Abstract
Background
Receptive endometrium is a restraining factor in the establishment of pregnancy in several estrogen-dependent gynecological disorders including uterine leiomyomas. Recently, data are beginning to accrue suggesting negative impact of non-cavity distorting intramural fibroids on molecular mediators of endometrial receptivity. The potential importance of the mammalian target of rapamycin (mTOR) signaling pathway has been suggested during embryo implantation. However, its exact role in fibroid-associated endometrium during the window of implantation is poorly defined. The objective of the study was to examine the expression and cellular distribution of key components of the mTOR signaling pathway during window of implantation in infertile women with non-cavity distorting intramural uterine leiomyomas (n = 24) as compared to fertile controls (n = 17). Relative gene expression analysis of mTOR, TSC1, and TSC2 was performed by real-time PCR. Immunohistochemistry was used to evaluate the expression of mTOR, phospho-mTOR (Serine 2448), TSC1, TSC2, phospho-TSC2 (Threonine 1462), and phospho-S6 ribosomal protein (Serines 235 and 236) and Ki67.
Results
In comparison to fertile controls, statistically significant upregulation of mTOR (8.97-fold; p < 0.001) and downregulation of TSC2 mRNA (− 6.01-fold; p < 0.01) levels and cell-specific upregulation of proteins phospho-mTOR, phospho-TSC2, and phospho-S6 and downregulation of TSC1 and TSC2 were observed in infertile women. The ratio of p-mTOR/mTOR and p-TSC2/TSC2 was significantly higher in infertile women. Pearson’s correlation analysis revealed significant negative correlation between p-mTOR and TSC2 and positive correlation between p-mTOR and p-S6 in the infertile group. Increased Ki67 labelling index was observed in the glandular epithelium (GE) and stroma of endometrium from infertile women as compared to controls.
Conclusions
Loss of TSC2 function and enhanced expression of activated mTOR and its downstream targets, observed in the infertile group, indicate heightened mTOR signaling which might contribute to impaired endometrial receptivity. Increased number of Ki67-positive nuclei suggests that enhanced mTOR signaling may help drive dysregulated proliferation of midsecretory endometrium leading to compromised fertility in women with non-cavity distorting intramural uterine leiomyomas. The present findings provide avenues for future investigation of mTOR pathway as a nonsurgical alternative for treatment of infertility in these patients.
Similar content being viewed by others
Background
Impaired endometrial receptivity, an important contributing factor to implantation failure, is one of the major causes of infertility and overall low success rate of assisted reproductive technologies (ART). Endometrial receptivity represents the brief window of time (days 6–10 post ovulation) during which the endometrium is optimally receptive to blastocyst acceptance and implantation. It is regulated by ovarian hormones and involves dynamic genotypic and phenotypic changes of the endometrial cells [1, 2].
Receptive endometrium is a restraining factor in the establishment of pregnancy in several estrogen-dependent gynecological disorders including endometriosis, uterine leiomyomas, and polycystic ovarian syndrome (PCOS) [3]. Uterine leiomyomas, or fibroids, are widely prevalent benign tumors of the uterus that affect approximately 35–77% of reproductive age women and are found more frequently in women with infertility [4]. While adverse effect of submucosal and intramural fibroids that distort endometrial cavity on reproductive outcomes is well established, unfavorable IVF/ICSI/ET outcomes have been reported in women having intramural leiomyomas not encroaching on the uterine cavity comparable to those of women without such leiomyomas [5,6,7,8].
Recent studies propose that physical and mechanical disruption of the endometrium is only one aspect of fibroid action; these tumors also have a global effect on the endometrium which is transmitted through molecular signaling. While several reports have highlighted the negative influence of submucosal leiomyomas on uterine receptivity, influence of non-cavity distorting intramural leiomyoma on molecular mediators of endometrial receptivity is poorly understood [9,10,11]. Positive impact of intramural myomectomy on receptivity markers and pregnancy outcome has been reported [12].
