Abstract
Background
Obstructive sleep apnea (OSA) is a multisystemic chronic disease with disabling symptoms, cardiometabolic comorbidities and reduction in physical activity. Continuous positive airway pressure (CPAP) is the standard treatment for OSA. Only a few studies have characterized trajectories of sleep parameters upon initiation of CPAP and these are limited to one or two nights of polysomnographic recording in a sleep laboratory. This is due to the cost of carrying out these studies and poor tolerance by patients of multiple nights of polysomnographic recordings. No study has characterized sleep over multiple nights before and after CPAP initiation, assessing the multidimensional efficacy of CPAP on patient reported outcomes, objective and subjective sleep quality, oximetry, glucose control and physical activity.
New digital technologies enable overnight sleep studies over several nights in the patient’s home, with a reliability of sleep characterization equivalent to polysomnographic recording.
The primary aim of this study is to investigate objective slow wave sleep (SWS or N3) quality before CPAP and during the first month of the treatment.
Secondary objectives are to assess changes in the following parameters before CPAP and during the first month of the treatment: other objective sleep parameters and sleep stages evolution (W, N1, N2 and REM), nocturnal oxygen desaturations, 24-h blood glucose profile, daily physical activity (the daily steps count), and patient reported outcomes.
Methods
Seventy patients prescribed CPAP for OSA will be recruited at Grenoble Alpes University Hospital (France) and monitored for 5 weeks using validated innovative wearable connected devices (the Dreem 3 headband, a pedometer, an oximeter, and a continuous glucose sensor) enabling them to track their own sleep and physiological parameters at home before and after CPAP initiation.
Discussion
By pooling data from the CPAP telemonitoring and other connected devices we should be able to follow the multidimensional trajectories of patients after the initiation of CPAP. This will enable us to determine whether objective changes in sleep parameters in the first few weeks of CPAP treatment are associated with improvements in daytime sleepiness, quality of life, treatment adherence, glucose control and physical activity. The data will provide integrated markers of treatment efficacy and will allow adapted personalized management of OSA in the short and long-term.
Trial registration
Clinicaltrials (NCT05197855).
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Obstructive sleep apnea (OSA) affects nearly a billion people worldwide [1]. It is characterized by repeated complete (apnea) or partial (hypopnea) collapse of the pharynx during sleep, resulting in chronic intermittent hypoxia responsible for daytime fatigue and sleepiness as well as numerous comorbidities such as the onset or worsening of hypertension, diabetes, and other cardiometabolic pathologies. Symptoms associated with OSA include snoring, daytime sleepiness, fatigue, morning headaches [2] and deterioration in quality of life [3]. OSA alters objective sleep parameters including sleep macro- and microstructure [4], and is frequently associated with poor glycemic control [5, 6] and a reduction in physical activity [7, 8].
Continuous positive airway pressure (CPAP) is the most effective treatment for patients with OSA; with over 1.6 million patients on CPAP in France [9, 10]. Randomized controlled trials have confirmed that CPAP treatment reduces OSA symptoms and improves patients’ quality of life [11]. CPAP has also been shown to improve sleep quality and architecture [12,13,14]. These studies suggest that sleep restoration, particularly slow wave sleep (SWS or N3) and REM sleep, may be linked to improvements in memory [12], thus prompting patients to maintain long-term adherence to the treatment. The main limitation of these studies is that pre-and post-treatment sleep patterns were assessed during isolated single nights of polysomnography (PSG). Little is known about the precise trajectories of this improvement, in part due to the cost and complexity of carrying out repeated recordings, and to the low acceptability by patients of multiple nights spent in a sleep laboratory. Also, other dimensions reflecting sleep apnea burden including glycemic control, physical activity and patient reported outcomes (PROMs) [15] have been poorly evaluated over several nights and days around CPAP initiation. One can hypothesize that an improvement in sleep architecture following CPAP initiation might in turn improve PROMs, CPAP adherence, glycemic control and physical activity.
