Abstract
Background
Left ventricular (LV) diastolic function (DF) may play an important role in predicting fluid responsiveness. However, few studies assessed the role of diastolic function in predicting fluid responsiveness. The aim of this pilot study was to assess whether parameters of right and left diastolic function assessed with transesophageal echocardiography, including the mitral E/e′ ratio, is associated with fluid responsiveness among patients undergoing elective bypass graft surgery. We also sought to compare other methods of fluid responsiveness assessment, including echocardiographic and hemodynamic parameters, pulse pressure variation, and stroke volume variation (SVV) (arterial pulse contour analysis, Flotrac/Vigileo system).
Results
We prospectively studied seventy patients undergoing coronary artery bypass grafting (CABG) monitored with a radial arterial catheter, transesophageal echocardiography (TEE), and a pulmonary artery catheter (for cardiac output measurements), before and after the administration of 500 mL of crystalloid over 10 min after the anesthetic induction. Thirteen patients were excluded (total of 57 patients). Fluid responsiveness was defined as an increase in cardiac index of ≥ 15%. There were 21 responders (36.8%) and 36 non-responders (63.2%). No difference in baseline pulsed wave Doppler echocardiographic measurements of any components of the mitral, tricuspid, and pulmonary and hepatic venous flows were found between responders and non-responders. There was no difference in MV tissue Doppler measurements between responders and non-responders, including E/e′ ratio (8.7 ± 4.1 vs. 8.5 ± 2.8 in responders vs. non-responders, P = 0.85). SVV was the only independent variable to predict an increase in cardiac index by multivariate analysis (P = 0.0208, OR = 1.196, 95% CI (1.028-1.393)).
Conclusions
In this pilot study, we found that no parameters of right and left ventricular diastolic function were associated with fluid responsiveness in patients undergoing CABG. SVV was the most useful parameter to predict fluid responsiveness.
Trial registration
ClinicalTrials.gov, NCT 02714244. Registered 21 March 2016—retrospectively registered.
Similar content being viewed by others
Background
Fluid responsiveness after volume expansion is better predicted by dynamic parameters compared to static parameters [1,2,3]. Among dynamic parameters, pulse pressure variation (PPV) and stroke volume variation (SVV) during mechanical ventilation as calculated by the FloTrac system algorithm has comparable sensitivity and specificity [4,5,6,7] to predict an increase in cardiac output after fluid administration.
The Frank-Starling curve illustrates fluid responsiveness, which depends on preload but also on systolic and diastolic ventricular function, venous compliance, and ventricular compliance [3, 8]. However, few studies assessed the role of diastolic function in predicting fluid responsiveness [9, 10]. Using TEE, the E velocity to mitral annular early diastolic velocity (e′) ratio (E/e′) is the measure of diastolic function that is best correlated with left ventricular filling pressure [11,12,13]. The E/e′ ratio is more representative of filling pressure, and potentially preload, because the e′ velocity corrects the effect of ventricular relaxation on E velocity [8]. So far, the association of the E/e′ ratio with fluid responsiveness has never been studied in cardiac surgical patients. In addition, no study evaluated multiple parameters of right and left diastolic function in their ability to predict fluid responsiveness. Our hypothesis is that parameters of right and left diastolic function measured with TEE, including the mitral E/e′ ratio, reflects preload reserve and is associated with preload responsiveness.
The aim of this pilot study was to assess whether parameters of right and left diastolic function, including the mitral E/e′ ratio, is associated with fluid responsiveness among patients undergoing elective bypass graft surgery. We also sought to compare other methods of fluid responsiveness assessment, including echocardiographic and hemodynamic parameters, PPV, and stroke volume variation (SVV).
Results
Seventy patients undergoing CABG were enrolled in this study. Thirteen patients were excluded from the analysis, for a total of 57 patients. The reasons for exclusion were initial PCWP ≥ 18 mmHg (n = 5), initial CVP ≥ 15 mmHg (n = 1), arrhythmia (n = 1), and scheduling conflict (n = 6). There were 50 men and 7 women included in the study. There was no difference in the demographic variables between the responders and non-responders (Table 1). There were 21 responders (36.8%) and 36 non-responders (63.2%). There were 48 patients (84%) without regional wall motion abnormalities. Among the 9 patients with regional motion abnormalities, only 2 patients (one in the responders and one in the non-responders group) had regional wall motion abnormalities in the lateral basal wall. There were no patients with moderate to severe mitral annular calcification. Among the 56 patients with available diastolic function evaluation (simplified algorithm [14]), 10 had normal diastolic function (3 responders and 7 non-responders), 14 had mild diastolic dysfunction (4 responders and 10 non-responders), 25 had moderate diastolic dysfunction (12 responders and 13 non-responders), and 7 patients had severe diastolic dysfunction (1 responders and 6 non-responders), without difference in the number of responders between each grade (P = 0.3212).