It is clear that many actions of ovarian hormones in regulating endometrial function and preparation for implantation are mediated by signaling pathways associated with proliferation and cell survival [1]. Latest studies have suggested important role of mammalian target of rapamycin (mTOR) signaling pathway during embryo implantation [13]. mTOR is a ubiquitously expressed and highly conserved serine/threonine kinase that is central to the control of cell proliferation, growth, and survival in mammalian cells. It is a downstream target of phosphatidylinositol 3-kinase (PI3K) and direct substrate for Akt kinase which activates it by phosphorylation at the serine 2448 residue. The activity of mTOR is negatively regulated by heterodimeric complex consisting of TSC1 (hamartin) and TSC2 (tuberin). It acts by directly activating ribosomal S6 kinase (S6K1) and inhibiting 4E-binding protein 1 (4E-BP1). S6K1 is a serine/threonine kinase that phosphorylates the S6 protein of the 40S ribosomal subunit at several sites, including serine’s 235 and 236 (phosphorylated S6 ribosomal protein), leading to initiation of protein synthesis. Phospho-S6 is the most studied effector of S6K1 [14].
The significance of mTOR in the implantation process has been corroborated mainly by studies on animal models. A recent study demonstrated increase in mTOR and associated signaling molecules during the implantation period in rats [15]. Chen et al. (2009) demonstrated a spatial–temporal expression pattern of mTOR in mouse endometria which reached the maximum level at the time of the implantation window [16]. A recent study in BALB/c mice demonstrated role of dexamethasone in altering endometrial receptivity through the modulation of mTOR pathway [17].
In view of increasing concern on fertility implications of non-cavity distorting intramural fibroids, we undertook the present study with the aim to investigate the role of mTOR pathway in fibroid-associated endometrium during the window of implantation. We studied the expression, cellular distribution, and activation status of important signaling components of the mTOR pathway along with the proliferative activity in midsecretory endometrium from infertile women with non-cavity distorting intramural uterine fibroids as compared to fertile women without uterine fibroids as controls. Association of p-mTOR expression with the expression of other proteins of the pathway was evaluated by Pearson’s correlation analysis.
Methods
Subjects
The study was approved by the ethics committee of King George Medical University, Lucknow, India, for use of human tissue for research and was based on informed patient consent. Subjects for the study included infertile women with intramural fibroids that cause no distortion (compression) of the uterine cavity (n = 24), aged between 26 and 38 years (mean ± SEM; 31.75 ± 0.79 years) and with mean duration of infertility as 6.25 ± 0.61 years (range 2–10 years). All infertile women and their partners were evaluated thoroughly for the etiology of infertility. While 22 women had primary infertility, 2 of them were cases of secondary infertility. All these women were enrolled in the Assisted Reproductive Technology (ART) Unit of the Obstetrics and Gynecology Department of King George’s Medical University, Lucknow. Male partners of all these women were fertile. Diagnosis of uterine fibroids was performed by transvaginal ultrasonography, magnetic resonance imaging, or hysteroscopy. A single intramural fibroid of > 3 cm in diameter (mean ± SEM; 5.15 ± 0.30 cm; range 3.3–7.5 cm) was present in each infertile woman. The fibroid was located in the posterior wall in 14 women and in the anterior wall in 10 women. The endometrial cavity in all infertile women was evaluated for mechanical distortion either by hysteroscopy, hysterosalpingography, or sonosalpingography.
Study controls were fertile healthy women volunteers without uterine leiomyomas (n = 17), aged between 24 and 36 years (mean ± SEM; 30.71 ± 1.02 years), with mean parity of 2.18 (range 1–4), and no history of infertility or habitual abortion.
All participants met the following inclusion criteria: (1) the presence of uterine leiomyoma as the only evident cause of infertility; (2) women having regular menstrual cycles, (3) women with normal early follicular FSH, LH, E2, TSH, and prolactin levels and with normal midluteal progesterone levels; (3) bilateral tubal patency in all infertile women as evidenced by normal hysterosalpingography, (4) no history of hormonal medication, intrauterine contraception or use of GnRH analogs, selective progesterone modulators, etc. within the last 6 months prior to enrollment in the study; and (5) women with midsecretory endometrium as confirmed by histological dating. The following were the exclusion criteria: (1) women with uterine abnormalities, the presence of submucous or pedunculated fibroids, previous history of polyps, and uterine septae; (2) women with diagnosis of hydrosalpinx, adenomyosis, or endometriosis at the time of the study; (3) previous surgeries, e.g., myomectomy, myolysis, metroplasty, and prior removal of part of uterus; (4) prior history of interventional radiological procedures such as uterine artery embolization and/or MRgFUS; (5) history of habitual abortion; (6) mechanical distortion of the uterine cavity by intramural leiomyoma; and (7) subjects with male factor infertility.