The commercially available innovative connected device the Dreem 3 headband (Beacon, Boston, Unites States), derives EEG signals allowing automatic sleep staging that has been demonstrated as being in good agreement with that measured by in-laboratory PSG [16,17,18]. In the present protocol we use the Dreem 3 headband in the real-life at-home sleeping conditions of sleep apnea patients during several nights before and after starting CPAP. The device enables us to characterize sleep before and after initiation of CPAP treatment, and thus the trajectories and stability of changes in sleep parameters (depending on the patient being adherent to the treatment).
As sleep apnea is often associated with the development of adverse metabolic conditions such as insulin resistance and/or type II diabetes, eliminating nocturnal desaturations and improving sleep quality should contribute to improved glycemic control [19,20,21]. Thus monitoring is completed by the addition of a non-invasive sensor enabling the collection of 24-h blood glucose readings [22,23,24]. The effect of CPAP initiation is assessed for a few nights every week during the first month of the treatment.
Over recent years, CPAP remote monitoring has transformed the management of OSA and produced a large amount of data. Accumulated CPAP data provide objective information regarding night-to-night treatment efficiency and patient adherence. CPAP efficacy is estimated through the residual apnea-hypopnoea index (rAHI) and the raw data flow curves provided by CPAP devices (allowing to validate or not the accuracy of reported rAHI) [25].
The pooled data from the CPAP device itself, the Dreem 3 headband, and other connected devices will provide original data on the multidimensional trajectories of OSA patients after the initiation of CPAP.
The primary objective of our study is to investigate the evolution of slow wave sleep (SWS or N3) before and after the initiation of CPAP treatment.
Secondary objectives will be to characterize the link between changes in sleep architecture (the other sleep stages: W, N1, N2 and REM) and the following parameters: nocturnal desaturations, 24-h blood-glucose profile, daily physical activity, the patient’s perception of sleep improvement, subjective sleep quality, insomnia severity, subjective daytime sleepiness, the patient’s chronotype [26], anxiety and depression, quality of life and CPAP adherence, objectively collected on a daily basis during the study.
Methods/design
Design and study setting
This is a single-center, prospective study of adults diagnosed with OSA at the Grenoble-Alpes University Hospital, France between October 2022 and November 2024. The consensual patient's care pathway is not modified, although the amount of data collected via connected devices is greater than usual and patients are required to wear and activate the devices, and to fill-in several questionnaires. The study was approved by the French bio-clinical research ethics committee (Comité de Protection des Personnes Ouest V (CPP)) on November 25, 2021. All participants are required to give written informed consent to participate in the study and for the collection and biobanking of blood samples.
Main experimental stages
Patient participation in the study requires 3 visits to the hospital.
Screening visit (V0): within 3 months before inclusion
Once a diagnosis of OSA has been made by PSG and CPAP treatment prescribed, the sleep physician informs the patient about the study and answers any questions about the objectives, constraints, foreseeable risks, expected benefits of participation in this research, and explains the patient’s rights during a face-to-face consultation at the hospital. The physician checks eligibility criteria (Table 1). The patient is given a maximum of seven days before deciding whether to participate or not.
Inclusion visit (V 1): fasted and at the hospital
The patient's informed consent to participate is obtained during a follow-up consultation with the sleep physician. For the purposes of the study, the patient completes questionnaires about their quality of sleep (Pittsburg) [27], insomnia severity (ISI) [28], daytime sleepiness (Epworth) [29], morning/nightlife questionnaire (rMEQ) and quality of life (SF36) questionnaires [30] online in the secured MARS database (https://epatient.mars-database.science/). The patient is supplied with a Dreem 3 headband, a pulse oximeter, a glucose sensor, and a pedometer, shown how to use them, and given instruction and follow-up booklets. The applications for the connected devices (Dreem 3 and the pedometer) are installed on the patient's smartphone, and a de-identified account is created.
