Abstract
Background
Homeostatic regulation of cardiomyocytes is indispensable in maintaining the normal physiological activity of cardiac tissue. Cardiotoxicity induced by drugs may lead to cardiac abnormalities such as arrhythmia, myocardial infarction and myocardial hypertrophy. Moreover, drug-induced cardiotoxicity confines the additional use of the implicated drugs. Several studies have reported that consumption of phytochemicals on regular intervals shall protect humans against numerous diseases such as diabetes, cardiovascular disease, inflammatory diseases and cancer.
Main body
Ferulic acid (FA) is a plant derived polyphenol abundantly found in vegetables, fruits and grains. FA is widely known for its antioxidant, anti-inflammatory, anticancer, nephroprotective and hepatoprotective effects. FA has been well documented for its cardioprotective activity against various drugs and toxic agents as well. However, the cardioprotective action of FA have remained a challenge with regard to understanding its mechanism in health and diseases.
Conclusion
The main purpose of this review is to explore the cardioprotective mechanisms of FA against several drugs and chemicals to recommend further studies to investigate the potential protective effect of FA.
Similar content being viewed by others
1 Background
Cardiotoxicity usually denotes toxicity that has a harmful effect on the heart, which may ultimately result in myocardiopathy such as arrhythmia, myocardial infarction and myocardial hypertrophy [1, 2]. In the last few years, more than 20% of clinical drugs were forced out of the market because of cardiovascular side effects, which hampered the drug development and extremely affected the improvement in patient health [3]. Several studies have showed that drug-induced cardiac dysfunction may be a stepwise process accompanied by the increase of cardiac biomarkers and structural myocardial deformation, finally resulting in reduced left ventricular ejection fraction (LVEF) [4, 5]. Recently, the most accepted term for cardiotoxicity is the reduction in LVEF of at least 10% to less than 55% [6]. Studies show that cardiac cell death or damage concurrently occurs with the progression of cardiotoxicity, demonstrating that drug-induced cardiomyocyte death may be the major reason for cardiotoxicity [7].
In the recent years, extensive research has been undertaken to investigate the action of phytochemical compounds found in the diet [8]. They are commonly found in fruits, vegetables, beverages and herbal remedies [9]. These alkaloid compounds contain numerous active substances like flavonoids, polyphenols, indoles and sulfur containing elements [10]. These phytochemicals have the tendency to safeguard humans against diabetes mellitus, hepatic disorders, cardiovascular disease, cancer, arthritis, and Alzheimer’s disease and many more [11, 12]. Findings from numerous studies have demonstrated that phenolic compounds are generally non-toxic when taken in smaller quantities and they possess extraordinary therapeutic values. [13, 14].
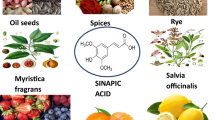
Ferulic acid (FA) is a polyphenol that is found in many staple foods such as grain bran, whole grain foods, citrus fruits, banana, coffee, orange juice, eggplant, bamboo shoots, beetroot, cabbage, spinach and broccoli [15]. FA exhibits an extensive range of therapeutic functions against diabetes [16], cancer [17], renal toxicity [18], liver toxicity [19], cardiotoxicity [20] and neurotoxicity [21].
In this review, the cardioprotective effects of FA against various drugs and toxins have been described in a comprehensive manner.
2 Main text
2.1 Sources
Ferula foetida is the primary source of 3-methoxy-4-hydroxycinnamic acid that was isolated first [22]. The chief sources of FA include rice, wheat and oats [22, 23]. Other sources of ferulic acid are fruits and vegetables [22, 23]. In some vegetables and fruits such as in coffee, cabbage, celery and carrots, FA is found to be in its conjugated form with hydroxyl acids. In grains, FA may form an ester with sterols. Forty to ninety percent of free FA is present in some vegetables such as burdock, water dropwort and eggplant [24, 25]. Additionally, 0.1–0.5% free FA is present in cereals [25].
2.2 Chemistry
Ferulic acid (FA) is the common name for 3-(4-hydroxy-3-methoxyphenyl)-2- propenoic acid. Other names include 3-methoxy-4-hydroxycinnamic acid, caffeic acid 3-methyl ether, and coniferic acid [26] (Fig. 1). Ferulic acid isolated from plants usually exists as the trans isomer. Due to its phenolic nucleus and an extended side chain, FA readily forms a resonance stabilized phenoxy radical which accounts for its free radical-scavenging effect [27].