All volunteers had regular menstrual cycles every 27 to 32 days and underwent transvaginal ultrasonographic monitoring of ovulation. Follicular growth was evaluated daily commencing on days 8 to 10 of menstrual cycle length using a 3.5–8.5 MHz vaginal transducer (Shimadzu SBU 350, Japan). The day of ovulation was designated as day of maximum follicular enlargement that was followed by sudden disappearance or filling in of follicle showing loss of clear demarcation of walls and intrafollicular echoes. The midsecretory phase was calculated + 6 to + 8 days after ovulation and was confirmed by endometrial histologic dating and serum P levels (> 10 ng/ml).
Endometrial histologic dating
Endometrial biopsy from each volunteer was collected from the uterine fundus during the midsecretory phase of menstrual cycle, using a standard endometrial suction curette (Gynetics, Belgium). Samples were divided into two portions; first portion was placed in 4% buffered formaldehyde for 24 h, embedded in paraffin, and cut into 5-mm-thick sections for histology and immunohistochemistry. The second portion was snap frozen and stored at − 80 °C until processed for isolation of RNA. Dating of each biopsy was performed by the same pathologist (M. M. G.) following the Noyes criteria [18]. Only midsecretory phase endometrial biopsies were included in the study.
RNA isolation and quantitative real-time PCR
RNA isolation and quantitative real-time PCR were performed as described previously [19]. The sequences of forward and reverse primers used in the study are as follows: (1) β-actin: forward 5′-GTGGGGCGCCCCAGGCACCA-3′; reverse 5′-CTCCTTAATGTCACGCACGATTTC-3′; (2) mTOR: forward 5′-AGTGGACCAGTGGAAACAGG-3′; reverse 5′-TTCAGCGATGTCTTGTGAGG-3′; (3) TSC1: forward 5′-CGCACTCTTTCATCGCCTTTAT-3′; reverse 5′-TCATTGGCTTGACCACTTCTTC-3′; and (4) TSC2: forward 5′-TGTGCAGGAGAAGACGAACC-3′; reverse 5′-GGCACCGACAGTGACTTGTA-3′. Relative gene expression was determined by the 2−ΔΔCT method and expressed as fold change from fertile control set at a value of 1.0.
Immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) endometrial biopsies were sectioned at 4 µm and mounted on 3-aminopropyl triethoxysilane-coated glass slides. Slides were incubated at 60 °C for 1 h followed by deparaffinization and rehydration. Antigen retrieval was performed in a microwave oven (EZ antigen retriever system; BioGenex, USA) by heating slides at 98 °C for 15 min in the following: (1) Tris–EDTA buffer (pH 9.0) for antibodies against p-mTOR (ab109268, Abcam, Cambridge, MA, USA), TSC1 (sc12082, Santa Cruz), TSC2 (sc893, Santa Cruz), pS6 ribosomal protein (no. 4857S, Cell Signaling Technology), and Ki67 (IS626, DAKO, Denmark) and (2) in citrate buffer (pH 6.0) for antibodies against mTOR (ab2732, Abcam) and p-TSC2 (T1462, ab59274, Abcam). Detection of p-mTOR, TSC1, TSC2, pS6 ribosomal protein, and Ki67 was performed using Dako EnVision FLEX detection system (DAKO, Denmark), while that of mTOR and p-TSC2 was performed using Novolink Min Polymer Detection System (Novacastra, Leica Biosystem, Newcastle Ltd., UK). Immunoreactivity was visualized using 3,3′-diaminobenzidine tetrahydrochloride chromogen followed by hematoxylin counterstain. Slides were dehydrated in a series of graded ethanol washes, mounted with DPX mountant (Rankem, India) and cover slipped for bright-field microscopy. Image acquisition was achieved using NIS-elements software (Nikon). Negative controls were slides processed without primary antibodies. Positive controls (viz., rat testis for mTOR; human breast carcinoma for p-mTOR, TSC2, P-S6, and Ki67; human prostate adenocarcinoma for TSC1) were selected according to the manufacturer’s instructions.