Baseline hemodynamic data, two-dimensional, pulsed wave, and tissue Doppler echocardiographic data after fluid bolus are shown for the responders and non-responders (Table 2). No difference in baseline hemodynamic parameters were found between responders and non-responders, except for SVV measured by Flotrac (13.95 ± 6.00% vs. 10.00 ± 4.35% for responders and non-responders respectively, P = 0.0076). No difference were found between responders and non-responders in baseline two-dimensional echocardiographic measurements of the right and left ventricle, except for left ventricular end-diastolic area (LVEDA) (13.58 ± 4.73 cm2 vs. 16.89 ± 5.38 cm2 for responders and non-responders respectively, P = 0.0335). No difference in baseline pulsed wave Doppler echocardiographic measurements of any components of the mitral, tricuspid, and pulmonary and hepatic venous flows were found between responders and non-responders. There was no difference in MV tissue Doppler measurements between responders and non-responders, including E/e′ ratio (8.68 ± 4.07 vs. 8.51 ± 2.83 in responders vs. non-responders, P = 0.8484).
Univariate logistic regressions models were used to assess for the influence of baseline hemodynamic parameters, two-dimensional, pulsed wave, and tissue Doppler echocardiographic measurements to predict fluid responsiveness. Only parameters with a P value < 0.20 in univariate analysis are shown in Table 3. Using this approach, we found that SVV variation measured by Flotrac and LVEDA were associated with fluid responsiveness. However, multivariate analysis revealed that SVV measured by Flotrac was the only independent variable to predict an increase in cardiac index more than 15% (OR = 1.196, 95% CI (1.028-1.393), P = 0.0208).
The performance of baseline hemodynamic and echocardiographic data to predict fluid responsiveness was evaluated by constructing ROC curves (Table 4). Area under the ROC curve was 0.70 for SVV measured by Flotrac using a threshold of 12% to predict fluid responsiveness (95% CI 0.55-0.85) and had a sensitivity of 66.7% with a specificity of 62.5%. However, there was a gray zone for SVV measured by Flotrac between 6 and 16%, which included 43% of the patients.
The change in right ventricular (RV) echocardiographic parameters, change in RV diastolic pressure over time (dP/dT), and RV end-diastolic pressure, from baseline to post fluid infusion are shown in Table 5. A statistically significant interaction term time × group for the LA diameter reveals a significant increase for the responder group but not for the non-responder group. For all other parameters, the interaction term was not significant. After fluid challenge, there was an overall (all groups) significant increase (all P < 0.05) in right atrial diameter, right ventricular end diastolic area, and left ventricular end diastolic area, without changes in right or left end systolic area and in right or left ventricular ejection fraction. There was also an increase in RV dP/dT and RV end diastolic pressure (all groups). A non-statistically significant interaction term (P = 0.7544) for the left ventricular E/e′ ratio of the mitral valve annulus was observed after fluid challenge, meaning that the two groups have the same evolution of this parameter in time (8.6 ± 4.07 to 9.44 ± 3.30 vs. 8.51 ± 2.83 to 9.03 ± 3.15 for responders and non-responders respectively, non-significant increase (P = 0.0835)).
Discussion
In this pilot study, we observed that predictor of fluid responsiveness was unrelated to left ventricular diastolic function parameters. Although left ventricular diastolic function may play a role in fluid responsiveness, our observations do not support the hypothesis that the evaluation of left ventricular diastolic function parameters is relevant in the prediction of fluid responsiveness. Indeed, there was neither difference in the E/e′ ratio of the mitral valve between responders and non-responders nor there was any difference in Doppler measurements of mitral valve inflow, pulmonary venous flow (PVF), and tissue Doppler components of MV annulus velocities. We also observed a lack of difference for Doppler measurements of tricuspid valve inflow, and hepatic venous flow (HVF) between responders and non-responders. The lack of difference in RV dP/dT of the diastolic filling and RV end-diastolic pressure between responders and non-responders are not in favor for a predominant role of RV diastolic function to predict preload responsiveness. Taken together, assessment of multiple parameters of biventricular diastolic function, including the E/e′ ratio of the mitral valve, does not increase our ability to predict fluid responsiveness in this population of patients undergoing CABG.