2.3 Bioavailability and metabolism
Several studies on absorption of FA have reported that FA can be absorbed from the stomach [28], jejunum [29] and ileum [30]. FA may be transported across the intestinal brush border membrane via carrier mediated sodium-dependent transport mechanism [31]. The amount of FA intake via consumption of cereals, vegetables, fruits, coffee and juices may reach up to 150–250 mg/day [30]. FA metabolites commonly found in circulation are FA-glucuronide, FA-sulfate, FA-diglucuronide, FA-sulfoglucuronide (FA-diconjugate with sulfate and glucuronide), m-hydroxyphenylpropionic acid, feruloylglycine, dihydroferulic acid, vanillic acid and vanilloylglycine [31]. Conjugated FA such as FA-glucuronide, FA-sulfate and FA-sulfoglucuronide are the predominant metabolites in the plasma and urine of rats [32]. It is reported that the bioavailability of FA is much greater compared to other dietary flavonoids and monophenolics. FA remains in blood for a longer time than other antioxidants like ascorbic acid [33].
2.4 Cardioprotective activity of FA
2.4.1 Effect of FA on Isoproterenol induced cardiotoxicity
Isoprenaline or isoproterenol (ISO) is a drug commonly employed for the treatment of bradycardia, heart block and sometimes for asthma [34]. It is categorized under non-selective β adrenoceptor agonist, which is the isopropylamine analog of adrenaline [35]. ISO acts as a synthetic β-adrenergic receptor agonist. One of the most common side effects of ISO is cardiotoxicity [36].
Several studies have shown the effect of FA on ISO induced cardiotoxicity [37,38,39,40,41]. Different doses of ISO (85, 150 mg/kg body weight) were used in these studies for inducing cardiotoxicity. FA (10, 20 and 40 mg/kg body weight) was given orally to see its protective effect. ISO induced rats showed abnormal alterations in oxidative stress parameters such as MDA, TBARS along with reduction in cellular antioxidants such as SOD, CAT, GPx, GST and GSH; activities of liver enzymes such as AST, ALT, CPK and LDH, pro-inflammatory cytokines TNF-α, IL-β and IL-6; serum-free cholesterol, esterified cholesterol, VLDL cholesterol, LDL cholesterol, HDL cholesterol and total cholesterol. Further, mitochondrial damage in ISO induced cardiotoxicity induced rats revealed alterations in the activities of TCA cycle and respiratory chain enzymes such as ICDH, SDH, MDH, αKGDH, NADH dehydrogenase and cytochrome C oxidase. In addition, the activities of serum lysosomal hydrolases such as β-D-glucuronidase, β-D-galactosidase, β-D-N acetyl glucosaminidase, cathepsin D and acid phosphatase were significantly increased in ISO induced animals. Further, the levels of Na+, Ca2+ were increased and K+ was decreased in the heart of ISO induced rats. All these abnormalities were markedly improved upon FA treatment revealing its strong hepatoprotective activity. The antioxidant, anti-hyperlipidemic, anti-inflammatory and mitochondrial stabilizing potential of FA were found to be key factors for its cardioprotective function.
In another study, Zhang et al. [42] have reported the effect of FA on ISO induced cardiotoxicity in Sprague Dawley rats. ISO (150 mg/kg body weight) was administered by intraperitoneal route for two days to induce cardiotoxicity. FA (5, 25 and 50 mg/kg body weight) treated rats effectively modulated heart rate, ejection fraction % (EF), fractional shortening (FS), and decreased left ventricular posterior wall thickness at end-systole (LVPWS), left ventricular internal diameter (LVID) along with decreased plasma NT-pro BNP levels. TUNEL assay and immunoblotting analysis were performed to highlight the effect of FA on apoptosis. Additionally, FA treatment effectively activated Nrf2 signaling pathway by significantly increasing the protein expressions of p-Nrf2, HO-1, NQ01 and decreasing the expression of Keap 1 compared to the ISO induced group. Reducing oxidative stress, apoptosis and activating Nrf2 signaling pathway was important for the cardioprotective efficacy of FA against ISO induced cardiotoxicity.
2.4.2 Effect of FA on Doxorubicin induced cardiotoxicity
Doxorubicin (DOX) is an anthracycline antibiotic used to treat various types of cancer, including solid tumors, hematologic malignancies and soft tissue sarcoma [43]. It acts by intercalating DNA and by arresting DNA replication by inhibiting topoisomerase II. The most deleterious effect of DOX is dilated cardiomyopathy leading to congestive heart failure [44].