Scoring of IHC staining
The samples were anonymized and scored independently by AM and MMG. In case of any discrepancy in scores, the slides were reexamined, and a consensus was reached by the observers. Immunostaining was evaluated in the surface epithelium (SE), glandular epithelium (GE), and stromal compartments. The localization of immunoreaction, that is, nuclear, cytoplasmic, or membranous, was also noted. Scoring of IHC staining was semiquantitative and assigned based on intensity and proportion of cells stained for each protein. The immunointensity was scored on the scale of 0: no staining, 1: light staining, 2: low intermediate, 3: high intermediate, and 4: darkest brown stain. The proportion of staining was scored on a scale of the following: 1: 0–25% of cells stain positive; 2: 26–50% of cells stain positive, 3: 51–75% of cells stain positive; and 4: 76–100% of cells stain positive. Immunointensity and immunopositivity scores were multiplied giving results that ranged from 0 to 16.
Statistical analysis
The real-time PCR results were evaluated by Student’s t-test. Immunohistochemistry data was analyzed by one-way ANOVA (unstacked) to compare means between two groups. Pearson’s correlation analysis was performed to see the association of p-mTOR expression with the expression of other proteins of the pathway. Minitab 13.0 statistical software (Minitab Inc., State College, PA, USA) was used for data analysis. A p-value of < 0.05 was considered as statistically significant.
Results
Relative gene expression analysis by real-time polymerase chain reaction for mTOR, TSC1, and TSC2 genes and immunohistochemistry for mTOR, p-mTOR, TSC1, TSC2, p-TSC2, pS6 ribosomal protein, and Ki67 was performed in midsecretory endometrial biopsies from infertile and fertile women.
mTOR, p-mTOR and p-mTOR/mTOR ratio
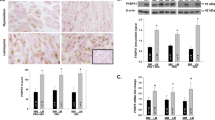
Significant upregulation of mTOR mRNA level (8.97-fold; p < 0.001) was observed in the infertile group as compared to fertile controls (Fig. 1a). However, no significant difference in immunohistochemical expression of mTOR was observed between the two groups in any endometrial compartment (Table 1). The staining for mTOR was mainly cytoplasmic (Fig. 2A, B).
Representative photomicrographs of immunostaining for mTOR, phospho-mTOR, TSC1, TSC2, phospho-TSC2 phospho-S6 ribosomal protein, and Ki67 from fertile women (A, C, E, G, I, K, M) and infertile women with intramural uterine leiomyomas (B, D, F, H, J, L, N) during the window of implantation. All microphotographs are shown at original magnification × 200
Cytoplasmic immunoreactivity for activated mTOR (p-mTOR, Serine 2448) was observed in both groups (Fig. 2C, D). Immunoscores for p-mTOR in the GE (11.10 ± 3.35 vs 8.13 ± 2.60; p < 0.05) and stroma (7.60 ± 2.35 vs 4.38 ± 1.06; p < 0.01) of infertile women with leiomyoma were significantly higher as compared to fertile controls (Table 1).
The p-mTOR/mTOR ratio, assessed to confirm activation status of mTOR, was significantly higher in the endometrial stroma of infertile group as compared to fertile controls (1.86 ± 0.74 vs 0.88 ± 0.19; p < 0.01) (Table 1).
TSC1
No significant difference in TSC1 transcript levels (− 1.18-fold; p = 0.50) was observed between the two groups (Fig. 1b). Immunostaining for TSC1 was both cytoplasmic and nuclear (Fig. 2E, F). Marginally significant downregulation of cytoplasmic TSC1 was observed only in the stromal compartment of endometrium from infertile women as compared to fertile controls (5.15 ± 3.45 vs 7.83 ± 3.43; p < 0.05) (Table 1).
TSC2, p-TSC2, and p-TSC2/TSC2 ratio
The TSC2 mRNA levels were significantly lower (− 6.01 fold; p < 0.01) in the infertile group as compared to fertile controls (Fig. 1c). Immunoexpression of TSC2 was cytoplasmic (Fig. 2G, H). The immunoscores for TSC2 were significantly lower in the GE (8.86 ± 4.33 vs. 12.13 ± 2.61; p < 0.05) and stroma (4.00 ± 1.37 vs. 6.96 ± 4.03; p < 0.05) of endometrium from infertile group as compared to fertile controls (Table 1).
