Abstract
Background
Streptozotocin (STZ) is a glucose analogue commonly used for inducing diabetes in experimental animals. This study is intended to investigate the ability of captopril (Cap) pretreatment to augment STZ-induced diabetogenic effect in an experimental rat model. If this hypothesis were proven, Cap administration to rats could reduce the dosage of STZ by augmenting its effect and resulting in a subsequent reduction in STZ cost. Forty-two adult male Wistar rats were randomly divided into seven groups: a control group that fed a normal diet, whereas the other six experimental groups were fed a high-fat diet (HFD). The six groups were then divided into STZ-30, STZ-30-Cap, STZ-40, STZ-40-Cap, STZ-50, and STZ-50-Cap. All Cap-received groups were supplemented with 50 mg/kg Cap orally one hour just before intraperitoneal (I.P.) injection of STZ. 30-STZ, 40-STZ, and 50-STZ-treated groups were injected once with STZ I.P. at doses of 30, 40, and 50 mg/kg, respectively. An intraperitoneal glucose tolerance test (IPGTT) was done. Pancreatic tissue was obtained to measure Tumor necrosis factor alpha (TNF-α), interleukin one beta (IL-1β), and nitric oxide (NO) by enzyme-linked immunosorbent assay (ELISA) and glucose transporter 2 (GLUT2) gene expression by reverse transcription polymerase chain reaction (RT-PCR). Pancreatic sections were examined by hematoxylin and eosin (H&E) stain, and immunohistochemical staining by anti-insulin and anti-TNF-α antibodies.
Results
Results indicated that administration of Cap before STZ in different doses significantly augmented the hyperglycemic state that was evident by intraperitoneal glucose tolerance test, and markedly increased pancreatic pro-inflammatory markers. Histological analysis of islets of Langerhans indicated degeneration with extensive vacuolations associated with a significant decrease in mean area % of insulin immunoreactivity and an increase in optical density of TNF-α immunoreactivity.
Conclusion
These findings pointed to the ability of captopril pretreatment to augment the hyperglycemic state and the diabetogenic response that was induced secondary to STZ injection in an experimental rat model.
Similar content being viewed by others
Introduction
The animal models played a crucial role in studying how disease pathogenesis develops and evaluating novel therapeutic agents. Diabetes mellitus (DM) was one of the disorders that necessitated ongoing creative therapy approaches [1]. DM refers to a set of metabolic illnesses that result in high blood glucose levels and a state of chronic hyperglycemia. In this context, the two most prevalent types of diabetes mellitus are type-1 diabetes and type-2 [2].
Type-1 diabetes is classified as an autoimmune disease due to the attack and destruction of insulin-producing pancreatic β-cells [3], while type-2 diabetes is accompanied by insulin resistance and insufficient insulin secretion due to a lack of appropriate compensation by the beta-cells. Consequently, the selection of appropriate animal models for diabetes research should be contingent upon the specific aspects of the disease under investigation [4].
To induce DM. in animal models, the traditional toxic chemicals that selectively target pancreatic β-cells have been used by many researchers. For instance, alloxan has been utilized to induce β-cell destruction through the production of free radicals. However, alloxan demonstrated several prominent drawbacks, including high animal mortality, the generation of animal ketosis due to the formation of free fatty acids, reversible diabetic states, species-specific resistance to its diabetogenic effects as in guinea pigs, and renal toxicity within a narrow effective dose range [5]. As a result, streptozotocin (STZ) has emerged as a substitute for alloxan due to its more favorable characteristics for diabetes induction [6]. STZ can be used to induce animal models of both type-1 and type-2 diabetes by applying either high or low doses [7]. High doses of STZ can severely impair insulin release resembling what happened in type-1 diabetes and can also lead to the formation of ketone bodies, a feature of uncontrolled type-1 diabetes. In contrast, low doses of STZ can cause mild impairment in insulin secretion that mimics type-2 diabetes. However, the low-dose STZ-induced animal model does not address insulin resistance which is in general a feature of type-2 diabetes [8].
A model, that combines a high-fat diet (HFD) followed by a low STZ dose to induce peripheral insulin resistance, would closely resemble human type-2 diabetes [9]. Although there are several experimental models of DM reported to date, the HFD-STZ-type-2 diabetes model is still considered the preferable one [10, 11]. The STZ dose-dependent direct and indirect toxicity is still the main significant drawback. Studies reported that some albino rats developed stomach ulcers, decreased bone volume and muscle mass, reproductive problems, hepatotoxicity, and nephrotoxicity at a dose of 65 mg/kg and 100% mortality in Wistar rats due to the lethal end-point at a dose ≥ 70 mg/kg [11].
Even while it appears to be easy to induce diabetes in many different animal species with STZ, it is difficult because of factors like unpredictability, high cost, and high mortality rates. Based on their particular experiences, the investigators employ different techniques when implementing this paradigm. The STZ dosage, the way it is administered, the animal species, the gender, and the body weight are all subject to variation. The investigators have made numerous attempts aiming to create more stable and consistent hyperglycemia [12].
The local renin–angiotensin system (RAS) has been discovered to play a crucial role in pancreatic physiology and is involved in pancreatic function. Angiotensin II receptors include two types 1 and 2 (AT1R and AT2R), they have been demonstrated to be present in pancreatic islets, whereas the function of AT2R is still controversial and not fully understood [13, 14].
Recent evidence revealed that RAS could regulate the endocrine and exocrine function of the pancreas and short-term Angiotensin II (Ag II) infusion changes the intra-islet blood flow while the chronic exposure effects are still unknown [15]. Another study found that Ang II has a significant vasoconstrictive effect on islet vasculature and reduces islet blood flow. In contrast, the angiotensin receptor blockers vasodilate islet arterioles and stimulates insulin secretion in response to high glucose levels [16, 17].
