Abstract
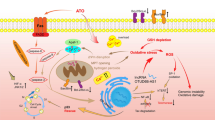
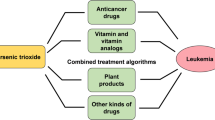
Arsenic trioxide (ATO) is among the first-line chemotherapeutic drugs used in oncological practice. It has shown substantial efficacy in treating patients with relapsed or refractory acute promyelocytic leukaemia. The clinical use of ATO is hampered due to cardiotoxicity and hence many patients are precluded from receiving this highly effective treatment. An alternative to this would be to use any drug that can ameliorate the cardiotoxic effects and allow exploiting the full therapeutic potential of ATO, with considerable impact on cancer therapy. Generation of reactive oxygen species is involved in a wide range of human diseases, including cancer, cardiovascular, pulmonary and neurological disorders. Hence, agents with the ability to protect against these reactive species may be therapeutically useful. The present review focuses on the beneficial as well as harmful effects of arsenic and ATO, the mechanisms underlying ATO toxicity and the possible ways that can be adopted to circumvent ATO-induced toxicity.


Adopted from http://ecgguru.com/ecg/ecg-waveform-illustration

Source: Roden and Viswanathan [91]

Similar content being viewed by others
References
Fox, E., Razzouk, B. I., Widemann, B. C., Xiao, S., O’Brien, M., Goodspeed, W., Reaman, G. H., Blaney, S. M., Murgo, A. J., Balis, F. M., & Adamson, P. C. (2008). Phase 1 trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphoma. Blood, 111, 566–573.
Mathews, V., Chendamarai, E., George, B., Viswabandya, A., & Srivastava, A. (2011). Treatment of acute promyelocytic leukemia with single-agent arsenic trioxide. Mediterranean Journal of Hematology and Infectious Diseases, 3, e2011056.
Drolet, B., Simard, C., & Roden, D. M. (2004). Unusual effects of a QT-prolonging drug, arsenic trioxide, on cardiac potassium currents. Circulation, 109, 26–29.
Mumford, J. L., Wu, K., Xia, Y., Kwok, R., Yang, Z., Foster, J., & Sanders, W. E. (2007). Chronic arsenic exposure and cardiac repolarization abnormalities with QT interval prolongation in a population-based study. Environmental Health Perspectives, 115, 690–694.
Ducas, R. A., Seftel, M. D., Ducas, J., & Seifer, C. (2011). Monomorphic ventricular tachycardia caused by arsenic trioxide therapy for acute promyelocytic leukaemia. The Journal of the Royal College of Physicians of Edinburgh, 41, 117–118.
Costa, V. M., Carvalho, F., Duarte, J., Bastos, M. L., & Remião, F. (2013). The heart as a target for xenobiotic toxicity: The cardiac susceptibility to oxidative stress. Chemical Research in Toxicology, 26, 1285–1311.
Vineetha, V. P., Soumya, R. S., & Raghu, K. G. (2015). Phloretin ameliorates arsenic trioxide induced mitochondrial dysfunction in H9c2 cardiomyoblasts mediated via alterations in membrane permeability and ETC complexes. European Journal of Pharmacology, 754, 162–172.
Gupta, S., Das, B., & Sen, S. (2007). Cardiac hypertrophy: Mechanisms and therapeutic opportunities. Antioxidants & Redox Signaling, 9, 623–652.
Hughes, M. F., Beck, B. D., Chen, Y., Lewis, A. S., & Thomas, D. J. (2011). Arsenic exposure and toxicology: A historical perspective. Toxicological Sciences, 123, 305–332.
Cervantes, C., Ji, G., Ramirez, J. L., & Silver, S. (1994). Resistance to arsenic compounds in microorganisms. FEMS Microbiology Reviews, 15, 355–367.
European Food Safety Authority (EFSA) and EFSA Panel on Contaminants in the Food Chain (CONTAM). (2009). Scientific opinion on arsenic in food. EFSA Journal, 7, 199.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17, 517–568.
Lantz, R. C., & Hays, A. M. (2006). Role of oxidative stress in arsenic-induced toxicity. Drug Metabolism Reviews, 38, 791–804.
Schuhmacher-Wolz, U., Dieter, H. H., Klein, D., & Schneider, K. (2009). Oral exposure to inorganic arsenic: Evaluation of its carcinogenic and non-carcinogenic effects. Critical Reviews in Toxicology, 39, 271–298.
National Research Council (NRC). (1999). Arsenic in drinking water. Washington, DC: National Academy Press.
Chen, C. J., Chuang, Y. C., Lin, T. M., & Wu, H. Y. (1985). Malignant neoplasms among residents of a blackfoot disease endemic area in Taiwan: High arsenic artesian well water and cancers. Cancer Research, 45, 5895–5899.
Argos, M., Kalra, T., Rathouz, P. J., Chen, Y., Pierce, B., Parvez, F., Islam, T., Ahmed, A., Rakibuz-Zaman, M., Hasan, R., Sarwar, G., Slavkovich, V., van Geen, A., Graziano, J., & Ahsan, H. (2010). Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): A prospective cohort study. The Lancet, 376, 252–258.
Nordstrom, D. K. (2002). Public health. Worldwide occurrences of arsenic in ground water. Science, 296, 2143–2145.