A single intraperitoneal dose of DOX (20 mg/kg body weight) induced cardiotoxicity in Wistar rats [45]. FA was given at different doses of 20, 40 mg/kg body weight for 7 days orally to evaluate its effects. DOX’s cardiotoxicity was revealed by abnormal increase in serum CK-MB, LDH, IL-1β, and IL-6; enhanced activities of Mg2+ATPase, Ca2+ATPase and altered gene expressions of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP). Histological analysis showed increased fibrosis and collagen deposition in the cardiotoxicity induced rats. FA treatment was able to revert all the above alterations seen during DOX induced cardiotoxicity. Modulation of oxidative stress, inflammation, ER stress, calcium homeostasis and renin played a major role in the cardioprotective efficacy of FA against DOX induced cardiotoxicity.
2.4.3 Effect of FA on Cyclophosphamide induced cardiotoxicity
Cyclophosphamide (CP) is a widely used chemotherapeutic agent belonging to the class of alkylating agents [46]. It is used to treat variety of cancers like lymphoma, leukemia and multiple myeloma. It is also used as an immune suppressive agent in graft-vs-host disease [47]. Administration of large dose of CP has been reported to cause hemorrhagic cell death, leading to heart failure [48].
Song et al. reported the influence of FA (200 mg/kg body weight) on CP induced cardiotoxicity in ICR mice [49]. Serum biomarkers such as ALT, AST, CK and LDH were increased, hematological parameters such as total WBC count, RBC count, hemoglobin and platelets were reduced; the levels of pro-inflammatory cytokines such as TNF-α, IL-β and IL-6 were markedly increased in animals with cardiac damage caused by CP. Further, the protein expression of p-NF-kB, p-IkBα, p-IKkα and IKkβ were significantly increased in CP challenged groups. FA (50 & 100 mg/kg body weight) intragastric treatment effectively regulated all the above alterations. This study showed that FA effectively ameliorated CP induced cardiotoxicity in mice by inhibiting IKk/IkB/NF-kB pathway and by reducing inflammation.
2.4.4 Effect of FA on Arsenic induced cardiotoxicity
Arsenic (Ars) is a naturally found metalloid that is universally present in both organic and inorganic forms. People are exposed to high levels of inorganic arsenic through contaminated drinking water, and food crops irrigated with excessive arsenic water sources. Ars exposure increases the risk of ischemia, arrhythmia and heart failure [50].
This study showed the protective function of FA on Ars induced cardiotoxicity in Wistar rats [51]. Sodium arsenite (5 mg/kg body weight) was dissolved in distilled water and administered orally for 30 days to induce cardiac damage. FA (10, 20 & 40 mg/kg body weight) was also supplemented orally as a treatment drug for 30 days to see its effect. The key parameters modulated by FA include serum cardiac markers such as CK-MB, LDH and ALT; protein carbonyls, LPO in myocardium and cellular antioxidants (SOD, CAT, GPx, GSH, ascorbic acid). Moreover, FA treatment modulated the protein expression of cytoskeleton intermediate filament proteins such as desmin, vimentin and AMPK signaling proteins such as pAMPKα, pAMPKβ (1/2), pACC. The modulatory role of FA against Ars induced cardiotoxicity could be because of its ability to improve antioxidants, ATP levels and modulation of AMPK signaling pathway.
Another study [52] reported the protective effects of FA in Ars induced cardiotoxicity in a Zebrafish model. Cardiotoxicity was induced by exposing to 1 mM Ars. FA supplementation (30 μM) markedly alleviated the changes in heartbeat, malformations such as pericardial edema, yolk sac edema, dorsal curvature, flat-head, and eye defects. Moreover, mRNA expression levels of some of the major genes of cardio genesis such as nkx2.5, bmp2b, myh6, gata4, gata5, myl7, and tnnt2 were appreciably regulated by FA. The cardioprotective function of FA reported in this study could be by modulating oxidative stress and regulating cardio genesis against Ars induced cardiotoxicity in Zebrafish model.
2.4.5 Effect of FA on streptozotocin induced diabetic cardiopathy
Streptozotocin (STZ) belongs to the class of glucosamine-nitrosourea compound. STZ is an alkylating antineoplastic agent that is mainly toxic to the pancreas and is widely used in experimental research for inducing type 1 or type 2 diabetes [53].