Fasted blood samples are collected. Glycemia, insulinemia, lipids (total cholesterol, HDL cholesterol, triglycerides, etc.) and glycated hemoglobin are measured immediately. Blood samples are bio-banked for future analyses.
Pre-CPAP period: 7 nights before CPAP initiation: at home
During the week preceding the initiation of CPAP, the patient wears the Dreem 3 headband, pulse oximeter for 7 consecutive nights, the glucose sensor continuously, and the pedometer during the day; and fills-in the follow-up logbook.
Initiation of CPAP treatment by health care provider: First month of CPAP, at home
The patient wears the Dreem 3 headband and pulse oximeter together with the CPAP device for at least:
-
- the first 3 nights of treatment.
-
- 2 nights during the second week.
-
- 2 nights during the third week,
-
- and the 2 last nights of the fourth week.
The glucose sensor is worn continuously and the pedometer is used on a daily basis throughout the study period.
End-of-study visit (V2): at the hospital
After at least one month of CPAP treatment, the headband, pulse oximeter, glucose sensor, pedometer, and logbook are returned to the investigating center. During this consultation, the patient will receive a detailed report on the evolution of their sleep and related parameters, based on data from the CPAP and connected devices. The patient will again complete the same questionnaires as at inclusion (V1) and fasted blood samples are collected for the same tests as before.
Table 2 shows the participant timeline.
Outcomes
Main outcome
Evolution of slow wave sleep (SWS or N3) recorded by the Dreem 3 headband, collected over 7 nights before and 9 nights after CPAP treatment initiation.
Secondary outcomes
Changes in the different sleep parameters and sleep stages evolution (W, N1, N2 and REM), the level of physical activity (daily steps count), glucose and other metabolic parameters (between before CPAP initiation and during the first month of the treatment).
Table 3 details the different outcomes of the study.
Description of connected devices
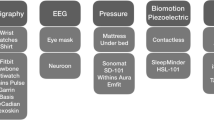
Figure 1 illustrates the medical devices used, when they are used and the data they collect.
-
- CPAP device (AirSense, Resmed, Lyon, France): This is the first-line therapy device for OSA. Daily adherence data, daily leaks, and residual AHI will be collected through CPAP telemonitoring.
-
- The Dreem 3 headband (Beacon, Biosignals Boston, United States) is a CE-marked health and wellness wireless device, commercially available in France.The headband is worn at home during sleep. It records, stores, and automatically analyzes EEG data in real time to characterize sleep architecture. The headband is connected to a smartphone application via low energy Bluetooth with analysis supported by artificial intelligence (AI). Every morning, data are directly and remotely accessible to healthcare professionals via a secured, password-protected medical platform. The main signals recorded from brain cortical activity by the six EEG dry electrodes yield seven derivations (FpZ-O1, FpZ-O2, FpZ-F7, F8-F7, F7-O1, F8-O2, FpZ-F8; 250 Hz with a 0.4–35 Hz bandpass filter) [31, 32]. The main signals recorded for brain cortical activity are six EEG dry electrodes yielding seven EEG derivations (FpZ-O1, FpZ-O2, FpZ-F7, F8-F7, F7-O1, F8-O2, FpZ-F8; 250 Hz with a 0.4–35 Hz bandpass filter) [31, 32]. The Dreem headband automatically derives sleep stages (Wake, N1, N2, N3 and REM), total sleep time and wake-after-sleep onset (WASO) (Fig. 2).
The patient will also use:
-
- A pulse oximeter (Nonin1350, Sleepinnov, France) to measure arterial hemoglobin oxygen saturation (SpO2) and pulse rate. This non-invasive and portable device is commonly used in healthcare settings and by individuals at home. The measurement process involves inserting the fingertip into the device, and within seconds the readings are displayed on the screen. Data are continuously recorded throughout the night every five seconds and downloaded by using specific software (blunight reader, sleepinnov, France) at the end of the patient’s study participation. The Nonin sampling time (5 s) can not be fully adjusted to the oximeter sampling recommendation of 3 s. This represents a potential limitation in the full interpretation of oximetry raw data. Oximetry data will be synchronized with the data of the headband.