International Agency for Cancer Research (IARC). (2004). Some drinking-water disinfectants and contaminants, including arsenic. In IARC monographs on the evaluation of carcinogenic risks to humans, Lyon: International Agency for Cancer Research (IARC), pp 39–267.
Smith, A. H., Lingas, E. O., & Rahman, M. (2000). Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bulletin of the World Health Organization, 78, 1093–1103.
World Health Organisation (WHO) (1993). Guidelines for drinking-water quality. 2nd edn. Geneva: WHO, ISBN 924, p. 154460.
Environmental protection Agency (EPA). (2001). Natural primary drinking water regulations; Arsenic and clarifications to compliance and new source contaminants monitoring; proposed rule. Federal Register, 66(78), 20580.
Murgo, A. J. (2001). Clinical trials of arsenic trioxide in hematologic and solid tumors: Overview of the national cancer institute cooperative research and development studies. The Oncologist, 6, 22–28.
Shibata, T., Solo-Gabriele, H. M., Fleming, L. E., Cai, Y., & Townsend, T. G. (2007). A mass balance approach for evaluating leachable arsenic and chromium from an inservice CCA-treated wood structure. Science of the Total Environment, 372, 624–635.
Smith, A. H., Hopenhayn-Rich, C., Bates, M. N., Goeden, H. M., Hertz-Picciotto, I., Duggan, H. M., Wood, R., Kosnett, M. J., & Smith, M. T. (1992). Cancer risks from arsenic in drinking water. Environmental Health Perspectives, 97, 259–267.
Greider, C. W. (1996). Telomere length regulation. Annual Review of Biochemistry, 65, 337–365.
Chou, W. C., Hawkins, A. L., Barrett, J. F., Griffin, C. A., & Dang, C. V. (2001). Arsenic inhibition of telomerase transcription leads to genetic instability. Journal of Clinical Investigation, 108, 1541–1547.
Bryan, T. M., Englezou, A., Gupta, J., Bacchetti, S., & Reddel, R. R. (1995). Telomere elongation in immortal human cells without detectable telomerase activity. EMBO Journal, 14, 4240–4248.
Gallagher, R. E. (1998). Arsenic: New life for an old potion. New England Journal of Medicine, 339, 1389–1391.
Shim, M. J., Kim, H. J., Yang, S. J., Lee, I. S., Choi, H. I., & Kim, T. (2002). Arsenic trioxide induces apoptosis in chronic myelogenous leukemia K562 cells: Possible involvement of p38 MAP kinase. Journal of Biochemistry and Molecular Biology, 35, 377–383.
Florea, A. M. (2005). Toxicity of alkylated derivatives of arsenic, antimony and tin: Cellular uptake, cytotoxicity, genotoxic effects, perturbation of Ca 2+ homeostasis and cell death. Aachen: Shaker Verlag.
Griffin, R. J., Williams, B. W., Park, H. J., & Song, C. W. (2005). Preferential action of arsenic trioxide in solid-tumour microenvironment enhances radiation therapy. International Journal of Radiation Oncology Biology Physics, 61, 1516–1522.
Florea, A. M., & Büsselberg, D. (2006). Metals and metal compounds: Occurrence, use, benefits and toxic cellular effects. BioMetals, 19, 419–427.
Ratnaike, R. N. (2003). Acute and chronic arsenic toxicity. Postgraduate Medical Journal, 79, 391–396.
Antman, K. H. (2001). Introduction: The history of arsenic trioxide in cancer therapy. The Oncologist, 6, 1–2.
Chen, C. J., Hsueh, Y. M., Lai, M. S., Shyu, M. P., Chen, S. Y., Wu, M. M., Kuo, T. L., & Tai, T. Y. (1995). Increased prevalence of hypertension and long-term arsenic exposure. Hypertension, 25, 53–60.
Tallman, M. S. (2007). Treatment of relapsed or refractory acute promyelocytic leukemia. Best Practice & Research Clinical Haematology, 20, 57–65.
Platanias, L. C. (2009). Biological responses to arsenic compounds. Journal of Biological Chemistry, 284, 18583–18587.
Iland, H. J., & Seymour, J. F. (2013). Role of arsenic trioxide in acute promyelocytic leukemia. Current Treatment Options in Oncology, 14, 170–184.
Mi, J. (2011). Current treatment strategy of acute promyelocytic leukemia. Frontiers in Medicine, 5, 341–347.
Baj, G., Arnulfo, A., Deaglio, S., Mallone, R., Vigone, A., De Cesaris, M. G., Surico, N., Malavasi, F., & Ferrero, E. (2002). Arsenic trioxide and breast cancer: Analysis of the apoptotic, differentiative and immunomodulatory effects. Breast Cancer Research and Treatment, 73, 61–73.
de The, H., Chomienne, C., Lanotte, M., Degos, L., & Dejean, A. (1990). The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature, 347, 558–561.
Borrow, J., Goddard, A. D., Sheer, D., & Solomon, E. (1990). Molecular analysis of acute promyelocytic leukemia break point cluster region on chromosome 17. Science, 249, 1577–1580.
Dyck, J. A., Maul, G. G., Miller, W. H. Jr., Chen, J. D., Kakizuka, A., & Evans, R. M. (1994). A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell, 76, 333–343.
Mu, Z. M., Chin, K. V., Liu, J. H., Lozano, G., & Chang, K. S. (1994). PML, a growth suppressor disrupted in acute promyelocytic leukemia. Molecular and Cellular Biology, 14, 6858–6867.