In this study, Wistar rats were induced with single intraperitoneal injection of STZ (50 mg/kg body weight) to induce hyperglycemia [54]. FA (50 mg/kg body weight) was supplemented orally for 8 weeks to assess its beneficial effect. Hyperglycemia induced rats showed abnormal variations in blood glucose, serum insulin levels, LPO and cellular antioxidants SOD, CAT, GR and GSH/GSSG ratio along with abnormalities in plasma total cholesterol, triglycerides and HDL cholesterol. In addition, the protein expression of insulin signaling proteins such as PI3K, Akt, GSK-3β, GLUT-4 and ER-stress dependent apoptotic cell death proteins such as calpain-1, cleaved caspase 12, GRP78, CHOP, cleaved caspase 3, cleaved PART and p-eIF2α/total eIF2α ratio were altered in STZ induced rats. FA supplementation effectively modulated all these changes. The findings of this study showed that FA alleviated STZ induced diabetic cardiopathy by modulating oxidative stress, hyperlipidemia and regulating PI3K/Akt dependent signaling cascade.
2.4.6 Effect of FA on N (ώ)-nitrol-L-arginine methyl ester induced cardiotoxicity
It is well recognized that nitric oxide (NO) produced in vascular endothelial cells has a potent vasodilator effect and plays an important role in vascular resistance and growth [55]. Administration of L-arginine analogues like N (ώ)-nitrol-L-arginine methyl ester hydrochloride (L-NAME) suppresses NO biosynthesis, resulting in hypertension and cardiac injury [56].
The effect of FA on L-NAME induced cardiotoxicity in male Wistar rats has been reported [57]. L-NAME (50 mg/kg body weight) was administered orally for eight weeks. FA (0.8 g/kg of powdered food) mixed with feed was supplemented parenterally. Major factors modulated by FA in this study include blood pressure, left ventricular weight, MDA, nitric oxide, MnSOD. Further, FA treatment reduced inflammatory cell infiltration, ferric iron accumulation, and collagen deposition in left ventricles and kidneys. The cardioprotective role of FA reported in this study is through its potent antioxidant and anti-inflammatory activity.
2.4.7 Effect of FA on TNF‑α/cycloheximide‑induced cardiac apoptosis
Cycloheximide (CHX) is a fungicide produced by Streptomyces griseus that is commonly used in research to hinder protein synthesis in eukaryotic cells [58]. TNF-α produces a sequence of biological effects that include immuno stimulation, mediation of host resistance to bacteria, activation of protein kinase C, and activation of the expression of a wide variety of genes generally involved in inflammation or cell growth [59, 60].
The protective efficacy of FA against TNF‑α (TNFA)/cycloheximide (CHX) ‑induced apoptosis in H9c2 cardiomyocytes as well as in acute myocardial infarction (AMI) induced C57BL/6 mice model has been reported [61]. In H9c2 cells, the concentration of TNFA was 10 ng/mL and CHX was 5 μg/mL were used. Anti-apoptotic effect of FA (50 μM) was evident through its ability to decrease the number of apoptotic cells and to reduce the levels of cleaved caspase-3 compared to the TNFA/CHX treated cells. Further, FA effectively modulated the levels/protein expressions of chief autophagy signaling proteins such as Akt/mTLC3B, p62 LC3B-II and LC3B1. In the in vivo study, FA was dissolved in saline and given by oral gavage route for 4 weeks at different doses (125 mg/kg/d, 62.5 mg/kg/d and 31.25 mg/kg/d) for 28 days to assess its protective role. The findings of the study clearly demonstrated that FA reduced oxidative stress induced autophagy and suppressed apoptosis by regulating Akt/mTOR signaling pathway.
2.4.8 Effect of FA on hydrogen peroxide and isoprenaline induced toxicity
The cardioprotective role of FA by modulating hydrogen peroxide and isoprenaline induced oxidative stress was reported [62]. In the cell line study, H9c2 cardiomyocytes were subjected to treatment by H2O2 (200 μM) to induce oxidative stress. FA (50 μM) treated cells showed reduced oxidative stress and decreased apoptosis. Moreover, FA treatment modified apoptosis and miR-499-5p/p21 pathway related proteins such as Bax, p-p38, cleaved caspase 3, Bcl-2 and p21. The in vivo study was carried out in the C5/BL/6 J mice heart injury model. Oxidative stress was induced using isoprenaline (IPL) (30 mg/kg body weight) per day in saline for 14 days. FA (30 mg/kg body weight) treatment efficiently attenuated all the abnormalities caused by IPL induced cardiotoxicity. In addition, FA treatment regulated the mRNA expression of miR-499-5p and p21. The results of the study showed that FA treatment effectively controlled oxidative stress induced by H2O2 and IPL by regulating the miR-499-5p/p21 signaling pathway both in vitro and in vivo.