As confirmed by other investigators, the absolute values of CVP and PCWP (static parameters) were not useful in predicting FR in these patients [15]. In contrast to another study [2], we found that LV end-diastolic area (static parameter) had a significant correlation with fluid responsiveness by univariate analysis. Only SVV measured by Flotrac was independently correlated with fluid responsiveness, as assessed with multivariate analysis. However, SVV had only a modest sensitivity and specificity to predict FR, and almost half of the patients are in a gray zone, which limits its usefulness as a predictor of FR.
There are very few studies evaluating the role of diastolic function to predict fluid responsiveness [9, 10]. In a previous study, Lattik et al. [10] observed that a mitral E/A ratio < 1.26 was superior to any hemodynamic variables in predicting fluid responsiveness in CABG patients (area under the ROC curve of 71%). They suggested that measurements of E/A ratio may be useful in determining the position of an individual patient on the LV pressure/volume curve. When that study was performed, TDI was not measured because unavailable on TEE equipment in 2002. In a subsequent study, Roy et al. [9] found that the a/v wave ratio of the PCWP tracing > 1 was associated with preload responsiveness (area under the ROC curve of 0.89%), which, according to the authors, is a reflection of a normal diastolic function. The difference in these studies and the present one could be explained by differences in population studied, the type and volume of fluid infused, the rate of fluid administration, and time of measurements. However, in neither of these previous studies, assessment of multiple parameters of biventricular diastolic function to predict preload responsiveness has been performed. Even if the hypothesis that a compliant heart can accommodate further volume and thus increase cardiac output is appealing, our analysis of multiple parameters of LV and RV diastolic function could not support these prior results [9, 10].
The hemodynamic response to a fluid loading is related to the Frank-Starling function curve. However, it depends also on the venous return to the heart, which determines if the administered volume is actually getting to the heart during diastole to increase preload [16]. Venous return is a function of stressed vascular volume, venous compliance and resistance, and the pressure gradient between the periphery mean systemic venous pressure (MSVP) and the right atrial pressure (RAP) [16]. The peripheral venous system has a large vascular capacitance and a linear compliance, whereas the diastolic pressure of the heart is curvilinear [16]. These differences in compliance could affect the venous return pressure gradient, because RAP could rise faster than MSVP [17], causing the net pressure gradient for venous return to decrease [17]. We did not measure the mean systemic venous pressure in our study, and consequently, we cannot determine if the MSVP-RAP gradient was different between responders and non-responders. Because preload responsiveness depends on many factors including the systolic and diastolic function, the position on the Frank Starling curve and venous return, for both of the right and left ventricle, and also on a decrease in afterload induced by hemodilution [18], it is difficult to assess the effect of RV and LV diastolic function in isolation on the hemodynamic response to fluid challenge. The effect of diastolic function may be less important than the combination of all the factors mentioned above.
This study has several limitations. First, no prestudy power analysis was performed in this exploratory study. Pilot study like this one serves to identify opportunities to test hypotheses with prospective registration trial with adequate sample size. Second, the results apply to patients undergoing CABG with a closed chest, under general anesthesia and mechanical ventilation, in normal sinus rhythm, preserved right ventricular function, and cannot be generalized to other situations. Third, although more recent guidelines have been published to assess diastolic function [19], it may be difficult to use because some of the parameters, such as left atrial volume, and peak tricuspid regurgitation measurements, are not validated with TEE. Instead, we evaluated TDI parameters of the mitral annulus as well as Doppler tricuspid and mitral flow parameters and used a simplified algorithm to grade the diastolic function [14]. The E/e′ ratio has been shown to correlate as an index of filling pressures and is a preload independent index of LV relaxation. As the E/e′ ratio increases, the severity of diastolic dysfunction becomes more pronounced [19]. The lack of differences in all these parameters between responders and non-responders suggests a minor role of right and left ventricular diastolic function to predict fluid responsiveness in our population. Moreover, there was no difference in the number of responders and non-responders between the different grades of diastolic dysfunction. Fourth, we used crystalloids instead of colloids, used in many of the preload responsiveness studies, which may explain the lower rate of fluid responsiveness obtained in our study. However, the fluid infusion was done within a short period (10 min), and measures were taken within 5 min after fluid infusion, which should limit the difference between colloids and crystalloids to increase preload [20]. Moreover, a meta-analysis by Toscani et al. [20] suggests that there is no difference in the proportion of responders when the time of measurements was assessed at the end of the fluid challenge or between 1 and 10 min after the fluid challenge. Our time of measurement was 5 min after the end of the fluid challenge and was consistent for all the patients.