Chang, K. S., Fan, Y. H., Andreeff, M., Liu, J., & Mu, Z. M. (1995). The PML gene encodes a phosphoprotein associated with the nuclear matrix. Blood, 85, 12.
Shao, W., Fanelli, M., Ferrara, F. F., Riccioni, R., Rosenauer, A., Davison, K., Lamph, W. W., Waxman, S., Pelicci, P. G., Lo Coco, F., Avvisati, G., Testa, U., Peschle, C., Gambacorti-Passerini, C., Nervi, C., & Miller, W. H. J. (1998). Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR alpha protein in acute promyelocytic leukemia cells. Journal of the National Cancer Institute (1988), 90, 124–133.
Zhang, X. W., Yan, X. J., Zhou, Z. R., Yang, F. F., Wu, Z. Y., Sun, H. B., Liang, W. X., Song, A. X., Lallemand-Breitenbach, V., Jeanne, M., Zhang, Q. Y., Yang, H. Y., Huang, Q. H., Zhou, G. B., Tong, J. H., Zhang, Y., Wu, J. H., Hu, H. Y., deThe, H., Chen, S. J., & Chen, Z. (2010). Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science, 328, 240–243.
Mi, J.-Q., Li, J.-M., Shen, Z.-X., Chen, S.-J., & Chen, Z. (2012). How to manage acute promyelocytic leukemia. Leukemia, 26, 1743–1751.
Sun, H. D. M. L., Hu, X. C., & Zhang, T. D. (1992). Thirty two cases of treating acute promyelocytic leukemia by Ailing I therapy combined with syndrome differentiation treatment of traditional Chinese medicine. Chinese Journal Combinat Traditional Chinese Medicine West Medicine, 1996, 170–171.
Zhang, P. (1999). The use of arsenic trioxide in the treatment of acute promyelocytic leukemia. Journal of Biological Regulators and Homeostatic Agents, 13, 195–200.
Soignet, S. L., Frankel, S. R., Douer, D., Tallmann, M. S., Kantarjian, H., Calleja, E., Stone, R. M., Kalaycio, M., Scheinberg, D. A., Steinherz, P., Sievers, E. L., Coutre, S., Dahlberg, S., Ellison, R., & Warrell, R. P. Jr. (2001). United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. Journal of Clinical Oncology, 19, 3852–3860.
Fenaux, P., Chomienne, C., & Degos, L. (2001). Treatment of acute promyelocytic leukaemia. Best Practice & Research: Clinical Haematology, 14, 153–174.
Zhu, J., Chen, Z., Lallemand-Breitenbach, V., & de The, H. (2002). How acute promyelocytic leukaemia revived arsenic. Nature Reviews Cancer, 2, 705–713.
Chen, C. J., Chiou, H. Y., Chiang, M. H., Lin, L. J., & Tai, T. Y. (1996). Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arteriosclerosis, Thrombosis, and Vascular Biology, 16, 504–510.
Abaza, Y., Kantarjian, H., Garcia-Manero, G., Estey, E., Borthakur, G., Jabbour, E., Faderl, S., O’Brien, S., Wierda, W., Pierce, S., Brandt, M., McCue, D., Luthra, R., Patel, K., Kornblau, S., Kadia, T., Daver, N., DiNardo, C., Jain, N., Verstovsek, S., Ferrajoli, A., Andreeff, M., Konopleva, M., Estrov, Z., Foudray, M., McCue, D., Cortes, J., & Ravandi, F. (2017). Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood, 129, 1275–1283.
Hosseini, R., Mandegary, A., Alimoghaddam, K., Ghavamzadeh, A., & Ghahremani, M. H. (2008). Pharmacokinetic of arsenic trioxide in newly diagnosed acute promyelocytic leukemia patients. Journal of Applied Sciences, 8, 4617–4623.
Shen, Y., Shen, Z. X., Yan, H., Chen, J., Zeng, X. Y., Li, J. M., Li, X. S., Wu, W., Xiong, S. M., Zhao, W. L., Tang, W., Wu, F., Liu, Y. F., Niu, C., Wang, Z. Y., Chen, S. J., & Chen, Z. (2001). Studies on the clinical efficacy and pharmacokinetics of low-dose arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia: A comparison with conventional dosage. Leukemia, 15, 735–741.
Wang, Z., Zhou, J., Lu, X., Gong, Z., & Le, X. C. (2004). Arsenic speciation in urine from acute promyelocytic leukemia patients undergoing arsenic trioxide treatment. Chemical Research in Toxicology, 17, 95–103.
Fukai, Y., Hirata, M., Ueno, M., Ichikawa, N., Kobayashi, H., Saitoh, H., Sakurai, T., Kinoshita, K., Kaise, T., & Ohta, S. (2006). Clinical pharmacokinetic study of arsenic trioxide in an acute promyelocytic leukemia (APL) patient: Speciation of arsenic metabolites in serum and urine. Biological & and Pharmaceutical Bulletin, 29, 1022–1027.
Fowler, B. A., Chou, S. J., Jones, R. L., & Chen, C. J. (2007). Arsenic. In G. F. Nordberg, B. A. Fowler, M. Nordberg, L. T. Freiberg (Eds.), Handbook on the toxicology of metals (pp. 367–443). Amsterdam: Elsevier.