-
- A continuous Freestyle glucose sensor (Abbott, France) to monitor glycemia by measuring real-time subcutaneous glucose levels over 24 h. The patient scans the sensor at least once every 8 h using a dedicated reader.
Final reports are downloaded at the end of the patient’s participation in the study using the reader which is connected to a secured medical platform, with a cloud-based data management solution for users of the FreeStyle Libre system (Libreview).
-
- A pedometer (Vivofit, Garmin, France) connected to the Garmin Connect™ phone application via Bluetooth to record daily physical activity data (number of steps and distance). Physical activity reports are readily accessible in the application.
Blood samples and biobanking
Blood samples will be collected for research purposes up to 15 mL for each visit with a maximum total over 30 consecutive days of 15 mL. The volume of blood collected respects the limitations recommended by French law for this type of study.
Authorization to collect blood samples and biobanking
The purpose of this blood sampling is to explore of the effect of CPAP treatment on cardiometabolic, inflammatory, and metabolic parameters. Collection and temporary storage (until the declaration of the end of the study) are made within the framework of this study provided the patient has given prior signed informed consent.
Where applicable, and if the patient has given prior consent for biobanking, samples collected will be stored in an authorized biobank (CRB02b AC-2021–4580) under responsibility the sponsor (in accordance with the regulations in force) at the end of the study.
Statistical analysis
Data analysis will take place at the EFCR Department of Grenoble Alpes University Hospital; and will be performed using SAS V9.4 and RStudio software under the responsibility of one of the senior co-authors (S. Bailly) an experienced biostatistician.
Statistical tests will be interpreted with the first-species risk α set at 5% in two-tailed situations.
Descriptive statistics for each parameter will be as follows:
-
- For quantitative variables, means, standard deviations, minimum and maximum values, medians, numbers, and numbers of missing values will be presented in tabular form.
-
- For qualitative variables, counts, numbers of missing values, and percentages will be presented in contingency tables.
Missing data will be the subject of a specific analysis to determine the reason for any missing data and identify an appropriate imputation method.
For all objectives, quantitative (t-test) and qualitative (McNemar Chi2) paired-data mean comparison tests will be used to compare parameter measurements before and after one month of CPAP treatment.
Analysis of primary outcomes
The comparison of slow wave sleep (SWS or N3) before and after CPAP will be carried out using a generalized linear regression model on the change in the percentage of slow wave sleep (SWS or N3) out of total sleep time (mean difference in measurements before and after 1 month of CPAP). Adjustments will be made for the main confounding factors identified by univariate analysis.
Analysis of secondary outcomes
The same approach will be applied to secondary outcomes. Analysis trajectories will be made for all outcomes, applying more advanced methodological approaches to explore the data in the form of trajectories [33].
Sample size calculation
This sample size calculation was based on the limited data available [34]. In this acute CPAP study, the improvement in N3 was closed to 10%. However, we expect to see an average increase of + 5% in slow wave sleep (SWS or N3) as a proportion of total sleep time. Assuming high measurement variability (SD = 12) and low intra-individual correlation between the two measurements (0.4), a sample size of 57 patients would enable us to demonstrate this minimal 5% difference in sleep time after 1 month on CPAP. To take into account missing data and study withdrawals (20%), a total of 70 patients will be included.
Data management
The participants’ data transmitted to the sponsor by the investigators (or any other specialist) is anonymized. Under no circumstance should the names or addresses of the persons concerned appear in clear text. Individuals included in the MARS database are coded using a database number (PAXXXX), the first two letters of their family name and date of birth (**/MM/YYYY), together with a code specific to the research project, indicating the order of inclusion.
The persons responsible for entering data in the MARS database are clearly identified in the MARS task delegation document.