In addition, we made the measurements with the legs raised, which adds around 150 mL of volume [21], to the fluid challenge. In this manner, we wanted to ensure that the volemic load was sufficient to increase venous return and cardiac output. Even if there is a possibility that this amount of fluid challenge may have produced right ventricular overload which may limit the fluid responsiveness [22] in our study (only a third were responders), we did not find differences between the two groups for right ventricular dimension, right ventricular ejection fraction, right ventricular diastolic pressure waveform analysis (dP/dT), CVP, and end diastolic right ventricular pressure after fluid challenge. Then, the possibility of RV overload is unlikely to explain preload unresponsiveness in this study. The effect of fluid challenge on stroke volume/cardiac output remains difficult to explain within the parameters measured. The concept of fluid responsiveness in itself does not mean that every fluid responder needs further fluid infusion, considering the deleterious effect of fluid overload on organs’ perfusion [23,24,25,26,27,28].
In conclusion, in this pilot study, assessment of multiple parameters of left and right ventricular diastolic function, including E/e′ ratio of the mitral valve annulus measured by TEE, is not a predictor of fluid responsiveness undergoing CABG. The SVV measured by the Flotrac/Vigileo system was the most useful parameter to predict fluid responsiveness; however, almost half of the patients are in a gray zone for this parameter, which decreases its usefulness to guide fluid therapy. Further studies are needed to confirm the results of this exploratory study.
Materials and methods
Population
After approval by the Montreal Heart Institute Research Ethics and New Technology Development Committee (F1-4651) and with informed consent, 70 patients over 18 years old undergoing elective coronary artery bypass grafting (CABG) surgery between January 2016 and September 2017 were enrolled. No prestudy power analysis was performed. After that period, the department elected to terminate this exploratory study and analyze the data. The study was retrospectively registered at Clinical Trials.gov (provided by the US National Library of Medicine) on March 21, 2016, with the Identifier: NCT02714244. Patients with more than mild valvular heart disease, intracardiac shunts, preoperative arrhythmia (non-sinus rhythm), decompensated heart failure, or pulmonary hypertension (mean pulmonary arterial pressure > 25 mmHg), renal insufficiency defined as creatinine clearance of less than 30 mL/min or any contraindications for TEE (such as esophageal disease or unstable cervical spine) were excluded. Central venous pressure (CVP) and pulmonary capillary wedge pressure (PWCP) were assessed before fluid administration and patients with high values (CVP ≥ 15 mmHg or PWCP ≥ 18 mmHg) were excluded because of risk of fluid overload in these patients.
Methods
Premedication, induction, and maintenance of anesthesia were achieved at the discretion of the attending anesthetist with the exception of the requirement for the use of isoflurane inspired concentration of 1%. Lungs were ventilated by intermittent positive-pressure ventilation with a fraction of inspired oxygen of 1.0 by use of an Ohmeda volume-cycled ventilator (Ohmeda, Helsinki, Finland). A respiratory rate of 8 breaths/min with an inspiratory/expiratory ratio of 1:2 and a tidal volume of 8 mL/kg were kept constant for the entire experiment. No positive end-expiratory pressure was applied. Usual monitoring was installed, including a 5-lead electrocardiogram, pulse oximeter, peripheral venous line, radial arterial line, 15-cm 3-lumen catheter (CS-12703, Arrow international Inc., Reading, CA), and fast-response thermodilution pulmonary artery catheter (Swan-Ganz catheter 7.5 Fr; Edwards Lifesciences Corporation, Irvine, CA). A FloTrac (Edwards Lifesciences Corporation, Irvine, CA) sensor was added to the arterial line and was analyzed by the Vigileo monitor (Edwards Lifesciences Corporation, Irvine, CA). A 5.0-MHz TEE omniplane probe (Vivid 7; GE Healtcare System, Milwaukee, WI) was inserted by the anesthesiologist after induction of general anesthesia.