Immunostaining for TSC2 phosphorylated at the threonine 1462 residue was both cytoplasmic and nuclear (Fig. 2I, J). The cytoplasmic p-TSC2 expression in the endometrial stroma of infertile women was significantly higher (8.58 ± 3.13 vs. 4.70 ± 2.10; p < 0.01) as compared to fertile controls (Table 1).
The cytoplasmic p-TSC2/TSC2 ratio in the GE (1.63 ± 0.90 vs. 0.83 ± 0.27; p < 0.05) and stroma (2.36 ± 1.06 vs. 0.84 ± 0.50; p < 0.001) of endometrium from infertile women was significantly higher as compared to controls (Table 1).
Phospho-S6
Immunoreactivity for S6 ribosomal protein (phosphorylated at the Serines 235 and 236), marker of mTOR activation, was localized to the cytoplasmic and/or nuclear compartments of the endometrial cells (Fig. 2K, L). The mean immunoscore for cytoplasmic p-S6 in the GE (9.50 ± 3.86 vs. 6.50 ± 3.25; p < 0.05) and stroma (7.83 ± 3.22 vs. 5.04 ± 2.55; p < 0.05) of endometrium from infertile women was significantly higher as compared to controls (Table 1).
Ki67
Significant increase in Ki67-positive nuclei was observed in the GE (23.38 ± 21.19 vs. 3.29 ± 3.46; p < 0.001) and stromal (36.67 ± 27.06 vs. 13.41 ± 10.37; p < 0.01) compartments of endometrium from infertile women as compared to controls (Table 1; Fig. 2M, N).
Association of active mTOR with other signaling components of mTOR pathway
Pearson’s correlation analysis was performed to assess the possible association of p-mTOR expression measured by immunohistochemistry with expression of other proteins of the mTOR pathway. IHC scores of p-mTOR had significant positive correlation with scores of mTOR and p-TSC2 across both fertile and infertile groups; however, the correlation tended to be stronger in the infertile group. Furthermore, a significant positive correlation of p-mTOR with p-S6 and Ki67 and negative correlation with TSC2 was observed only in the infertile group (Table 2).
Discussion
Uterine leiomyoma or fibroids affect a woman’s quality of life, as well as her fertility and obstetrical outcomes. Reports in literature have shown that besides their cavity distorting effects, submucous or intramural leiomyomas may also compromise fertility by altering endometrial receptivity, a state of uterine differentiation that is crucial for blastocyst attachment and subsequent implantation. The data on molecular mechanisms associated with impaired fertility in patients with non-cavity encroaching intramural fibroids is, however, limited.
Growing evidence has demonstrated the involvement of mTOR signaling pathway during embryo implantation [15]. However, its exact role in fibroid-associated endometrium during the window of implantation is poorly defined. The present study is aimed at characterizing the expression, cellular distribution, and activation status of mTOR and its downstream targets in midsecretory endometrium from infertile women with non-cavity distorting intramural uterine fibroids as compared to fertile controls.
We found significant upregulation of mTOR gene expression in the infertile group as compared to fertile group. Although no significant difference in mTOR protein expression was observed immunohistochemically between groups, the phosphorylated form of mTOR, a measure of activated mTOR, was significantly elevated in the glandular epithelium and stroma of endometrium from infertile women as compared to fertile controls. Furthermore, the p-mTOR/mTOR ratio, considered to reflect the mTOR activity, was also significantly higher in the stroma of infertile group as compared to fertile controls. Additionally, we observed that the localization of p-mTOR was cytoplasmic in all endometrial samples. Previous studies have shown that p-mTOR expressed in cytoplasm indicates the activated state of mTOR and is directly or indirectly critically involved in complex signaling networks regulating various cellular events including initiation of translation [20, 21].