The data collected may be used and transmitted for scientific purposes in the context of sleep apnea research, as well as for exploitation for regulatory submission purposes (by the study partners; in the event of withdrawal of consent, and unless otherwise specified by the patient, the data collected up to that date may be used.
Data access by study team members
All authors and medical staff implicated in this study will have access to the final and fully anonymized data set. Further access will be authorized under the supervision of the principal investigator.
Discussion and perspectives: The place of digital health in OSA management
Digital medicine currently offers new tools for the management of OSA from diagnosis to long-term follow up. The new ambulatory management pathways will provide a reliable alternative to in-laboratory sleep clinics in a near future [35, 36]. This is required to improve access to diagnosis and care, reduce waiting times, and to allow multidisciplinary integrated care supported by appropriate in-home monitoring [37].
Our study will generate a wide range of original data using in-home multidimensional multi-night monitoring. This proof-of-concept study aims to assess the interest and clinical relevance of combining several digital devices for collecting physiological parameters along with CPAP telemonitoring, and subjective measures based on patient reported outcomes.
In general, this project assesses the effectiveness and reliability of digital medicine, and whether the use of connected digital devices can be successfully integrated into the patient's routine care pathway and improve the follow-up and care of patients. As patients will have access to their own data, we hope this will increase the level of patient engagement in their long-term treatment.
Figure 3 depicts a proposal for a reinvented patient pathway for OSA diagnosis and treatment with a simplified home follow-up with expected good patient acceptance (Fig. 3).
The reinvented patient’s pathway for OSA treatment with in-home follow-up. The virtual sleep laboratory would collect measurements made by the patient themselves such as patient reported outcome measures (PROMS), heart rate (HR), oxygen saturation (SPO2), and physical activity over several days using innovative sensors. Data will be analyzed using automated ML algorithms with recourse to the sleep physician when in doubt. The treatment follow-up could be made at a face-to-face or video consultation with the sleep physician in the light of all available data.
Modified from JL Pépin et al. [38]
The study findings should ultimately incite healthcare systems and policymakers to support future digital innovations and will assist researchers and clinicians in identifying and reinventing well defined pathways for personalized sleep apnea management.
Availability of data and materials
The datasets generated by this study will be made available to the sleep research community.
Abbreviations
- CPAP:
-
Continuous Positive Airway Pressure
- HR:
-
Heart rate
- LDL:
-
Low-density lipoprotein
- OSA:
-
Obstructive Sleep Apnea
- PSG:
-
Polysomnography
- PROMs:
-
Patient reported outcome measures
- SPO2 :
-
Oxygen saturation
References
Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–98.
Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323:1389–400.
Pauletto P, Réus JC, Bolan M, Massignan C, Flores-Mir C, Maia I, et al. Association between obstructive sleep apnea and health-related quality of life in untreated adults: a systematic review. Sleep Breath. 2021;25:1773–89.
Pase MP, Harrison S, Misialek JR, Kline CE, Cavuoto M, Baril A-A, et al. Sleep architecture, obstructive sleep apnea, and cognitive function in adults. JAMA Netw Open. 2023;6:e2325152.
Pépin J-L, Bailly S, Texereau JB, Sonnet E, Picard S, Vergès B, et al. Prevalence of sleep apnoea in patients with type 1 diabetes and its association with comorbidities and diabetic complications: A French nationwide prospective study. Diabetes Obes Metab. 2023;25:1624–31.
Sterling KL, Cistulli PA, Linde-Zwirble W, Malik A, Benjafield AV, Malhotra A, et al. Association between positive airway pressure therapy adherence and health care resource utilization in patients with obstructive sleep apnea and type 2 diabetes in the United States. J Clin Sleep Med. 2023;19:563–71.
Mendelson M, Marillier M, Bailly S, Flore P, Borel J-C, Vivodtzev I, et al. Maximal exercise capacity in patients with obstructive sleep apnoea syndrome: a systematic review and meta-analysis. Eur Respir J. 2018;51:1702697.