Captopril (Cap), an angiotensin-converting enzyme (ACE) inhibitor medication, is used in controlling hypertension, management of left ventricular dysfunction after myocardial infarction, and diabetic nephropathy. Many researchers discovered its role in increased glucose uptake by pancreatic tissues, the exact mechanism is unclear, but a vasodilation effect may be incorporated [18].
This study aimed to investigate the possibility of developing a modified experimental model of diabetes mellitus by estimating the adding effect of captopril on different low doses of STZ in HFD adult Wistar rats. The authors compared the changes in serum glucose levels in response to the intraperitoneal glucose tolerance test (IPGTT), and local pro-inflammatory markers in pancreatic tissues secondary to injecting different doses of STZ that were given either alone or in combination with captopril for inducing animal model diabetes.
Material and methods
Experimental animal & grouping
Forty-two adult male Wistar rats (8–10 weeks in age and 180–200 g weight) were purchased from and housed in the Animal House of the Faculty of Medicine, Cairo University. Animals were left for seven days to acclimate to ordinary environmental living conditions regarding humidity, temperature, and dark/light cycles. Rats had free access to food and water to ensure normal growth and behavior; they were kept in wire mesh cages (three rats in each) before starting the experimental procedures. All animal procedures were done in accordance with the highest International Criteria for Animal Experimentation of Helsinki. The Faculty of Medicine, Cairo University Ethical Approval No is; CU/III/F/38/22.
Drugs and chemicals
STZ was purchased from Sigma‐Aldrich, MO, USA, and was given at doses of (30, 40 &50 mg/kg). STZ was dissolved in freshly prepared 0.1 M citrate buffer (pH 4.5; Talpate, Bhosale, Zambare, &Somani). Captopril purchased from Sigma-Aldrich, MO, USA, as a powder, was dissolved in sterile distilled water and was given at 50 mg/kg orally. All other chemicals and reagents were of high analytical grade.
Investigation protocols
Rats were randomly divided into seven groups (six rats each), Group I; control normal rats (fed a normal rat chow (12% fat, 60% carbohydrate, and 28% protein), and the other six main experimental groups were maintained on a HFD; 41% fat, 41% carbohydrate, and 18% protein) for 2 weeks. Then, the overnight‐fasted rats in the experimental groups were injected with STZ. The rats were allocated to: Group II (STZ-30); the rats in this group received STZ at a dose of 30 mg/kg (I.P.) [19], Group III (STZ-30-Cap); the rats were subjected to the intake of captopril (50 mg/kg orally) [20, 21] one hour just before injecting STZ at a dose of 30 mg/kg, group IV (STZ-40); the rats in this group were subjected to STZ injection at a dose of 40 mg/kg I.P.), group V (STZ-40-Cap): the rats were subjected to oral administration of captopril at the same of group III one hour just before injecting 40 mg/kg of STZ, group VI (STZ-50); the rats were subjected to STZ injection at a dose of 50 mg/kg (I.P.), and group VII (STZ-50-Cap); the rats were subjected to oral administration of captopril (at the same of group III, and V one hour just before injecting 50 mg/kg of STZ.
At the end of the study, after 28 days (one week allowed for acclimatization of the experimental rats, followed by a two-week span during which the rats were maintained on a HFD). The final week was dedicated to the implementation of the treatment protocol and served as the end-point of the study, the blood pressure of each rat was measured using the tail-cuff method. The animals were fasted overnight for about 12 h, and then the blood samples were obtained from the femoral vein under ketamine/xylazine (60/7.5 mg/kg, i.p) [22] anesthesia using non-heparinized capillary tubes for separation of sera. Samples were kept immediately in ice-chilled, siliconized disposable glass tubes. The serum samples were obtained by centrifuging blood samples at 4,000 g for 15 min at 4 °C and kept at − 80 °C until further analysis. After that, the rats were sacrificed by cervical dislocation under anesthesia, and the pancreatic tissues were excised and fixed in a 10% buffered formalin solution for histopathologic analysis. More treatment protocol clarification is shown in Fig. 1.
Noninvasive blood pressure measurement
The noninvasive rat tail systolic arterial blood pressure was recorded at the end of the study using a noninvasive blood pressure meter (LE 5002, Harvard Apparatus). The rat is placed in a restrainer and warmed. A pneumatic pulse sensor is attached to its tail. A cuff is placed around the tail, and slowly inflated above the systolic pressure until it causes pulsations to cease, measured by the piezo-electric pulse sensor. The cuff pressure at which pulsations cease is taken to be the SBP in the tail.
Verification of diabetes
Intraperitoneal glucose tolerance test (IPGTT)
All rats were subjected to IPGTT [23, 24]. Male rats were fasted overnight and injected with glucose at a dose of 2 g/kg (I.P.). Blood glucose levels were measured at times of fasting (time zero), 30, 60, and 120 min with a glucometer (GlucoDrTM, All Medicus Co. Ltd, Gyeonggi, Korea) [25].
Biochemical measurement
TNF-α in pancreatic tissue by ELISA
TNF-α in pancreatic tissue protein was assessed by ELISA technique according to kit instructions; Rat TNF-α ELISA Kit, Catalog No: MBS2507393. MyBioSource, Inc. San Diego, CA 92195–3308, USA.
IL-1β in pancreatic tissue by ELISA
IL-1β in pancreatic tissue protein was assessed by ELISA technique according to kit instructions; Rat IL-1β ELISA Kit. Catalog No: MBS824956.MyBioSource, Inc. San Diego, CA 92195–3308.
NO level in pancreatic tissue
NO level in pancreatic tissue was assessed according to kit instruction; Nitric Oxide Assay Kit (Colorimetric). Catalog No: ab65328, Abcam, Waltham, USA.