Hemodynamic parameters
After the induction of anesthesia, central line insertion and hemodynamic stability, the following parameters were recorded: arterial blood pressure, cardiac output (CO), right ventricular pressure, CVP, pulmonary artery pressure (PAP), PCWP, and respiratory-induced SVV. The CO was assessed with thermodilution technique using three injections of dextrose 5% (10 mL) at room temperature at end expiration. The average of three measurements with less than 10% difference between each was noted. Cardiac output response measured with the Swan-Ganz catheter was considered the gold standard to assess preload responsiveness. FloTrac was used to determine SVV. The PPV was calculated from the arterial line tracing on the monitor (Philips, Intellivue G5 M1019A).
Echocardiographic parameters
We obtained a complete baseline TEE exam, including the evaluation of diastolic function of the right and left ventricle, according to the ASE/SCA Guidelines for performing a comprehensive intraoperative multiplane TEE examination [8, 29]. The lateral mitral annulus (e′) was measured using TDI, along with measurement of the E wave peak diastolic velocity with pulsed-wave Doppler, and the E/e′ was derived. We also obtained a TEE exam (four chamber, trans-gastric, mitral flow Doppler, pulmonary venous flow Doppler, hepatic venous flow Doppler, and tricuspid Doppler flow) before and after volume infusion. In addition, we determined diastolic dysfunction using a simplified algorithm previously described by Swaminathan et al. [14].
-
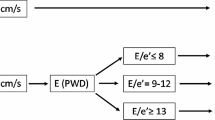
Normal diastolic function: e′ ≥ 10 cm·s−1
-
Grade 1 diastolic dysfunction (mild): e′ < 10 cm·s−1; E/e′ ≤ 8
-
Grade 2 diastolic dysfunction (moderate): e′ < 10 cm·s−1; E/e′ = 9-12
-
Grade 3 diastolic dysfunction (severe): e′ < 10 cm·s−1; E/e′ > 13
A single National Board of Echocardiography (NBE) board-certified anesthetist obtained the echocardiographic images. If any doubt arose about the interpretation of an echocardiographic measurement or hemodynamic waveform interpretation, the advice of a second anesthetist was obtained.
Protocol
All parameters were measured before chest opening and during hemodynamic stability in a supine position. The first measurement (T1) was carried within the 15 min that preceded a rapid volume infusion of 500 mL of Lactate Ringer® over 10 min. The second measurement (T2) was obtained within a maximum of 5 min after the bolus. Because T2 coincided with the time of asepsis, patients’ legs were raised, causing further increase of venous return. The legs were raised for the duration of the measurements for each patient. Fluid infusion was stopped if the PCWP was ≥ 20 mmHg during the protocol and such patients were excluded from the study. Fluid responsiveness was defined as an increase in cardiac index of more than 15% [30].
Statistics
Continuous variables are summarized using mean ± standard deviation, or median (Q1, Q3), according to the distribution of the variable, while categorical variables are described using frequencies and percentages. Demographic data were compared between responders and non-responders using Student t test or Mann-Whitney-Wilcoxon test as appropriate for continuous variables, and chi-square test for categorical variables. The number of responders and non-responders for each grade of diastolic dysfunction (normal to severe) was compared using chi-squared test. Comparison of groups for diastolic function parameters (including mitral E/e′ ratio), 2-D and Doppler echocardiographic measurements, and hemodynamic measurements was performed with a Students t test. Then, using univariate logistic regression models, we explored the potential predictors of preload responsiveness among the same echocardiographic, and hemodynamic values. Only parameters with a P value < 0.20 in univariate analysis were included in the stepwise selection procedure of a multivariate logistic regression model. Receiver operating characteristic (ROC) curves were also generated for hemodynamic and echocardiographic parameters. Because the ROC curve methodology does not take into account, the existence of overlap between positive and negative fluid challenges, we also use the gray zone approach. This methodology avoids the binary response proposed by the ROC curves and proposed a low cut-off value that excludes positive fluid challenge in 90% of patients, whereas a high cut-off value predicts positive fluid challenge in 90% of cases [31]. Two-way repeated measures ANOVA model was used to compare the evolution of the two groups in terms of right and left ventricular and atrial parameters, right ventricular pressure over time (dP/dT), right ventricular end-diastolic pressure, and E/e′ ratio of the mitral valve before and after fluid challenge. The interaction term was tested, and comparison pre vs. post fluid challenge was produced in each group in case of statistically significant interaction. A two-tailed P value < 0.05 was considered statistically significant. All statistical analysis was performed using the SAS version 9.4 software (SAS Institute, NC).