Mendelson M, Bailly S, Marillier M, Flore P, Borel JC, Vivodtzev I, et al. Obstructive sleep apnea syndrome, objectively measured physical activity and exercise training interventions: a systematic review and meta-analysis. Front Neurol. 2018;9:73.
Epstein LJ, Kristo D, Strollo PJ, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76.
Pépin J-L, Bailly S, Rinder P, Adler D, Szeftel D, Malhotra A, et al. CPAP therapy termination rates by OSA phenotype: a French nationwide database analysis. J Clin Med. 2021;10:936.
McDaid C, Durée KH, Griffin SC, Weatherly HLA, Stradling JR, Davies RJO, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev. 2009;13:427–36.
Quan SF, Budhiraja R, Kushida CA. Associations between sleep quality, sleep architecture and sleep disordered breathing and memory after continuous positive airway pressure in patients with obstructive sleep apnea in the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep Sci. 2018;11:231–8.
Somiah M, Taxin Z, Keating J, Mooney AM, Norman RG, Rapoport DM, et al. Sleep quality, short-term and long-term CPAP adherence. J Clin Sleep Med. 2012;8:489–500.
McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1459–63.
Cistulli PA, Armitstead JP, Malhotra A, Yan Y, Vuong V, Sterling KL, et al. Relationship between self-reported sleepiness and positive airway pressure treatment adherence in obstructive sleep apnea. Ann Am Thorac Soc. 2023. https://doi.org/10.1513/AnnalsATS.202206-482OC.
Peter-Derex L, Berthomier C, Taillard J, Berthomier P, Bouet R, Mattout J, et al. Automatic analysis of single-channel sleep EEG in a large spectrum of sleep disorders. J Clin Sleep Med. 2021;17:393–402.
Sabil A, Vanbuis J, Baffet G, Feuilloy M, Le Vaillant M, Meslier N, et al. Automatic identification of sleep and wakefulness using single-channel EEG and respiratory polygraphy signals for the diagnosis of obstructive sleep apnea. J Sleep Res. 2019;28:e12795.
Herman J. Living without health–a challenge to patient and doctor. J R Coll Gen Pract. 1987;37:50.
Tatti P, Tahrani A, Passali D, Reutrakul S, Kanagasabai T. The relationship between disturbed sleep, OSAS, and metabolic diseases. J Diabetes Res. 2019;2019:1463045.
Kothari V, Cardona Z, Chirakalwasan N, Anothaisintawee T, Reutrakul S. Sleep interventions and glucose metabolism: systematic review and meta-analysis. Sleep Med. 2021;78:24–35.
Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152:1070–86.
Banghøj AM, Krogager C, Kristensen PL, Hansen KW, Laugesen E, Fleischer J, et al. Effect of 12-week continuous positive airway pressure therapy on glucose levels assessed by continuous glucose monitoring in people with type 2 diabetes and obstructive sleep apnoea; a randomized controlled trial. Endocrinol Diabetes Metab. 2021;4:e00148.
Byun J-I, Cha KS, Jun JE, Kim T-J, Jung K-Y, Jeong I-K, et al. Dynamic changes in nocturnal blood glucose levels are associated with sleep-related features in patients with obstructive sleep apnea. Sci Rep. 2020;10:17877.
Saito K, Okada Y, Torimoto K, Takamatsu Y, Tanaka Y. Blood glucose dynamics during sleep in patients with obstructive sleep apnea and normal glucose tolerance: effects of CPAP therapy. Sleep Breath. 2022;26:771–81.
Bottaz-Bosson G, Midelet A, Mendelson M, Borel J-C, Martinot J-B, Le Hy R, et al. Remote monitoring of positive airway pressure data: challenges, pitfalls, and strategies to consider for optimal data science applications. Chest. 2023;163:1279–91.
Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307.
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5.
Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83.