GLTU2 gene expression in pancreatic tissue by RT-PCR
The pancreatic tissues from all studied groups were homogenized and total RNA was isolated with Gene JET Kit (Thermo Fisher Scientific Inc., Germany, #K0732). A one-step qRT-PCR reaction was done; for reverse transcription; about 5 µl from the total RNA from each sample (once for the GLUT2 gene and once for ß actin gene) was used with subsequent amplification with Bioline, A median Life Science company, UK (SensiFASTTM SYBR R Hi-ROX) One-step Kit (catalog number PI-50217 V) in a 48-well plate using the Step-one instrument (Applied Biosystems, USA). Thermal profile was as follows: 45 °C for 15 min in one cycle (for cDNA synthesis), 10 min at 95 °C for reverse transcriptase enzyme inactivation, followed by 40 cycles of PCR amplification. 10 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C were adjusted for each cycle.
RNA quantitation assessment was carried out using a spectrophotometer the Nano Drop® (ND)-1000 (Nano Drop Technologies, Inc.) by measuring the absorbance at 260 nm.
RNA purity was assessed by comparing the absorbance ratio using a spectrophotometer the Nano Drop® (ND)-1000 (Nano Drop Technologies, Inc.) as we measured the absorbance at 280 to exclude contamination with protein and reagent that absorbed near 280, the 260/280 ratio was 2, also the absorbance at 230 was measured to exclude contamination with another compound as carbohydrate and phenol that absorb near 230, the 260/230 ratio was 2.1
From the expression of the studied genes, genes were normalized relative to the mean critical threshold (CT) values of ß actin as the housekeeping gene by the Ct method. Primers’ sequences for studied genes are listed in Table 1.
Pancreatic tissues histological study
The Pancreas was dissected, fixed in 10% formaldehyde solution, and processed into paraffin blocks. Five-micron sections were cut and mounted on glass slides for hematoxylin and eosin (H&E) staining to examine structural changes in islets of Langerhans in the pancreas.
Immunohistochemical staining of the pancreatic tissues
Pancreatic sections were de-paraffinized, hydrated with descending grades of alcohol, and treated with citrate buffer pH 6 in the microwave for antigen retrieval, then incubated overnight with: 1-anti-insulin antibody (ab63820; rabbit polyclonal antibody, dilution (1:100), Abcam, Cambridge, UK). 2-anti-TNF-α receptor (ab220210; mouse monoclonal antibody, dilution (1:100), Abcam, Cambridge, UK). Two drops of biotinylated secondary antibody were applied for 20 min and then two drops of streptavidin peroxidase for 10 min. The reaction was visualized with diaminobenzidine (DAB) as a chromogen and Mayer’s hematoxylin as a counter-stain. The primary antibodies were replaced in negative control serial sections with PBS.
Morphometric assessment
The “Leica Qwin 500 C” image analyzer computer system Ltd. (Cambridge, UK) was used to collect the data. The image analyzer was made up of an Olympus color video camera, a colored monitor, and an IBM hard drive that was attached to a light microscope and ran “Leica Qwin 500 C” software. To automatically translate the measurement units (pixels) generated by the image analyzer program into actual micrometer units, the image analyzer was first calibrated.
The mean diameter of the islet of Langerhans, area % of anti-insulin immunoreactivity, and optical density of TNF-α immunoreactivity were assessed in 10 non-overlapping randomly selected high-power fields (× 400).
Statistical analysis
The results were obtained as mean ± standard deviation (SD). Statistical analysis for parametric measures was done via one-way analysis of variance (ANOVA) version 25, followed by the Tukey–Kramer multiple comparisons test as a post hoc test. A comparison of glucose levels in each group with time was done using repeated measure ANOVA. Statistical significance was considered when P < 0.05. Statistical analysis will be carried out using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, N.Y., USA).
Results
The intraperitoneal glucose tolerance test (IPGTT) and the systolic and diastolic blood pressure result in all studied groups
The blood pressure was recorded both before and after the administration of STZ and Cap to confirm any changes in blood pressure. The findings showed that there were no significant differences in the reading among all studied groups.
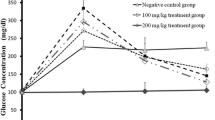
As shown in Table 2, all treated groups showed a significant increase in fasting, and estimated blood glucose was measured after 30, 60, and 120 min following I.P. injection of glucose compared to the control group. Moreover, receiving captopril before injecting STZ at 30 mg/kg and 40 mg/kg was able to significantly increase the levels of blood glucose measured at different times compared to STZ-40 and STZ-50, respectively. Additionally, the maximum results of estimated blood glucose were obtained STZ-50-Cap group at different times compared to other groups.
Captopril administration was able to enhance the expression of GLUT2 and increased nitric oxide levels in pancreatic tissues in the studied groups
The expression levels of GLUT2 were estimated in all studied groups and the pre-intake of captopril before the administration of STZ was able to enhance its expression levels that were documented by PCR. Our data documented enhanced expression levels in the STZ-30-Cap compared to STZ-30, and a significant increase in the same parameter in the STZ-40-Cap compared to its corresponding values in the STZ-40 group, additionally significant increase in GLUT2 expression was recorded in the STZ-50-Cap compared to its corresponding values in the STZ-50 group, without recording any significant difference between STZ-30-Cap, STZ-40-Cap, STZ-50-Cap groups (Table 3). Additionally, the levels of nitric oxide were significantly increased in all STZ-treated groups compared to the control group, and captopril administration before STZ injection was able to significantly increase the levels of NO in these groups compared to the corresponding values of the STZ-treated groups (Table 3).
Augmenting pro-inflammatory state secondary to captopril pre-intake with an increased dose of STZ
As shown in Fig. 2, the levels of pro-inflammatory cytokines (TNF-α and IL-1β) were significantly increased in all STZ and STZ-Cap-treated groups compared to the corresponding results of the control group that denotes the effectiveness of STZ to produce the pro-inflammatory condition. Moreover, the captopril intake before STZ administration was able to augment the production of both TNF-α and IL-1β in STZ-30-Cap compared to STZ-30, and STZ-40-Cap compared to STZ-40 and STZ-50-Cap compared to SZT-50.