2.5 Clinical studies on FA
The studies of FA on human subjects are limited. A study by Bumrungpert et al. [63] showed that FA treatment (1000 mg/day) for six weeks improved lipid profiles, oxidative stress, oxidized LDL-C and inflammation in patients with hyperlipidemia. In another study, laser-assisted delivery of FA along with vitamin C and E was found to be helpful in wound healing [64]. A study from Wu et al. proved that topical antioxidant complex containing vitamins C, E and FA can protect solar-simulated ultraviolet irradiation induced acute photodamage in human skin [65].
2.6 Toxicity of FA
FA was found to be well tolerated in most of the animal studies. Investigations have shown that oral dose of FA up to 150 mg/kg body weight was tolerable in rats and did not induce any toxic effects [38, 42]. A study by Xu et al. reported that intravenous administration of FA (866 ± 28 mg/kg) to mice resulted in spasticity, tremor, ankylosis of hind limbs and death within 6 h [66] Additionally, a study from Peng et al. displayed that long-term administration of FA may cause renal damage; however, the mechanism is unclear [67]. In vitro studies showed that FA (300 μg/mL) treatment had no noticeable toxicity to red blood cells, platelets and white blood cells [68]. Truzzi et al. reported that FA (40 mg/L) elicited toxic effects in human monocytes (U937) and human colon cancer cells [69]. In human studies, a dosage of 1000 mg/day has been found to be safe and tolerable [63].
3 Conclusions
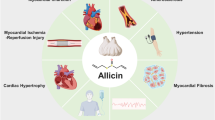
This review has briefed the cardioprotective effects of FA against cardiotoxicity induced by various drugs and chemical agents using preclinical studies and cell line models (Table 1) (Fig. 2). It is understood that FA was basically involved in regulating ROS mediated oxidative stress, cellular antioxidants, apoptosis, inflammation, autophagy and energy metabolism in most of the studies. Some of the major signaling pathways regulated by FA were Nrf2 signaling pathway, Akt/mTOR signaling pathway, PI3K/Akt dependent signaling, AMPK signaling and miR-499-5p/p21 pathways. It is also to be recalled that most of the studies to assess the cardioprotective effects of FA were demonstrated in animals (rats, mice, zebrafish) and cell line models; cardioprotective role of FA in human subjects is extremely limited. Therefore, the bioavailability, toxicity and mode of administration of FA requires further research and development. Moreover, since most of the findings reported in the current work are based on animal studies, extensive research on human subjects would be highly recommended in order to implement this compound as a potential cardioprotective agent.
Availability of data and materials
Not applicable.
References
Vater LB, Lefebvre B, Turk A, Clasen SC (2022) Fluoropyrimidine cardiotoxicity: Incidence, outcomes, and safety of rechallenge. Curr Oncol Rep. https://doi.org/10.1007/s11912-022-01256-6
Leong Bin Abdullah MFI, Singh D (2021) The adverse cardiovascular effects and cardiotoxicity of Kratom (Mitragyna speciosa Korth.): a comprehensive review. Front Pharmacol 27;12:726003.
Bhagat A, Kleinerman ES (2020) Anthracycline-Induced cardiotoxicity: causes, mechanisms, and prevention. Adv Exp Med Biol 1257:181–192
Pistillucci G, Ciorra AA, Sciacca V, Raponi M, Rossi R, Veltri E (2015) Troponina I e Peptide Natriuretico Cerebrale (BNP) come biomarcatori predittivi di cardiotossicità nelle pazienti affette da carcinoma della mammella in terapia adiuvante con antracicline e trastuzumab [Troponin I and B-type Natriuretic Peptide (BNP) as biomarkers for the prediction of cardiotoxicity in patients with breast cancer treated with adjuvant anthracyclines and trastuzumab]. Clin Ter 166(1):e67–71. Italian.