Shen, Z. X., Chen, G. Q., Ni, J. H., Li, X. S., Xiong, S. M., Qiu, Q. Y., Zhu, J., Tang, W., Sun, G. L., Yang, K. Q., Chen, Y., Zhou, L., Fang, Z. W., Wang, Y. T., Ma, J., Zhang, P., Zhang, T. D., Chen, S. J., Chen, Z., & Wang, Z. Y. (1997). Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood, 89, 3354–3360.
Ni, J., Chen, G., Shen, Z., Li, X., Liu, H., Huang, Y., Fang, Z., Chen, S., Wang, Z., & Chen, Z. (1998). Pharmacokinetics of intravenous arsenic trioxide in the treatment of acute promyelocytic leukemia. Chinese Medical Journal, 111, 1107–1110.
Hua, H., Qin, S., Rui, J., & Li, J. (2011). Pharmacokinetics of arsenic trioxide (As2O3) in Chinese primary hepatocarcinoma patients. Asian Pacific Journal of Cancer Prevention, 12, 61–65.
Schoen, A., Beck, B., Sharma, R., & Dube, E. (2004). Arsenic toxicity at low doses: Epidemiological and mode of action considerations. Toxicology and Applied Pharmacology, 198, 253–267.
Singh, A. P., Goel, R. K., & Kaur, T. (2011). Mechanisms pertaining to arsenic toxicity. International Journal of Toxicology, 18, 87–93.
Miller, W. H., Schipper, H. M., Lee, J. S., Singer, J., & Waxman, S. (2002). Mechanisms of action of arsenic trioxide. Cancer Research, 62, 3893–3903.
Lau, A. T., Li, M., Xie, R., He, Q. Y., & Chiu, J. F. (2004). Opposed arsenite-induced signalling pathways promote cell proliferation or apoptosis in cultured lung cells. Carcinogenesis, 25, 21–28.
Benbrahim-Tallaa, L., Webber, M. M., & Waalkes, M. P. (2007). Mechanisms of acquired androgen independence during arsenic-induced malignant trans-formation of human prostate epithelial cells. Environmental Health Perspectives, 115, 243–247.
Chen, H., Liu, J., Zhao, C. Q., Diwan, B. A., Merrick, B. A., & Waalkes, M. P. (2001). Association of c-myc overexpression and hyperproliferation with arsenite-induced malignant transformation. Toxicology and Applied Pharmacology, 175, 260–268.
Benbrahim-Tallaa, L., Webber, M. M., & Waalkes, M. P. (2005). Acquisition of androgen independence by human prostate epithelial cells during arsenic-induced malignant transformation. Environmental Health Perspectives, 113, 1134–1139.
Kitchin, K. T. (2001). Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicology and Applied Pharmacology, 172, 249–261.
Zhao, C. Q., Young, M. R., Diwan, B. A., Coogan, T. P., & Waalkes, M. P. (1997). Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proceedings of the National Academy of Sciences of the United States of America, 94, 10907–10912.
Jensen, T. J., Novak, P., Eblin, K. E., Gandolfi, A. J., & Futscher, B. W. (2008). Epigenetic remodeling during arsenical-induced malignant transformation. Carcinogenesis, 29, 1500–1508.
Chow, S. K., Chan, J. Y., & Fung, K. P. (2004). Suppression of cell proliferation and regulation of estrogen receptor alpha signalling pathway by arsenic trioxide on human breast cancer MCF-7 cells. Journal of Endocrinology, 182, 325–337.
Chen, G. C., Guan, L. S., Hu, W. L., & Wang, Z. Y. (2002). Functional repression of estrogen receptor a by arsenic trioxide in human breast cancer cells. Anticancer Research, 22, 633–638.
Roboz, G. J., Dias, S., Lam, G., Lane, W. J., Soignet, S. L., Warrell, R. P., & Rafii, S. (2000). Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood, 96, 1523–1530.
Qian, Y., Castranova, V., & Shi, X. (2003). New perspectives in arsenic-induced cell signal transduction. Journal of Inorganic Biochemistry, 96, 271–278.
Barbey, J. T., & Soignet, S. (2001). Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Annals of Internal Medicine, 135, 842–843.
Westervelt, P., Brown, R. A., Adkins, D. R., Khoury, H., Curtin, P., Hurd, D., Luger, S. M., Ma, M. K., Ley, T. J., & DiPersio, J. F. (2001). Sudden death among patients with acute promyelocytic leukemia treated with arsenic trioxide. Blood, 98, 266–271.
Ning, Y., Shen, Q., Herrick, K., Mikkelsen, R., Anscher, M., Houlihan, R., & Lapane, K. (2012). Abstract LB-339: Cause of death in cancer survivors. In: Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research, 72(8) (Suppl. 1). https://doi.org/10.1158/1538-7445.AM2012-LB-339.
Driver, J. A., Djousse´, L., Logroscino, G., Gaziano, J. M., & Kurth, T. (2008). Incidence of cardiovascular disease and cancer in advanced age: Prospective cohort study. The BMJ 337, a2467.
Albini, A., Pennesi, G., Donatelli, F., Cammarota, R., De Flora, S., & Noonan, D. M. (2010). Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. Journal of the National Cancer Institute (1988), 102, 14–25.
Bovelli, D., Plataniotis, G., Roila, F.,& On behalf of the ESMO Guidelines Working Group (2010). Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO clinical practice guidelines. Annals of Oncology 21, v277–v282.