Changes in tissue levels of TNF-α and IL-1β in all studied groups. Data presented as mean values and SD. No = 6. Statistical analysis was done using one-way ANOVA test followed by Tukey–Kramer post hoc test. * denotes significant difference compared to control, # denotes significant difference compared to STZ-30, $ denotes significant difference compared to STZ-30-Cap, € denotes significant difference compared to STZ-40, ¥ denotes significant difference compared to STZ-40-Cap, and @ denotes significant difference compared to STZ-50. TNF-α Tumor necrosis alpha, IL-1β Interleukin one beta, STZ Streptozotocin, Cap Captopril
Effect of STZ and captopril pre-intake on pancreatic tissue degeneration
As shown in Fig. 3, the histological examination using H&E staining of the pancreatic tissues demonstrated that the control group showed normal architecture of the pancreas with the islet of Langerhans surrounded by pancreatic acini. The experimental groups, STZ-30; STZ-30-Cap; and STZ-40, exhibited a degenerated islet of Langerhans. However, STZ-40-Cap; STZ-50; and STZ-50-Cap groups revealed massive degeneration of islets of Langerhans with large areas of vacuolations and numerous depleted β-cells.
Representative histological photomicrographs of H&E-stained rat pancreatic Sects. (400x). A The control group shows a pale-stained islet of Langerhans surrounded by a darkly stained closely packed pancreatic acini. The acini are formed of pyramidal cells displaying apical acidophilia and basal basophilia with vesicular nuclei and prominent nucleoli. B & C Groups STZ-30 and STZ-30-Cap illustrate degenerated islets of Langerhans with vacuolations (arrows) and a few depleted β-cells that display darkly stained nuclei (arrow heads). D & E Group STZ-40 illustrates a degenerated islet of Langerhans with few vacuolations (arrows) and few depleted β-cells that display darkly stained nuclei (arrow heads). Group STZ-40-Cap exhibits more vacuolations (arrows) in islets of Langerhans and more depleted B-cells with darkly stained nuclei (arrow heads). F & G. Group STZ-50 displays marked degeneration of islets of Langerhans with vacuolations (arrows) and many depleted β-cells. Group STZ-50-Cap shows massive degeneration with vacuolations (arrows) and most of the cells are depleted (arrow heads). H. Histogram representing mean area % Islet of Langerhans size; *denotes significant difference compared to control, # denotes significant difference compared to STZ-30, $ denotes significant difference compared to STZ-30-Cap, € denotes significant difference compared to STZ-40, ¥ denotes significant difference compared to STZ-40-Cap, and @ denotes significant difference compared to STZ-50. STZ Streptozotocin, Cap Captopril
Immunohistochemical assessment of the pancreas stained with anti-insulin
As shown in Fig. 4; the control group revealed strong widespread insulin immunostaining in the islet of Langerhans. All STZ-treated groups exhibited a significant decrease in the mean area % of insulin immunoreactivity in comparison with the control group. β-cells in experimental groups, STZ-30; STZ-30-Cap; and STZ-40, demonstrated moderate heterogeneous insulin immunostaining. STZ-40-Cap group showed weak insulin immunostaining. Moreover, STZ-50 and STZ-50-Cap groups exhibited faint heterogeneous insulin immunostaining in β-cells, revealing a significant decrease in mean area % of insulin immunoreactivity when compared to groups STZ-30; STZ-30-Cap; and STZ-40. Furthermore, the STZ-50-Cap group demonstrated a significant decrease in mean area % in comparison with all experimental groups. A significant difference was recorded between STZ-30 and STZ-30-Cap groups as well as between STZ-40 and STZ-40-Cap groups in addition between STZ-50 and STZ-50-Cap groups.
Representative photomicrographs of anti-insulin immunohistochemistry-stained rat pancreatic Sects. (400x). A Control group shows a strong homogeneous distribution of insulin immunostaining in β cells. B & C Groups STZ-30 and STZ-30-Cap illustrate moderate heterogeneous insulin immunostaining in β cells. D STZ-40 group exhibits moderate insulin immunostaining in β cells. E Group STZ-40-Cap demonstrates weak insulin immunostaining of β cells. F & G STZ-50 and STZ-50-Cap exhibit faint heterogeneous insulin immunostaining in β cells. H Histogram representing mean area % anti-insulin immunostained sections; * denotes significant difference compared to control, # denotes significant difference compared to STZ-30, $ denotes significant difference compared to STZ-30-Cap, € denotes significant difference compared to STZ-40, ¥ denotes significant difference compared to STZ-40-Cap, and @ denotes significant difference compared to STZ-50. STZ Streptozotocin, Cap Captopril
Immunohistochemical assessment of the pancreas stained with anti-TNF-α
As shown in Fig. 5, β-cells of the control group exhibited negative TNF-α immunostaining. The optical density of TNF-α immunostaining in all experimental groups revealed a significant increase when compared to the control group. β cells of STZ-30; STZ-30-Cap; and STZ-40 groups revealed moderate TNF-α immunostaining. In addition, groups STZ-40-Cap; STZ-50; and STZ-50-Cap illustrated strong TNF-α immunostaining of β cells. STZ-40 and STZ-40-Cap groups revealed a significant increase in the optical density of TNF-α immunostaining when compared to STZ-30 and STZ-30-Cap groups. Moreover, STZ-50 and STZ-50-Cap groups demonstrated a significant increase of optical density of TNF-α immunostaining in comparison with all experimental groups. A significant difference was recorded between STZ-30 and STZ-30-Cap groups as well as between STZ-40 and STZ-40-Cap groups in addition between STZ-50, and STZ-50-Cap groups.