Patel VG, Cornell RF (2019) Cardiovascular complications associated with multiple myeloma therapies: incidence, pathophysiology, and management. Curr Oncol Rep 21(4):29
Nicol M, Baudet M, Cohen-Solal A (2019) Subclinical left ventricular dysfunction during chemotherapy. Card Fail Rev 5(1):31–36
Ma W, Wei S, Zhang B, Li W (2020) Molecular mechanisms of cardiomyocyte death in drug-induced cardiotoxicity. Front Cell Dev Biol 3(8):434
Ganesan K, Jayachandran M, Xu B (2020) Diet-derived phytochemicals targeting colon cancer stem cells and microbiota in colorectal cancer. Int J Mol Sci 21(11):3976
Zhu F, Du B, Xu B (2018) Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: a review. Crit Rev Food Sci Nutr 58(8):1260–1270
Strzępek-Gomółka M, Gaweł-Bęben K, Kukula-Koch W (2021) Achillea species as sources of active phytochemicals for dermatological and cosmetic applications. Oxid Med Cell Longev 25(2021):6643827
Balakrishnan R, Azam S, Cho DY, Su-Kim I, Choi DK (2021) Natural phytochemicals as novel therapeutic strategies to prevent and treat parkinson’s disease: current knowledge and future perspectives. Oxid Med Cell Longev 25:6680935
Xu DP, Li HB (2015) Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 20(12):21138–21156
Lyu JI, Ryu J, Jin CH, Kim DG, Kim JM, Seo KS et al (2020) Phenolic compounds in extracts of Hibiscus acetosella (Cranberry Hibiscus) and their antioxidant and antibacterial properties. Molecules 25(18):4190
Dudonné S, Dal-Pan A, Dubé P, Varin TV, Calon F, Desjardins Y (2016) Potentiation of the bioavailability of blueberry phenolic compounds by co-ingested grape phenolic compounds in mice, revealed by targeted metabolomic profiling in plasma and feces. Food Funct 7(8):3421–3430
Kumar N, Pruthi V (2014) Potential applications of ferulic acid from natural sources. Biotechnol Rep (Amst) 16(4):86–93
Zhao J, Gao J, Li H (2020) Ferulic acid confers protection on islet β cells and placental tissues of rats with gestational diabetes mellitus. Cell Mol Biol (Noisy-le-grand) 66(1):37–41
Senthil Kumar C, Thangam R, Mary SA, Kannan PR, Arun G, Madhan B (2020) Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr Polym 1(231):115682
Kelainy EG, Ibrahim Laila IM, Ibrahim SR (2019) The effect of ferulic acid against lead-induced oxidative stress and DNA damage in kidney and testes of rats. Environ Sci Pollut Res Int 26(31):31675–31684
Roghani M, Kalantari H, Khodayar MJ, Khorsandi L, Kalantar M, Goudarzi M, Kalantar H (2020) Alleviation of liver dysfunction, oxidative stress and inflammation underlies the protective effect of ferulic acid in methotrexate-induced hepatotoxicity. Drug Des Devel Ther 20(14):1933–1941
Mancuso C, Santangelo R (2014) Ferulic acid: pharmacological and toxicological aspects. Food Chem Toxicol 65:185–195
Turkez H, Arslan ME, Barboza JN, Kahraman CY, de Sousa DP, Mardinoğlu A (2021) Therapeutic potential of ferulic acid in Alzheimer’s disease. Curr Drug Deliv. https://doi.org/10.2174/1567201819666211228153801
Graf E (1992) Antioxidant potential of ferulic acid. Free Radic Biol Med 13:435–448
Zhao Z, Moghadasian MH (2008) Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem 109:691–702
Sakakibara H, Honda Y, Nakagawa S, Ashida H, Kanazawa K (2002) Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J Agric Food Chem 51:571–581
Adom KK, Liu RH (2002) Antioxidant activity of grains. J Agric Food Chem 50:6182–6187
Li D, Rui YX, Guo SD, Luan F, Liu R, Zeng N (2021) Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci 1(284):119921
Graf E (1992) Antioxidant potential of ferulic acid. Free Radic Biol Med 13(4):435–448
Zhao Z, Egashira Y, Sanada H (2004) Ferulic acid is quickly absorbed from rat stomach as the free form and then conjugated mainly in liver. J Nutr 134(11):3083–3088
Wolffram S, Weber T, Grenacher B, Scharrer E (1995) A Na(+)-dependent mechanism is involved in mucosal uptake of cinnamic acid across the jejunal brush border in rats. J Nutr 125(5):1300–1308
Spencer JP, Chowrimootoo G, Choudhury R, Debnam ES, Srai SK, Rice-Evans C (1999) The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett 458(2):224–230