Availability of data and materials
The datasets used and/or analyzed during this current study are available from the corresponding author on reasonable request.
References
Thys D, Hillel Z, Goldman M et al (1987) A comparison of hemodynamic indices derived by invasive monitoring and two-dimensional echocardiography. Anesthesiology 67(5):630–634. https://doi.org/10.1097/00000542-198711000-00003
Tousignant CP, Walsh F, Mazer CD (2000) The use of transesophageal echocardiography for preload assessment in critically ill patients. Anesth Analg 90(2):351–355. https://doi.org/10.1097/00000539-200002000-00021
Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 121(6):2000–2008. https://doi.org/10.1378/chest.121.6.2000
Le Manach Y, Hofer CK, Lehot JJ et al (2012) Can changes in arterial pressure be used to detect changes in cardiac output during volume expansion in the perioperative period? Anesthesiology 117(6):1165–1174. https://doi.org/10.1097/ALN.0b013e318275561d
Cannesson M, Musard H, Desebbe O, Boucau C, Simon R, Hénaine R, Lehot JJ (2009) The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg 108(2):513–517. https://doi.org/10.1213/ane.0b013e318192a36b
Slagt C, Malagon I, Groeneveld AB (2014) Systematic review of uncalibrated arterial pressure waveform analysis to determine cardiac output and stroke volume variation. Br J Anaesth 112(4):626–637. https://doi.org/10.1093/bja/aet429
Monnet X, Marik PE, Teboul JL (2016) Prediction of fluid responsiveness: an update. Ann Intensive Care 6(1):111. https://doi.org/10.1186/s13613-016-0216-7
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133. https://doi.org/10.1016/j.echo.2008.11.023
Roy S, Couture P, Qizilbash B, Toupin F, Levesque S, Carrier M, Lambert J, Denault AY (2013) Hemodynamic pressure waveform analysis in predicting fluid responsiveness. J Cardiothorac Vasc Anesth 27(4):676–680. https://doi.org/10.1053/j.jvca.2012.11.002
Lattik R, Couture P, Denault AY, Carrier M, Harel F, Taillefer J, Tardif JC (2002) Mitral Doppler indices are superior to two-dimensional echocardiographic and hemodynamic variables in predicting responsiveness of cardiac output to a rapid intravenous infusion of colloid. Anesth Analg 94(5):1092–1099. https://doi.org/10.1097/00000539-200205000-00007
Rivas-Gotz C, Manolios M, Thohan V, Nagueh SF (2003) Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol 91(6):780–784. https://doi.org/10.1016/S0002-9149(02)03433-1
Bouhemad B, Nicolas-Robin A, Benois A, Lemaire S, Goarin JP, Rouby JJ (2003) Echocardiographic Doppler assessment of pulmonary capillary wedge pressure in surgical patients with postoperative circulatory shock and acute lung injury. Anesthesiology 98(5):1091–1100. https://doi.org/10.1097/00000542-200305000-00011
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102(15):1788–1794. https://doi.org/10.1161/01.CIR.102.15.1788
Swaminatham M, Nicoara A, Phillips-Bute BG et al (2011) Cardiothoracic Anesthesia Research Endeavors (CARE) Group. Utility of simple algorithm to grade diastolic dysfunction and predict outcome after coronary artery bypass graft surgery. Ann Thorac Surg 91(6):1844–1850. https://doi.org/10.1016/j.athoracsur.2011.02.008
Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, Teboul JL (2007) Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 35(1):34–68. https://doi.org/10.1097/01.CCM.0000249851.94101.4F
Monnet X, Pinsky MJ (2015) Predicting the determinants of volume responsiveness. Intensive Care Med 41(2):354–356. https://doi.org/10.1007/s00134-014-3637-5
Kinsky M, Ribeiro N, Canesson M et al (2016) Peripheral venous pressure as an indicator of preload responsiveness during volume resuscitation from hemorrhage. Anesth Analg 123(1):114–122. https://doi.org/10.1213/ANE.0000000000001297
Ueyama H, Kionaka S (2017) Predicting the need for fluid therapy: does fluid responsiveness work? J Intensive Care 5(1):34. https://doi.org/10.1186/s40560-017-0210-7
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011
Toscani L, Hollman DA, Antonakaki D et al (2017) What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit Care 21(1):207. https://doi.org/10.1186/s13054-017-1796-9