Representative photomicrographs of anti-TNF-α immunohistochemistry-stained rat pancreatic Sects. (400x). A The control group shows negative immunostaining of β cells with TNF-α. B & C The groups STZ-30 and STZ-30-Cap exhibit moderate TNF-α immunostaining in β cells. D Group STZ-40 exhibits moderate TNF-α immunostaining. E Group STZ-40-Cap demonstrates strong TNF-α immunostaining of β cells. F STZ-50 illustrates strong TNF-α immunostaining of β cells. G STZ-50-Cap exhibits intensive immunostaining of β cells with TNF-α. H Histogram illustrating optical density of anti-TNF-α immunostained sections. * denotes significant difference compared to control, # denotes significant difference compared to STZ-30, $ denotes significant difference compared to STZ-30-Cap, € denotes significant difference compared to STZ-40, ¥ denotes significant difference compared to STZ-40-Cap, and @ denotes significant difference compared to STZ-50. TNF-α Tumor necrosis alpha, STZ Streptozotocin, Cap Captopril
Mean area % of insulin antibody immunoreactivity result
In addition, STZ-30-Cap, STZ-40-Cap, and STZ-50-Cap revealed a significant decrease when compared to group STZ-30, STZ-40, and STZ-50, respectively (Fig. 4).
Optical density of TNF-α antibody immunoreactivity result
The optical density of TNF-α immunoreactivity in all experimental groups revealed a significant increase when compared to the control group. Groups STZ-30-Cap, STZ-40-Cap, and STZ-50-Cap exhibited a significant increase in comparison with groups STZ-30, STZ-40, and STZ-50, respectively (Fig. 5).
Discussion
To our knowledge, this study is the first to investigate the possibility of developing a modified experimental model of DM by estimating the adding effect of captopril on different low doses of STZ in HFD adult Wistar rats. Our goal was to reduce the high doses of STZ and to reduce the cost of the experimental protocols.
The components of RAS have been proven to be expressed locally in rodent and human pancreatic islets a long time ago. Twenty years ago, researchers [26] discovered the intrinsic angiotensin system in dog exocrine pancreas, and numerous data support the idea of an intrinsic RAS in the rat pancreas indicating its role in pancreatic function regulation and insulin secretion [27].
In this study, we tried to explore a new modified experimental model for the induction of DM in which we investigated the effect of the addition of ACE inhibitor, captopril, to different doses (30, 40, & 50 mg/kg) of STZ aiming to augment the diabetic response caused by low doses of STZ by combining it with captopril. This approach assists in achieving a more efficient model of diabetes while reducing the hazards associated with giving high doses of STZ; which can sometimes be lethal to the animals.
Our results were consistent with our working hypothesis as the administration of 50 mg/kg of captopril just an hour before the I.P. injection of 30 mg/kg STZ to the HFD rats resulted in a significant increase in both fasting and postprandial glucose levels more than in groups that were injected with STZ alone and all animals were still alive till the end of the experiment. However, there is some mortality at the other two higher doses (40 & 50 mg/kg) in STZ combined with captopril. Captopril is a medication used as an antihypertensive drug that prevents the conversion of angiotensin I to the potent vasoconstrictor angiotensin II [28]. However, its precise mechanism in producing hyperglycemia is not evident but a long time ago, several studies have suggested the relationship between RAS and both pancreatic and islet blood flow.
Studies revealed that islet RAS exhibits a significant role in regulating islet glucose-stimulated insulin release and the locally produced Ang II markedly impair islet blood flow and blocks glucose-induced insulin secretion. RAS antagonists, such as enalaprilat and irbesartan (an AT1 receptor antagonist), can selectively augment pancreatic islet blood flow and improve glucose-stimulated insulin release [29].
A study was done by Olsson et al. [30] to investigate the effect of Ang II on revascularization and local blood flow regulation in transplanted rat pancreatic islets and they concluded that Ang II decreased the vascular conductance of the islet grafts in both nondiabetic and diabetic animals. Carlsson et al. [29] suggested that RAS has marked impacts on the growth, differentiation, secretion, and insulin sensitivity of pancreatic islets, which in turn affect glucose uptake, metabolism, and pathogenesis of DM. Campbell and his colleague [31] 1986 have reported that mRNA encoding angiotensinogen, renin, and Ang II have been discovered in the pancreas of various species. This Ang II can adversely influence pancreatic and islet blood flow through the vasoconstrictive mechanism. A further study was conducted by Lau et al. [32], which used a double immunostaining technique to detect high-affinity binding sites for Ang. Results showed that Ang II was specifically located in islet beta-cells, and it was found to block glucose-induced insulin release either directly by inhibiting β-cell insulin exocytosis or indirectly through suppressing islet blood perfusion. This effect was fully reversed by losartan indicating a potential role of locally produced pancreatic Ang II in glucose homeostasis. Based on these earlier studies, RAS inhibitors may have a promising role in increasing local pancreatic and islet blood flow which may increase glucose and glucose analog (STZ) uptake through the GLUT2 mechanism. According to these prior studies, the authors of the current study found that the biochemical and both histological and immunohistochemical findings support this theory.
In the present study, the histological examination of pancreatic sections revealed degeneration of islets of Langerhans with β-cell necrosis after STZ administration which is augmented with captopril intake. These results agree with Raza and John [33], stating that administration of STZ at low doses results in β-cell dysfunction and apoptosis while at higher doses leads to β-cell necrosis. Various data explained that STZ induces diabetes in experimental animals through different pathways, The first mechanism involves DNA fragmentation due to the STZ nitrosourea moiety, the second is releasing nitric oxide (NO), the third is creating reactive oxygen species (ROS) and inducing oxidative stress, the fourth mechanism is inhibiting the activity of the glycoside hydrolase O-GlcNAcase enzyme in the β-cell, which is responsible for removing O-GlcNA from proteins. This produces irreversible O-glycosylation of intracellular proteins causing β-cell apoptosis [34].