Morganroth, J., Brozovich, F. V., McDonald, J. T., & Jacobs, R. A. (1991). Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. American Journal of Cardiology, 67, 774–776.
Unnikrishnann, D., Dutcher, J. P., Varshneya, N., Lucariello, R., Api, M., Garl, S., Wiernik, P. H., & Chiaramida, S. (2001). Torsades de pointes in 3 patients with leukemia treated with arsenic trioxide. Blood, 97, 1514–1516.
Ficker, E., Kuryshev, Y. A., Dennis, A. T., Obejero-Paz, C., Wang, L., Hawryluk, P., Wible, B. A., & Brown, A. M. (2004). Mechanisms of arsenic-induced prolongation of cardiac repolarization. Molecular Pharmacology, 66, 33–44.
Clancy, C. E., Kurokawa, J., Tateyama, M., Wehrens, X. H. T., & Kass, R. S. (2003). K+ channel structure-activity relationships and mechanisms of drug-induced QT prolongation. Annual Review of Pharmacology and Toxicology, 43, 441–461.
Taglialatela, M., Castaldo, P., Iossa, S., Pannaccione, A., Fresi, A., Ficker, E., & Annunziato, L. (1997). Regulation of the human ether-a-go go related gene (HERG) K+ channels by reactive oxygen species. Proceedings of the National Academy of Sciences of the United States of America, 94, 11698–11703.
Berube, J., Caouette, D., & Daleau, P. (2001). Hydrogen peroxide modifies the kinetics of HERG channel expressed in a mammalian cell line. Journal of Pharmacology and Experimental Therapeutics, 297, 96–102.
Roden, D. M., & Viswanathan, P. C. (2005). Genetics of acquired long QT syndrome. Journal of Clinical Investigation, 115, 2025–2032.
van Noord, C., Eijgelsheim, M., & Stricker, B. H. (2010). Drug- and non-drug-associated QT interval prolongation. British Journal of Clinical Pharmacology, 70, 16–23.
Ott, M., Gogvadze, V., Orrenius, S., & Zhivotovsky, B. (2007). Mitochondria, oxidative stress and cell death. Apoptosis, 12, 913–922.
Rana, S. V. (2008). Metals and apoptosis: Recent developments. Journal of Trace Elements in Medicine and Biology, 22, 262–284.
Young, I., & Woodside, J. (2001). Antioxidants in health and disease. Journal of Clinical Pathology, 54, 176–186.
Willcox, J. K., Ash, S. L., & Catignani, G. L. (2004). Antioxidants and prevention of chronic disease. Critical Reviews in Food Science and Nutrition, 44, 275–295.
Fang, Y. Z. (2002). Free radicals and nutrition. In Y. Z. Fang, Zheng & RL (Eds.), Theory and application of free radical biology (p. 647). Beijing: Scientific Press.
Shi, H., Hudson, L. G., Ding, W., Wang, S., Cooper, K. L., Liu, S., Chen, Y., Shi, X., & Liu, K. J. (2004). Arsenite causes DNA damage in keratinocytes via generation of hydroxyl radicals. Chemical Research in Toxicology, 17, 871–878.
Woo, S. H., Park, I. C., Park, M. J., Lee, H. C., Lee, S. J., Chun, Y. J., Lee, S. H., Hong, S. I., & Rhee, C. H. (2002). Arsenic trioxide induces apoptosis through a reactive oxygen species-dependent pathway and loss of mitochondrial membrane potential in HeLa cells. International Journal of Oncology, 21, 57–63.
Han, Y. H., Moon, H. J., You, B. R., Kim, S. Z., Kim, S. H., & Park, W. H. (2010). Effects of arsenic trioxide on cell death, reactive oxygen species and glutathione levels in different cell types. International Journal of Molecular Medicine, 25, 121–128.
Sun, Y., Wang, C., Wang, L., Dai, Z., & Yang, K. (2018). Arsenic trioxide induces apoptosis and the formation of reactive oxygen species in rat glioma cells. Cellular & Molecular Biology Letters, 23, 13.
Hagen, T. M., Wehr, C. M., & Ames, B. N. (1998). Mitochondrial decay in aging. Reversal through supplementation of acetyl-Lcarnitine and N-tert-butyl-alpha-phenyl-nitrone. Annals of the New York Academy of Sciences, 854, 214–223.
Bahorun, T., Soobratte, M. A., Luximon-Ramma, V., & Aruoma, O. I. (2006). Free radicals and antioxidants in cardiovascular health and disease. Internet Journal of Medical Update, 1, 25–41.
Cadenas, E., & Davies, K. J. A. (2000). Mitocondrial free radical generation, oxidative stress, and aging. Free Radical Biology and Medicine, 29, 222–230.
Mieyal, J. J., Gallogly, M. M., Qanungo, S., Sabens, E. A., & Shelton, M. D. (2008). Molecular mechanisms and clinical implications of reversible protein s-glutathionylation. Antioxidants & Redox Signaling, 10, 1941–1988.
Anderson, T. J., Meredith, I. T., Yeung, A. C., Frei, B., Selwyn, A. P., & Ganz, P. (1995). The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. New England Journal of Medicine, 332, 488–493.
Guzik, T. J., West, N. E. J., Black, E., McDonald, D., Ratnatunga, C., Pillai, R., & Channon, K. M. (2000). Vascular superoxide production by NAD(P)H oxidase: Association with endothelial dysfunction and clinical risk factors. Circulation Research, 86, e85–e90.