Zhao Z, Moghadasian MH (2008) Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem 109(4):691–702
Rondini L, Peyrat-Maillard MN, Marsset-Baglieri A, Berset C (2002) Sulfated ferulic acid is the main in vivo metabolite found after short-term ingestion of free ferulic acid in rats. J Agric Food Chem 50(10):3037–3041
Srinivasan M, Sudheer AR, Menon VP (2007) Ferulic Acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 40(2):92–100
Higashida H, Egorova A, Higashida C, Zhong ZG, Yokoyama S, Noda M, Zhang JS et al (1999) Sympathetic potentiation of cyclic ADP-ribose formation in rat cardiac myocytes. J Biol Chem 274(47):33348–33354
Ng SP, Nomura W, Mohri S, Takahashi H, Jheng HF, Ara T et al (2019) Soy hydrolysate enhances the isoproterenol-stimulated lipolytic pathway through an increase in β-adrenergic receptor expression in adipocytes. Biosci Biotechnol Biochem 83(9):1782–1789
Steen EM, Noronha-Dutra AA, Woolf N (1982) The response of isolated rat heart cells to cardiotoxic concentrations of isoprenaline. J Pathol 137(2):167–176
Yogeeta SK, Gnanapragasam A, Kumar SS, Subhashini R, Sathivel A, Devaki T (2006) Synergistic interactions of ferulic acid with ascorbic acid: its cardioprotective role during isoproterenol induced myocardial infarction in rats. Mol Cell Biochem 283(1–2):139–146
Yogeeta SK, Hanumantra RB, Gnanapragasam A, Senthilkumar S, Subhashini R, Devaki T (2006) Attenuation of abnormalities in the lipid metabolism during experimental myocardial infarction induced by isoproterenol in rats: beneficial effect of ferulic acid and ascorbic acid. Basic Clin Pharmacol Toxicol 98(5):467–472
Yogeeta SK, Raghavendran HR, Gnanapragasam A, Subhashini R, Devaki T (2006) Ferulic acid with ascorbic acid synergistically extenuates the mitochondrial dysfunction during beta-adrenergic catecholamine induced cardiotoxicity in rats. Chem Biol Interact 163(1–2):160–169
Yogeeta SK, Gnanapragasam A, Senthilkumar S, Subhashini R, Devaki T (2006) Synergistic salubrious effect of ferulic acid and ascorbic acid on membrane-bound phosphatases and lysosomal hydrolases during experimental myocardial infarction in rats. Life Sci 80(3):258–263
Jain PG, Mahajan UB, Shinde SD, Surana SJ (2018) Cardioprotective role of FA against isoproterenol induced cardiac toxicity. Mol Biol Rep 45(5):1357–1365
Zhang XJ, Cui ZH, Zhao YX, He TT, Wang L, Liang XW (2021) Ferulic acid ameliorates isoproterenol-induced heart failure by decreasing oxidative stress and inhibiting cardiocyte apoptosis via activating Nrf2 signaling pathway in rats. Biol Pharm Bull 44(3):396–403
Tacar O, Sriamornsak P, Dass CR (2013) Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 65(2):157–170
Chaterjee K, Jianqing Z, Norman H, Joel SK (2010) Doxorubicin cardiomyopathy. Cardiology 115(2):155–162
Aswar U, Mahajan U, Kandhare A, Aswar M (2019) Ferulic acid ameliorates doxorubicin-induced cardiac toxicity in rats. Naunyn Schmiedebergs Arch Pharmacol 392(6):659–668
Shulman LN (1993) The biology of alkylating-agent cellular injury. Hematol Oncol Clin North Am 7(2):325–335
Ahlmann M, Hempel G (2016) The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol 78(4):661–671
Iqubal A, Iqubal MK, Sharma S, Ansari MA, Najmi AK, Ali SM (2019) Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: old drug with a new vision. Life Sci 1(218):112–131
Song Y, Zhang C, Wang C, Zhao L, Wang Z, Dai Z, Lin S, Kang H, Ma X (2016) Ferulic acid against cyclophosphamide-induced heart toxicity in mice by inhibiting NF-κB pathway. Evid Based Complement Alternat Med 2016:1261270
Vineetha VP, Raghu KG (2019) An overview on arsenic trioxide-induced cardiotoxicity. Cardiovasc Toxicol 19(2):105–119
Panneerselvam L, Raghunath A, Ravi MS, Vetrivel A, Subramaniam V, Sundarraj K, Perumal E (2020) Ferulic acid attenuates arsenic-induced cardiotoxicity in rats. Biotechnol Appl Biochem 67(2):186–195
Perumal E, Eswaran S, Parvin R, Balasubramanian S (2021) Mitigation of arsenic induced developmental cardiotoxicity by ferulic acid in zebrafish. Comp Biochem Physiol C Toxicol Pharmacol 244:109021
Furman BL (2015) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 70:5.47.1–5.47.20.