In addition, STZ contains deoxyglucose molecules that are very reactive and can produce direct cytotoxic damage on pancreatic β-cells. It targets the GLUT2 receptor, which is copious on β-cells plasma membranes making them its primary target [35]. Hence the STZ mechanism begins with GLUT2, a transmembrane carrier protein found in β-cells of the pancreas, the absorptive epithelial cells of the intestinal mucosa, liver, and renal cells [36]. The current results showed that the administration of captopril before STZ exposure resulted in more damage of β-cells, this may be due to enhanced expression of GLUT2 in pancreatic tissues of all groups regardless of the dosage compared to the groups that only received STZ.
Furthermore, this could be attributed to the fact that pancreatic β-cells selectively accumulate STZ via GLUT2 and STZ damage to the β-cells is dependent on GLUT2 expression [37].
The contribution of NO and oxidative stress is a crucial aspect of STZ toxicity [38]. Since β-cells have lower levels of enzymes that scavenge NO and free radicals, they are particularly vulnerable to damage caused by NO and free radicals [39]. In our study, we found that NO levels in pancreatic tissues were increased significantly in all groups that administered STZ. This increase was particularly significant in the STZ-Cap groups, and this may be related to the marked uptake of STZ by captopril. Results were supported by the work done by Eleazu et al. [40] who explained the mechanism of increased NO as the nitrosamine group found in STZ serves as an intracellular donor of NO in pancreatic β-cells, and the amount of NO released locally in pancreatic tissues increased with a high level of STZ. When released in excessive amounts NO hinders the action DNA enzyme, aconitase, which ultimately leads to alkylation and damage of DNA. Aconitase is an enzyme that safeguards mitochondrial DNA from degradation. This enzyme can be inhibited by various ROS as well as reactive nitrogen species [41].
Selective and progressive destruction of insulin-secreting β-cells secondary to the diabetogenic action of STZ can enhance local pancreatic infiltration with inflammatory cells and cytokines, such as TNF-α, IL-1β, and interferon-γ (IFN-γ). Furthermore, IFN-γ and TNF-α, either alone or in combination, produce high levels of NO in pancreatic cells [42, 43].
The current results reported a significant increase in pancreatic TNF-α and IL-1β levels in STZ and STZ-Cap-treated groups which reflect the inflammatory state of the pancreatic tissues. Moreover, the captopril intake before STZ administration was able to augment the production of both TNF-α and IL-1β in STZ-Cap which might be due to increased STZ uptake. Also, histopathological findings of the pancreas showed a significant degeneration of islets of Langerhans and an increase in the optical density of TNF-α immunoreactivity at the doses 30 mg/kg in both STZ and STZ-Cap and the degree of degeneration increased by increase STZ-Cap doses. Also, the mean area % of anti-insulin immunoreactivity had a significant decrease in all groups STZ and STZ-Cap. This decrease ranges from moderate heterogeneous insulin immunostaining in β-cells to faint immunostaining according to STZ doses. Our results are consistent with Akash et al. [44] who denoted that the first pro-inflammatory cytokine that was recognized due to its involvement in insulin resistance pathogenesis and T2DM was TNF-α and the release of other cytokines like IL-6 and IL-1β is enhanced by the pro-inflammatory TNF-α. During acute inflammation, the elevation in both TNF-α and IL-1β impairs β-cell activity and disrupts the insulin signaling pathway.
In conclusion, our study suggests that captopril may be a crucial regulator of glucose analog uptake (STZ) by β-cell. We found that the prior administration of captopril significantly enhanced the effect of low-dose STZ, helped the induction of diabetes, and decreased the mortality rate that occurred at the other higher doses this could make it a potential novel modified experimental model for the induction of diabetes in experimental animals.
Limitation and future recommendations
Future work is highly recommended to elucidate the long-term effect of coadministration of captopril and STZ regarding the state of hyperglycemia. We combined captopril with different doses of STZ, and we found that the intake of STZ at a dose of 40 mg/kg produced the same results of 50 mg/kg. By introducing the appropriate dose and route of administration for STZ and Alloxan chemical compounds, it could be possible to produce an optimal experimental model for type II DM with a low dose of STZ in rats. However, the long-term effect on plasma glucose and insulin levels, and islet morphology was not investigated.
In addition, it is highly recommended to investigate the effect of the combined treatment protocol versus STZ on the liver and kidney.
Availability of data and materials
The data that support the findings of this study are available from the authors upon reasonable request.