Heymes, C., Bendall, J. K., Ratajczak, P., Cave, A. C., Samuel, J. L., Hasenfuss, G., & Shah, A. M. (2003). Increased myocardial NADPH oxidase activity in human heart failure. Journal of the American College of Cardiology, 41, 2164–2171.
Halliwell, B. (2011). Free radicals and antioxidants-quo vadis? Trends in Pharmacological Sciences, 32, 125–130.
Chance, B., Sies, H., & Boveris, A. (1979). Hydroperoxide metabolism in mammalian organs. Physiological Reviews, 59, 527–605.
Hwang, J. T., Kwon, D. Y., Park, O. J., & Kim, M. S. (2008). Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes & Nutrition, 2, 323–326.
Gomes, E. C., Silva, A. N., & de Oliveira, M. R. (2012). Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxidative Medicine and Cellular Longevity, 2012, 12.
Ursini, F., Maiorino, M., & Brigelius-Flohé, R. (1995). The diversity of glutathione peroxidase. Methods in Enzymology, 252, 38–63.
Vineetha, V. P., Girija, S., Soumya, R. S., & Raghu, K. G. (2014). Polyphenol-rich apple (Malus domestica L.) peel extract attenuates arsenic trioxide induced cardiotoxicity in H9c2 cells via its antioxidant activity. Food & Function, 5, 502–511.
Brookes, P. S., Yoon, Y., Robotham, J. L., Anders, M. W., & Sheu, S. S. (2004). Calcium, ATP, and ROS: A mitochondrial love-hate triangle. American Journal of Physiology. Cell Physiology, 287, 817–833.
Yano, M., Ikeda, Y., & Matsuzaki, M. (2005). Altered intracellular Ca2+ handling in heart failure. Journal of Clinical Investigation, 115, 556–564.
Berridge, M. J., Lipp, P., & Bootman, M. D. (2000). The versatility and universality of calcium signalling. Nature Reviews Molecular Cell Biology, 1, 11–21.
Orrenius, S., Zhivotovsky, B., & Nicotera, P. (2003). Regulation of cell death: The calcium-apoptosis link. Nature Reviews Molecular Cell Biology, 4, 552–565.
Berridge, M. J., Bootman, M. D., & Roderick, H. L. (2003). Calcium signalling: Dynamics, homeostasis and remodelling. Nature Reviews Molecular Cell Biology, 4, 517–529.
Rizzuto, R., Brini, M., Murgia, M., & Pozzan, T. (1993). Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighbouring mitochondria. Science, 262, 744–747.
Wang, Y., & Goldhaber, J. I. (2004). Return of calcium: Manipulating intracellular calcium to prevent cardiac pathologies. Proceedings of the National Academy of Sciences of the United States of America, 101, 5697–5698.
Sitsapesan, R., & Williams, A. J. (2000). Do inactivation mechanisms rather than adaptation hold the key to understanding ryanodine receptor channel gating? Journal of General Physiology, 116, 867–872.
Eisner, D. A., Trafford, A. W., Diaz, M. E., Overend, C. L., & O’Neill, S. C. (1998). The control of Ca release from the cardiac sarcoplasmic reticulum: Regulation versus autoregulation. Cardiovascular Research, 38, 589–604.
Florea, A. M., Yamoah, E. N., & Dopp, E. (2005). Intracellular calcium disturbances induced by arsenic and its methylated derivatives in relation to genomic damage and apoptosis induction. Environmental Health Perspectives, 113, 659–664.
Zhang, J., Sun, G., Wang, M., Liao, P., Du, Y., Yang, K., & Sun, X. (2016). Arsenic trioxide triggered calcium homeostasis imbalance and induced endoplasmic reticulum stress-mediated apoptosis in adult rat ventricular myocytes. Toxicology Research, 5, 682–688.
Ohnishi, K., Yoshida, H., Shigeno, K., Nakamura, S., Fujisawa, S., Naito, K., Shinjo, K., Fujita, Y., Matsui, H., & Takeshita, A. (2000). Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Annals of Internal Medicine, 133, 881–885.
Yedjou, C. G., & Tchouwou, P. B. (2007). In-vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell electrophoresis (comet) assays. Molecular and Cellular Biochemistry, 301, 123–130.
Michel, L., Dupuy, A., Jean-Louis, F., Sors, A., Poupon, J., Viguier, M., Musette, P., Dubertret, L., Degos, L., Dombret, H., & Bachelez, H. (2003). Arsenic trioxide induces apoptosis of cutaneous T cell lymphoma cells: Evidence for a partially caspase-independent pathway and potentiation by ascorbic acid (vitamin C). The Journal of Investigative Dermatology, 121, 881–893.
Huang, H. S., Chang, W. C., & Chen, C. J. (2002). Involvement of reactive oxygen species in arsenite-induced down regulation of phospholipid hydroperoxide glutathione peroxidase in human epidermoid carcinoma A431 cells. Free Radical Biology and Medicine, 33, 864–873.
Graham-Evans, B., Cohly, H. H. P., Yu, H., & Tchounwou, P. B. (2004). Arsenic-induced genotoxic and cytotoxic effects in human keratinocytes, melanocytes and dendritic cells. International Journal of Environmental Research and Public Health, 1, 83–89.