Jabot J, Teboul JL, Richard C, Monnet X (2009) Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med 35(1):85–90. https://doi.org/10.1007/s00134-008-1293-3
Vieillard-Baron A, Naeije R, Haddad F, Bogaard HJ, Bull TM, Fletcher N, Lahm T, Magder S, Orde S, Schmidt G, Pinsky MR (2018) Diagnostic workup, etiologies and management of acute right ventricle failure. Intensive Care Med 44(6):774–790. https://doi.org/10.1007/s00134-018-5172-2
Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA (2011) Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 39(2):259–265. https://doi.org/10.1097/CCM.0b013e3181feeb15
Stein A, de Souza LV, Belettini CR, Menegazzo W, Viégas J, Costa Pereira E, Eick R, Araújo L, Consolim-Colombo F, Irigoyen M (2012) Fluid overload and changes in serum creatinine after cardiac surgery: predictors of mortality and longer intensive care stay. A prospective cohort study. Crit Care 16(3):R99. https://doi.org/10.1186/cc11368
Silva JM Jr, de Oliveira AM, Nogueira FA et al (2013) The effect of excess fluid balance on the mortality rate of surgical patients: a multicenter prospective study. Crit Care 17(6):R288. https://doi.org/10.1186/cc13151
Hassinger AB, Wald EL, Goodman DM (2014) Early postoperative fluid overload precedes acute kidney injury and is associated with higher morbidity in pediatric cardiac surgery patients. Pediatr Crit Care Med 15(2):131–138. https://doi.org/10.1097/PCC.0000000000000043
Lee J, de Louw E, Niemi M, Nelson R, Mark RG, Celi LA, Mukamal KJ, Danziger J (2015) Association between fluid balance and survival in critically ill patients. J Intern Med 277(4):468–477. https://doi.org/10.1111/joim.12274
Gambardella I, Gaudino M, Ronco C, Lau C, Ivascu N, Girardi LN (2016) Congestive kidney failure in cardiac surgery: the relationship between central venous pressure and acute kidney injury. Interact Cardiovasc Thorac Surg 23(5):800–805. https://doi.org/10.1093/icvts/ivw229
Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, Reeves ST, Shanewise JS, Siu SC, Stewart W, Picard MH, American Society of Echocardiography, Society of Cardiovascular Anesthesiologists (2014) Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg 118(1):21–68. https://doi.org/10.1213/ANE.0000000000000016
Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P (1998) Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology 89(6):1313–1321. https://doi.org/10.1097/00000542-199812000-00007
Cannesson M, Le Manach Y, Hofer CK et al (2011) Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a gray zone approach. Anesthesiology 115(2):231–241. https://doi.org/10.1097/ALN.0b013e318225b80a
Acknowledgements
Not applicable
Funding
This research did not receive any specific grant from funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AC: Conceptualization; data curation; formal acquisition; investigation; methodology; project administration; validation; writing original; writing: review and editing. CPH: Data curation; formal acquisition; investigation; validation; writing: review and editing. II: Data curation; formal acquisition; investigation; validation; writing: review and editing. GD: Data curation; formal acquisition; investigation; validation; writing: review and editing. AF: Data curation; formal acquisition; investigation; validation; writing: review and editing. AD: Data curation; formal acquisition; investigation; validation; writing: review and editing. AD: Data curation; formal acquisition; investigation; validation; writing: review and editing. PC: Conceptualization data curation; formal acquisition; investigation; methodology; project administration; validation; writing original; writing: review and editing. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval by the Montreal Heart Institute Research Ethics and New Technology Development Committee (F1-4651) and with informed consent, patients were included.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Courbe, A., Perrault-Hébert, C., Ion, I. et al. Should we use diastolic function parameters to determine preload responsiveness in cardiac surgery? A pilot study. J Anesth Analg Crit Care 1, 12 (2021). https://doi.org/10.1186/s44158-021-00014-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44158-021-00014-7