Abbreviations
- STZ:
-
Streptozotocin
- Cap:
-
Captopril
- IPGTT:
-
Intraperitoneal glucose tolerance test
- TNF-α:
-
Tumor necrosis factor alpha
- IL-1β:
-
Interleukin one beta
- ELISA:
-
Enzyme-linked immunosorbent assay
- GLUT2:
-
Glucose transporter 2
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- H&E:
-
Hematoxylin and eosin
- DM:
-
Diabetes mellitus
- RAS:
-
Renin–angiotensin system
- AT1R:
-
Angiotensin II receptor types 1
- AT2R:
-
Angiotensin II receptor types 2
- ACE:
-
Angiotensin converting enzyme
- ANOVA:
-
Analysis of variance
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
5. References
Mukherjee P, Roy S, Ghosh D, Nandi SK (2022) Role of animal models in biomedical research: a review. Lab Anim Res 38(1):18. https://doi.org/10.1186/s42826-022-00128-1
Harreiter J, Roden M (2019) Diabetes mellitus-definition, classification, diagnosis, screening and prevention. Wien Klin Wochenschr 131:6–15. https://doi.org/10.1007/s00508-019-1450-4
Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A (2021) Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol 17:150–161. https://doi.org/10.1038/s41574-020-00443-4
Zhao X, An X, Yang C, Sun W, Ji H, Lian F (2023) The crucial role and mechanism of insulin resistance in metabolic disease. Front Endocrinol 14:1149239. https://doi.org/10.3389/fendo.2023.1149239
Rais N, Ved A, Ahmad R, Parveen K, Gautam GK, Bari DG, Shukla KS, Gaur R, Singh AP (2022) Model of streptozotocin-nicotinamide induced type 2 diabetes: a comparative review. Curr Diabetes Rev 18(8):e171121198001. https://doi.org/10.2174/1573399818666211117123358
Kottaisam CPD, Raj DS, Prasanth Kumar V, Sankaran U (2021) Experimental animal models for diabetes and its related complications-a review. Lab Anim Res 37(1):23. https://doi.org/10.1186/s42826-021-00101-4
Furman BL (2021) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc 1(4):e78. https://doi.org/10.1002/cpz1.78
Zhang M, Lv XY, Li J, Xu ZG, Chen L (2008) The characterization of high-fat diet and multiple low-dose streptozotocin-induced type 2 diabetes rat model. Exp Diabet Res 2008:704045. https://doi.org/10.1155/2008/704045
Asrafuzzaman M, Cao Y, Afroz R, Kamato D, Gray S, Little PJ (2017) Animal models for assessing the impact of natural products on the aetiology and metabolic pathophysiology of type 2 diabetes. Biomed Pharma 89:1242–1251. https://doi.org/10.1016/j.biopha.2017.03.010
Di Leo MA, Santini SA, Silveri NG, Giardina B, Franconi F, Ghirlanda G (2004) Long term taurine supplementation reduces mortality rate in streptozotocin induced diabetic rats. Amino Acids 2:187–191. https://doi.org/10.1007/s00726-004-0108-2
Dalco LJ, Dave KR (2023) Diabetic rodent models for chronic stroke studies. Methods Mol Biol 2616:429–439. https://doi.org/10.1007/978-1-0716-2926-0_30
Goyal SN, Reddy NM, Patil KR, Nakhate KT, Ojha S, Patil CR, Agrawal YO (2016) Challenges and issues with streptozotocin-induced diabetes —a clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem Biol Interact 25(244):49–63. https://doi.org/10.1016/j.cbi.2015.11.032
Wang L, Leung PS (2013) The role of renin-angiotensin system in cellular differentiation: implications in pancreatic islet cell development and islet transplantation. Mol Cell Endocrinol 381:261–271. https://doi.org/10.1016/j.mce.2013.08.008
Sadik NA, Metwally NS, Shaker OG, Soliman MS, Mohamed AA, Abdelmoaty MM (2017) Local renin-angiotensin system regulates the differentiation of mesenchymal stem cells into insulin-producing cells through angiotensin type 2 receptor. Biochimie 137:132–138. https://doi.org/10.1016/j.biochi.2017.03.002
Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME (2004) Improved islet morphology after blockade of the renin-angiotensin system in the ZDF rat. Diabetes 53(4):989–997. https://doi.org/10.2337/diabetes.53.4.989
Ihoriya C, Satoh M, Kuwabara A, Sasaki T, Kashihara N (2014) Angiotensin II regulates islet microcirculation and insulin secretion in mice. Microcirculation 21(2):112–123. https://doi.org/10.1111/micc.12094
Batista JPT, Faria AOV, Ribeiro TFS, Simões E, Silva AC (2023) The role of renin-angiotensin system in diabetic cardiomyopathy: a narrative review. Life 13(7):1598. https://doi.org/10.3390/life13071598
Elshimy G, Techathaveewat P, Alsayed M, Jyothinagaram S, Correa R (2019) ACE Inhibitor-induced severe recurrent hypoglycemia in a nondiabetic patient. Cureus 11(8):e5449. https://doi.org/10.7759/cureus.5449
Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM (2000) A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism 49(11):1390–1394. https://doi.org/10.1053/meta.2000.17721
Zhu B, Sun Y, Sievers RE, Browne AE, Pulukurthy S, Sudhir K, Lee RJ, Chou TM, Chatterjee K, Parmley WW (2000) Comparative effects of pretreatment with captopril and losartan on cardiovascular protection in a rat model of ischemia-reperfusion. J Am Coll Cardiol 35:787–795. https://doi.org/10.1016/s0735-1097(99)00592-6
Akbar Nekooeian A, Rasti Pour A, Dehghani F, Mashghoolozekr E, Esmaeilpour T (2021) Effects of captopril and losartan on cardiac stereology in rats with renovascular hypertension. Iran J Med Sci 46(3):169–179. https://doi.org/10.30476/ijms.2020.81948.0
El-Sayed K, Ali DA, Maher SA, Ghareeb D, Selim S, Albogami S, Fayad E, Kolieb E (2022) Prophylactic and ameliorative effects of PPAR-γ agonist pioglitazone in improving oxidative stress, germ cell apoptosis and inflammation in gentamycin-induced testicular damage in adult male albino rats. Antioxidants 11(2):191. https://doi.org/10.3390/antiox11020191
Watada S, Yu YM, Fischman AJ, Kurihara T, Shen CA, Tompkins RG, Fagan S (2014) Evaluation of intragastric vs intraperitoneal glucose tolerance tests in the evaluation of insulin resistance in a rodent model of burn injury and glucagon-like polypeptide-1 treatment. J Burn Care Res 35(1):e66-72. https://doi.org/10.1097/BCR.0b013e31828a8ede