Debellemaniere E, Chambon S, Pinaud C, Thorey V, Dehaene D, Léger D, et al. Performance of an ambulatory dry-EEG device for auditory closed-loop stimulation of sleep slow oscillations in the home environment. Front Hum Neurosci. 2018;12:88.
Chouraki A, Tournant J, Arnal P, Pépin J-L, Bailly S. Objective multi-night sleep monitoring at home: variability of sleep parameters between nights and implications for the reliability of sleep assessment in clinical trials. Sleep. 2023;46:zsac319.
Bottaz-Bosson G, Hamon A, Pépin J-L, Bailly S, Samson A. Continuous positive airway pressure adherence trajectories in sleep apnea: clustering with summed discrete Fréchet and dynamic time warping dissimilarities. Stat Med. 2021;40:5373–96.
Li Y, Li Q, Zou X, Zhong Z, Ouyang Q, Zeng Q, et al. Effects of CPAP treatment on electroencephalographic activity in patients with obstructive sleep apnea syndrome during deep sleep: Preliminary findings of a cross-sectional study. Chron Respir Dis. 2023;20:14799731231215094.
Baumert M, Cowie MR, Redline S, Mehra R, Arzt M, Pépin J-L, et al. Sleep characterization with smart wearable devices: a call for standardization and consensus recommendations. Sleep. 2022;45:zsac183.
Verhaert DVM, Betz K, Gawałko M, Hermans ANL, Pluymaekers NAHA, van der Velden RMJ, et al. A VIRTUAL sleep apnoea management pathway for the work-up of atrial fibrillation patients in a digital remote infrastructure: VIRTUAL-SAFARI. Europace. 2022;24:565–75.
Pépin J-L, Baillieul S, Tamisier R. Reshaping sleep apnea care: time for value-based strategies. Ann Am Thorac Soc. 2019;16:1501–3.
Pépin J-L, Tamisier R, Baillieul S, Ben Messaoud R, Foote A, Bailly S, et al. Creating an optimal approach for diagnosing sleep apnea. Sleep Med Clin. 2023;18:301–9.
Acknowledgements
We thank Alison Foote (an independent medical writer based in Grenoble, France) for critical reading and very substantial editing of the manuscript.
Funding
JLP, S Bailly and RT are supported by the French National Research Agency in the framework of the “Investissements d’avenir” program (ANR-15-IDEX-02) and the “e-health and integrated care and trajectories medicine and MIAI artificial intelligence” chairs of excellence from the Grenoble Alpes University Foundation. This work was partially supported by MIAI @ Grenoble Alpes, (ANR-19-P3IA-0003) and the APMC (Action for Chronic Illnesses). SFRMS (French society for sleep medicine and research) funded RBM for one year. However, no reports of independent project evaluation are available.
Author information
Authors and Affiliations
Contributions
JLP is the principal investigator of the study. JLP, MJF, and RBM contributed to the conception and design of the study. JLP, SB, and RT are responsible for the inclusion of patients. RBM, RTe organized the study procedure and will carry out the clinical research. RBM and MJF wrote the first draft of the manuscript. S Bailly wrote the statistical analysis section of the manuscript. JLP, MJF, SB, and RT critically revised the manuscript for important intellectual content. RBM and SB designed the figures 1-2. All authors had full access to the study documents. All authors contributed to the manuscript revision, read and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is performed in accordance with all relevant guidelines and regulations (Declarations of Helsinki and French regulations).
The study was approved by the French bio-clinical research ethics committee (Comité de Protection des Personnes Ouest V in France (CPP)) on November 25, 2021. All participants are required to give written informed consent to participate and for the collection and biobanking of blood samples.
Consent for publication
Not applicable.
Competing interests
None of the authors declare competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ben Messaoud, R., Baillieul, S., Tamisier, R. et al. Digital sleep clinic: assessing efficacy of continuous positive airway pressure through sleep staging via connected devices: a study protocol. BMC Digit Health 2, 23 (2024). https://doi.org/10.1186/s44247-024-00077-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44247-024-00077-w