Waclavicek, M., Berer, A., Oehler, L., Stöckl, J., Schloegl, E., Majdic, O., & Knapp, W. (2001). Calcium ionophore: A single reagent for the differentiation of primary human acute myelogenous leukaemia cells towards dendritic cells. British Journal of Haematology, 114, 466–473.
Parag, H. A., Raboy, B., & Kulka, R. G. (1987). Effects of heat shock on protein degradation in mammalian cells involvement of the ubiquitin system. EMBO Journal, 6, 55–61.
Matsui, M., Nishigori, C., Toyokuni, S., Takada, J., Akaboshi, M., Ishikawa, M., Imamura, S., & Miyachi, Y. (1999). The role of oxidative DNA damage in human arsenic carcinogenesis: Detection of 8-hydroxy-2′- deoxyguanosine in arsenic-related Bowen’s disease. The Journal of Investigative Dermatology, 113, 26–31.
Alarifi, S., Ali, D., Alkahtani, S., Siddiqui, M. A., & Ali, B. A. (2013). Arsenic trioxide-mediated oxidative stress and genotoxicity in human hepatocellular carcinoma cells. OncoTargets and Therapy, 6, 75–84.
Nordenson, I., & Beckman, L. (1991). Is the genotoxic effect of arsenic mediated by oxygen free radicals? Human Heredity, 41, 71–73.
Barchowsky, A., Klei, L. R., Dudek, E. J., Swartz, H. M., & James, P. E. (1999). Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radical Biology and Medicine, 27, 1405–1412.
Smith, K. R., Klei, L. R., & Barchowsky, A. (2001). Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology, 280, L442–L449.
Bunderson, M., Brooks, D. M., Walker, D. L., Rosenfeld, M. E., Coffin, J. D., & Beall, H. D. (2004). Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicology and Applied Pharmacology, 201, 32–39.
Lee, P. C., Ho, I. C., & Lee, T. C. (2005). Oxidative stress mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte chemoattractant protein-1, and interleukin-6 in vascular smooth muscle cells. Toxicological Sciences, 85, 541–550.
Pysher, M. D., Chen, Q. M., & Vaillancourt, R. R. (2008). Arsenic alters vascular smooth muscle cell focal adhesion complexes leading to activation of FAK-src mediated pathways. Toxicology and Applied Pharmacology, 231, 135–141.
Tsai, S. H., Hsieh, M. S., Chen, L., Liang, Y. C., Lin, J. K., & Lin, S. Y. (2001). Suppression of Fas ligand expression on endothelial cells by arsenite through reactive oxygen species. Toxicology Letters, 123, 11–19.
Chen, S. C., Tsai, M. H., Wang, H. J., Yu, H. S., & Chang, L. W. (2007). Involvement of substance P and neurogenic inflammation in arsenic-induced early vascular dysfunction. Toxicological Sciences, 95, 82–88.
Pereira, F. E., Coffin, J. D., & Beall, H. D. (2007). Activation of protein kinase C and disruption of endothelial monolayer integrity by sodium arsenite-potential mechanism in the development of atherosclerosis. Toxicology and Applied Pharmacology, 220, 164–177.
Tsou, T. C., Tsai, F. Y., Hsieh, Y. W., Li, L. A., Yeh, S. C., & Chang, L. W. (2005). Arsenite induces endothelial cytotoxicity by down-regulation of vascular endothelial nitric oxide synthase. Toxicology and Applied Pharmacology, 208, 277–284.
Balakumar, P., & Kaur, J. (2009). Arsenic exposure and cardiovascular disorders: An overview. Cardiovascular Toxicology, 9, 169–176.
Lee, M. Y., Lee, Y. H., Lim, K. M., Chung, S. M., Bae, O. N., Kim, H., Lee, C. R., Park, J. D., & Chung, J. H. (2005). Inorganic arsenite potentiates vasoconstriction through calcium sensitization in vascular smooth muscle. Environmental Health Perspectives, 113, 1330–1335.
Raghu, K. G., & Cherian, O. L. (2009). Characterization of cytotoxicity induced by arsenic trioxide (a potent anti-APL drug) in rat cardiac myocytes. Journal of Trace Elements in Medicine and Biology, 23, 61–68.
Zhao, J. L., Sun, B. G., Wen, Q. Z., Zhang, J. J., Jin, W., Xue, J. X., & Zhuang, W. Y. (2007). Effect of early and non-early controlled-release of arsenic-trioxide eluting stents on restenosis inhibition in a canine model. Zhonghua Xin Xue Guan Bing Za Zhi, 35, 571–574.
Gill, C., Mestril, R., & Sali, A. (2002). Losing heart; The role of apoptosis in heart disease-noval therapeutic target. The FASEB Journal, 16, 135–146.
Nerheim, P., Krishnan, S. C., Olshansky, B., & Shivkumar, K. (2001). Apoptosis in the genesis of cardiac rhythm disorders. Cardiology Clinics, 19, 155–163.
James, T. N. (1996). Long reflections on the QT interval: The sixth annual Gordon K. Moe Lecture. Journal of Cardiovascular Electrophysiology, 7, 738–759.
Best, P. J., Hasdai, D., Sangiorgi, G., Schwartz, R. S., Holmes, D. R. Jr., & Simari, R. D. (1999). Apoptosis. Basic concepts and implications in coronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 19, 14–22.