Chowdhury S, Ghosh S, Rashid K, Sil PC (2016) Deciphering the role of ferulic acid against streptozotocin-induced cellular stress in the cardiac tissue of diabetic rats. Food Chem Toxicol 97:187–198
Bohlen HG (2015) Nitric oxide and the cardiovascular system. Compr Physiol 5(2):808–823
Mahmoody SA, Gharakhanlou R, Roshan VD, Hedayati M (2013) Individual and concomitant effects of cardioprotective programs on cardiac apelinergic system and oxidative state in L-NAME-induced hypertension. Clin Exp Hypertens 35(1):20–27
Alam MA, Sernia C, Brown L (2013) Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J Cardiovasc Pharmacol 61(3):240–249
Gordon RY, Mugantseva EA, Khutzian SS, Podolski IY (2009) Cycloheximide-induced inhibition of protein synthesis in hippocampal pyramidal neurons is time-dependent: differences between CA1 and CA3 areas. Neurosci Lett 461(3):249–251
Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T (2010) Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 49(7):1215–1228
Zelová H, Hošek (2013) TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm Res 62(7):641–651
Li C, Chen L, Song M, Fang Z, Zhang L, Coffie JW et al (2020) Ferulic acid protects cardiomyocytes from TNF-α/cycloheximide-induced apoptosis by regulating autophagy. Arch Pharm Res 43(8):863–874
Sun S, Ruan Y, Yan M, Xu K, Yang Y, Shen T, Jin Z (2021) Ferulic acid alleviates oxidative stress-induced cardiomyocyte injury by the regulation of miR-499-5p/p21 signal cascade. Evid Based Complement Alternat Med 7(2021):1921457
Bumrungpert A, Lilitchan S, Tuntipopipat S, Tirawanchai N, Komindr S (2018) Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: a randomized, double-blind. Placebo-Control Clin Trial Nutr 10(6):713
Waibel JS, Mi QS, Ozog D, Qu L, Zhou L, Rudnick A, Al-Niaimi F, Woodward J, Campos V, Mordon S (2016) Laser-assisted delivery of vitamin C, vitamin E, and ferulic acid formula serum decreases fractional laser postoperative recovery by increased beta fibroblast growth factor expression. Lasers Surg Med 48(3):238–244
Wu Y, Zheng X, Xu XG, Li YH, Wang B, Gao XH, Chen HD, Yatskayer M, Oresajo C (2013) Protective effects of a topical antioxidant complex containing vitamins C and E and ferulic acid against ultraviolet irradiation-induced photodamage in Chinese women. J Drugs Dermatol 12(4):464–468
Xu J, Li YK, Liang ZJ (1992) Effects of tetramethylpyrazine and ferulic acid alone or combined on vascular smooth muscle, blood viscosity and toxicity. Zhongguo Zhong Yao Za Zhi 17(11):680–2, 703–704
Peng CC, Hsieh CL, Wang HE, Chung JY, Chen KC, Peng RY (2012) Ferulic acid is nephrodamaging while gallic acid is renal protective in long term treatment of chronic kidney disease. Clin Nutr 31(3):405–414
Choi JH, Park JK, Kim KM, Lee HJ, Kim S (2018) In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. J Biochem Mol Toxicol 32(1):e22004
Truzzi F, Valerii MC, Tibaldi C, Zhang Y, Abduazizova V, Spisni E, Dinelli G (2020) Are supplements safe? Effects of gallic and ferulic acids on in vitro cell models. Nutrients 12(6):1591
Acknowledgements
None.
Funding
This work is NOT funded by any agencies.
Author information
Authors and Affiliations
Contributions
AP searched the literature, and designed the manuscript. MHR and VMK participated in discussions and suggested useful additions in the manuscript. NC helped in preparing the tables and figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study does not involve any experimental procedures. Only a review. So ethical approval may be exempted.
Consent for publication
All authors give their consent for publication in this journal.
Competing interests
Authors declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pandi, A., Raghu, M.H., Chandrashekar, N. et al. Cardioprotective effects of Ferulic acid against various drugs and toxic agents. Beni-Suef Univ J Basic Appl Sci 11, 92 (2022). https://doi.org/10.1186/s43088-022-00273-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00273-5