Jørgensen MS, Tornqvist KS, Hvid H (2017) Calculation of glucose dose for intraperitoneal glucose tolerance tests in lean and obese mice. J Am Assoc Lab Anim Sci 56(1):95–97
Morrow TJ (2004) Animal models of painful diabetic neuropathy: the STZ rat model. Curr Protoc Neurosci 29(1):9–18
Chappell MC, Jacobsen DW, Tallant EA (1995) Characterization of angiotensin II receptor subtypes in pancreatic acinar AR42J cells. Peptides 16(4):741–747. https://doi.org/10.1016/0196-9781(95)00044-k
Leung PS, Chan WP, Wong TP, Sernia C (1999) Expression and localization of the renin-angiotensin system in the rat pancreas. J Endocrinol 160(1):13–19. https://doi.org/10.1677/joe.0.1600013
Zheng W, Liu Z, Zhou C, Liao W, Li J, Ren C (2022) Small molecule angiotensin converting enzyme inhibitors: a medicinal chemistry perspective. Front Pharmacol 1(13):968104. https://doi.org/10.3389/fphar.2022.968104
Carlsson PO, Berne C, Jansson L (1998) Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia 41(2):127–133. https://doi.org/10.1007/s001250050880
Olsson R, Jansson L, Andersson A, Carlsson PO (2000) Local blood flow regulation in transplanted rat pancreatic islets: influence of adenosine, angiotensin II, and nitric oxide inhibition. Transplantation 70:280–287. https://doi.org/10.1097/00007890-200007270-00007
Campbell DJ, Habener JF (1986) Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J Clin Invest 78(1):31–39. https://doi.org/10.1172/JCI112566
Lau T, Carlsson PO, Leung PS (2004) Evidence for a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia 47:240–248. https://doi.org/10.1007/s00125-003-1295-1
Raza H, John A (2012) Streptozotocin-induced cytotoxicity, oxidative stress and mitochondrial dysfunction in human hepatoma HepG2 cells. Int J Mol Sci 13(5):5751–5767. https://doi.org/10.3390/ijms13055751
Wu J, Yan LJ (2015) Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes 2(8):181–188. https://doi.org/10.2147/DMSO.S82272
Bao S, Zheng H, Chen C, Zhang Y, Bao L, Yang B, Hou Y, Chen Y, Zhang Q, Pi J, Fu J (2021) Nfe2l1 deficiency mitigates streptozotocin-induced pancreatic β-cell destruction and development of diabetes in male mice. Food Chem Toxicol 158:112633. https://doi.org/10.1016/j.fct.2021.112633
Sharari S, Abou-Alloul M, Hussain K, Ahmad KF (2020) Fanconi–bickel syndrome: a review of the mechanisms that lead to dysglycaemia. Int J Mol Sci 21(17):6286. https://doi.org/10.3390/ijms21176286
Kim J, Kim JH, Sohn KY, Yoon SY, Kim JW (2019) Palmitoyl-2-Linoleoyl-3-Acetyl- rac-glycerol attenuates streptozotocin-induced pancreatic beta cell damage by promoting glucose transporter 2 endocytosis. Mol Cell Biol 39(21):e00157-e219. https://doi.org/10.1128/MCB.00157-19
Stanojevic JB, Zeljkovic M, Dragic M, Stojanovic IR, Ilic TV, Stevanovic ID, Ninkovic MB (2023) Intermittent theta burst stimulation attenuates oxidative stress and reactive astrogliosis in the streptozotocin-induced model of Alzheimer’s disease-like pathology. Front Aging Neurosci 15:1161678. https://doi.org/10.3389/fnagi.2023.1161678
Eguchi N, Vaziri ND, Dafoe DC, Ichii H (2021) The role of oxidative stress in pancreatic β cell dysfunction in diabetes. Int J Mol Sci 22(4):1509
Eleazu CO, Eleazu KC, Chukwuma S, Essien UN (2013) Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord 12(1):60. https://doi.org/10.1186/2251-6581-12-60
Chen Y, Cai GH, Xia B, Wang X, Zhang CC, Xie BC, Shi XC, Liu H, Lu JF, Zhang RX, Zhu MQ, Liu M, Yang SZ, Yang Zhang D, Chu XY, Khan R, Wang YL, Wu JW (2020) Mitochondrial aconitase controls adipogenesis through mediation of cellular ATP production. FASEB J 34(5):6688–6702. https://doi.org/10.1096/fj.201903224RR
Eizirik DL, Colli ML, Ortis F (2009) The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol 5(4):219–226. https://doi.org/10.1038/nrendo.2009.21
Toren E, Burnette KS, Banerjee RR, Hunter CS, Tse HM (2021) Partners in crime: beta-cells and autoimmune responses complicit in type 1 diabetes pathogenesis. Front Immunol 7(12):756548. https://doi.org/10.3389/fimmu.2021.756548
Akash MSH, Rehman K, Liaqa A (2018) Tumor necrosis factor-alpha: Role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem 119(1):105–110. https://doi.org/10.1002/jcb.26174
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The authors declare that all data were generated in-house and that no paper mill was used. All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ateyya Hayam; Harb Inas; ShamsEldeen Asmaa; Adel Sara; Samir Samaa; Rashed Laila; Mostafa Abeer. HI., AH., and S A. conceived and designed research. AH, HI, and HS conducted experiments. RL, SA, and KS contributed new reagents or analytical tools. AH, HI, AM, and AH analyzed data. AH and SA wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was done in accordance with the highest International Criteria of Animal Experimentation of Helsinki and was approved by the animal house of the faculty of medicine, Cairo University, approval No; CU/III/F/38/22. The authors confirm that neither the manuscript nor any parts of its content are currently under consideration or published in another journal.
Consent for publication
Not applicable.
Competing interests
We would like to declare that there were no conflicts of interest in conducting this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ateyya, H., ShamsEldeen, A.M., Hosny, S.A. et al. Captopril pretreatment augments diabetogenic response to streptozotocin administration: experimental in vivo rat model. Futur J Pharm Sci 10, 47 (2024). https://doi.org/10.1186/s43094-024-00620-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-024-00620-6