Jacobson, M. D. (1996). Reactive oxygen species and programmed cell death. Trends in Biochemical Sciences, 21, 83–86.
Zhao, X., Feng, T., Chen, H., Shan, H., Zhang, Y., Lu, Y., & Yang, B. (2008). Arsenic trioxide-induced apoptosis in H9c2 cardiomyocytes: Implications in cardiotoxicity. Basic & Clinical Pharmacology & Toxicology, 102, 419–425.
Hei, T. K., Liu, S. X., & Waldren, C. (1998). Mutagenicity of arsenic in mammalian cells: Role of reactive oxygen species. Proceedings of the National Academy of Sciences of the United States of America, 5, 8103–8107.
Szeto, H. H. (2006). Cell permeable, mitochondrial-target, peptide antioxidants. American Association of Pharmaceutical Scientists Journal, 8, 277–278.
Dillard, C. J., & German, J. B. (2000). Phytochemicals: Nutraceuticals and human health. Journal of the Science of Food and Agriculture, 80, 1744–1756.
Gu, J., Gui, Y., Chen, L., Yuan, G., Lu, H., & Xu, X. (2013). Use of natural products as chemical library for drug discovery and network pharmacology. PLoS ONE, 8, e62839.
Nampoothiri, S. V., Binilraj, S. S., Prathapan, A., Abhilash, P. A., Arumughan, C., & Sundaresan, A. (2011). In vitro antioxidant activities of the methanol extract and its different solvent fractions obtained from the fruit pericarp of Terminalia bellerica. Natural Product Research, 25, 277–287.
Prathapan, A., Vineetha, V. P., Abhilash, P. A., & Raghu, K. G. (2013). Boerhaavia diffusa L. attenuates angiotensin II-induced hypertrophy in H9c2 cardiac myoblast cells via modulating oxidative stress and down-regulating NF-κB and transforming growth factor β1. British Journal of Nutrition, 110, 1201–1210.
Prathapan, A., Vineetha, V. P., & Raghu, K. G. (2014). Protective effect of Boerhaavia diffusa L. against mitochondrial dysfunction in angiotensin II induced hypertrophy in H9c2 cardiomyoblast cells. PLoS ONE, 9(4), e96220.
Vineetha, V. P., Prathapan, A., Soumya, R. S., & Raghu, K. G. (2013). Arsenic trioxide toxicity in H9c2 myoblasts—damage to cell organelles and possible amelioration with Boerhaavia diffusa. Cardiovascular Toxicology, 13, 123–137.
Bouayed, J., & Bohn, T. (2010). Exogenous antioxidants-double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Medicine and Cellular Longevity, 3, 228–237.
Diplock, A. T., Charleux, J. L., Crozier-Willi, G., Kok, F. J., Rice-Evans, C., Roberfroid, M., Stahl, W., & Viña-Ribes, J. (1998). Functional food science and defence against reactive oxygen species. British Journal of Nutrition, 80, S77–S112.
Howard, B. V., & Kritchevsky, D. (1997). Phytochemicals and cardiovascular disease. A statement for healthcare professionals from the American Heart Association. Circulation, 95, 2591–2593.
Hu, F. B. (2003). Plant-based foods and prevention of cardiovascular diseases: An overview. American Journal of Clinical Nutrition, 78, 544S–551S.
Reddy, V. B. M., Sasikala, P., Karthik, A., Sudheer, S. D., & Murthy, L. N. (2012). Protective role of curcumin against arsenic trioxide toxicity during gestation and lactational periods. Global Veterinaria, 9, 270–276.
Zhao, X.-Y., Li, G.-Y., Liu, Y., Chai, L.-M., Chen, J.-X., Zhang, Y., Du, Z.-M., Lu, Y.-J., & Yang, B.-F. (2008). Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. British Journal of Pharmacology, 154, 105–113.
Kumazaki, M., Ando, H., Kakei, M., Ushijima, K., Taniguchi, Y., Yoshida, M., Yamato, S., Washino, S., Koshimizu, T. A., & Fujimura, A. (2013). α-Lipoic acid protects against arsenic trioxide-induced acute QT prolongation in anesthetized guinea pigs. European Journal of Pharmacology, 705, 1–10.
Zhang, J.-Y., Wang, M., Wang, R.-Y., Sun, X., Du, Y.-Y., Ye, J.-X., Sun, G.-B., & Sun, X.-B. (2018). Salvianolic acid A ameliorates arsenic trioxide-induced cardiotoxicity through decreasing cardiac mitochondrial injury and promotes its anticancer activity. Frontiers in Pharmacology, 9, 487.
Sinha, M., Manna, P., & Sil, P. C. (2008). Arjunolic acid attenuates arsenic-induced nephrotoxicity. Pathophysiology, 15, 147–156.
Bors, W., Heller, W., Michel, C., & Saran, M. (1990). Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods in Enzymology, 186, 343–355.
Acknowledgements
The first author thank Kerala State Council for Science, Technology and Environment (KSCSTE), Woman Scientist Division (WSD) – Project Grant No.1107/2017/KSCSTE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest existing among the authors.
Additional information
Handling Editor: Dipak K Dube.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vineetha, V.P., Raghu, K.G. An Overview on Arsenic Trioxide-Induced Cardiotoxicity. Cardiovasc Toxicol 19, 105–119 (2019). https://doi.org/10.1007/s12012-018-09504-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-018-09504-7