagy P, Dinya E, Kispélyi B, et al. Effects of disinfection and sterilization on the dimensional changes and mechanical properties of 3D printed surgical guides for implant therapy - pilot study. BMC Oral Health. 2020;20(1):1–12.
Nguyen A, Goering P, Reipa V, Narayan R. Toxicity and photosensitizing assessment of gelatin methacryloyl-based hydrogels photoinitiated with human primary renal proximal tubule epithelial cells:1–22.
Sabnis A. Cytocompatibility Studies of an in situ photopolymerized thermoresponsive hydrogel nanoparticle system using human aortic smooth muscle cells. 2010;91(1):52–9.
Choi G, Cha HJ. Recent advances in the development of nature-derived photocrosslinkable biomaterials for 3D printing in tissue engineering. 2019;1–7.
Bergonzi C, Natale A Di, Zimetti F, Marchi C, Bianchera A, Bernini F, et al. Study of 3D-printed chitosan scaffold features after different post-printing gelation processes. 2019;(February):1–11.
510(k) Premarket notification. US Food and Drug Administration [Internet]. Available from. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm.
Vaz VM, Kumar L. 3D printing as a promising tool in personalized medicine. AAPS PharmSciTech. 2021;22:1.
FDA. Technical considerations for additive manufactured devices draft guidance for industry and [Internet]. 2016. 28. Available from: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM499809.pdf
Di Prima M, Coburn J, Hwang D, Kelly J, Khairuzzaman A, Ricles L. Additively manufactured medical products – the FDA perspective. 3D Print Med [Internet]. 2016;2(1):4–9. Available from. https://doi.org/10.1186/s41205-016-0005-9.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Guest Editors: Diane Burgess, Marilyn Morris and Meena Subramanyam
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ivone, R., Yang, Y. & Shen, J. Recent Advances in 3D Printing for Parenteral Applications. AAPS J 23, 87 (2021). https://doi.org/10.1208/s12248-021-00610-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00610-z