Although not much information is available on expression of mTOR in women during the period of endometrial receptivity, significantly high mTOR expression has been reported in mouse endometrial stromal cells. Increased levels of translationally controlled tumor protein (TCTP), a highly conserved growth-related protein, have been observed in mouse endometria during the implantation window. It was suggested that upregulated TCTP might evoke stromal cell proliferation by increasing the mTOR expression [22]. Pertinently, Chen et al. reported that expression of mTOR mRNA and protein, located mainly in the stromal cells of mouse uterus increased gradually during days 3 to 5 of pregnancy, reached maximum level at the time of implantation window and decreased dramatically on days 6 to 7 of pregnancy. The authors suggested that mTOR may participate in the proliferation of uterine stromal cells, and that reduction in mTOR expression on days 6–7 might reduce growth and proliferation of endometrial cells. They further observed that endometrial receptivity in these animals was compromised by the intrauterine injection with rapamycin which is an inhibitor of mTOR [16]. Inhibition of embryo implantation by intrauterine injection of rapamycin has also been reported in rat model, confirming thereby participation of mTOR in the implantation process [23, 24]. Li et al. reported that mTOR signaling may contribute to impaired endometrial receptivity during maternal hyperinsulinemia and insulin resistance in mouse [25]. Activation of ERK1/2-mTOR pathway was found to be one mechanism through which fludrocortisone affects uterine receptivity in BALB/c mice [26].
We analyzed the expression of TSC1 and TSC2, the negative regulators of mTOR signaling. While no significant change in TSC1 transcript levels was found among the groups, marginally significant decrease in immunohistochemical expression of TSC1 was observed in the stromal compartment of endometria from infertile women as compared to fertile controls. Previous studies have shown that conditional deletion of Tsc1 in mouse uterus alters uterine receptivity by enhancing mTOR signaling [27]. Tsc1 deletion in endometrial stroma blocked implantation resulting in infertility in conditional Tsc1 knockout mice [28].
TSC2 has been described in literature as the gatekeeper for mTOR activation. PI3K in a variety of cell types regulates mTOR pathway via the activation of Akt, which directly phosphorylates (inactivates) TSC2 and thereby allows mTOR activation to proceed [14]. While no reports are available on expression of TSC2 during implantation window in women, loss of TSC2 expression or functional inactivation of Tuberin via Akt-mediated phosphorylation at Threonine 1462 has been reported in infertile women [29]. Frequent TSC2 inactivation via loss of expression or phosphorylation, and activated mTOR signaling, has been reported in endometrial carcinoma [30]. In the present study, we observed significant downregulation of TSC2 gene along with decreased expression of TSC2 protein in the glandular epithelium and stroma of infertile women as compared to fertile controls. Besides, Pearson’s correlation analysis revealed a significant negative correlation between p-mTOR and TSC2 in the infertile group.
Immunohistochemical staining for TSC2 phosphorylated at Threonine 1462, in the present study, revealed significantly higher p-TSC2 immunoscores as well as p-TSC2/TSC2 ratio in the stromal compartment of fibroid-associated endometrium as compared to endometrium from fertile controls. The observations indicate functional inactivation of the tumor suppressor that may be related to hyperactivation of mTOR observed in the infertile group.
The expression levels of phospho-S6 ribosomal protein (a major downstream target and effector of mTOR pathway) have been described as an indicator of mTOR activation. We observed significant upregulation of p-S6 in the GE and stroma of fibroid-associated endometrium as compared to endometrium from fertile controls. Interestingly, Pearson’s correlation analysis revealed a significant positive correlation of p-mTOR with p-S6 in the infertile group. The findings lend support to heightened mTOR signaling in infertile women with non-cavity encroaching intramural leiomyomas. Furthermore, increased expression of both p-mTOR and p-S6 in the GE and stroma may be attributed to the suggested role of mTOR pathway in decidualization of stroma and hence in blastocyst implantation. Pertinently, it is a well-established fact that while SE is involved mainly in the adhesion and invasion of the trophoblast, the endometrial gland secretions play vital biological roles in regulating uterine receptivity and stromal cell decidualization [16, 31].
Considering the crucial role of mTOR in regulating cell proliferation, growth, and survival, we analyzed immunoexpression of Ki67, a marker of cell proliferation. In line with immunoexpression of components of the mTOR pathway, the Ki67 labelling index was also significantly higher in the GE and stroma of fibroid-associated endometria as compared to controls. The observations suggest that enhanced mTOR signaling may help drive dysregulated proliferation of midsecretory endometrium in infertile women with non-cavity distorting intramural leiomyomas. Pertinently, Wang et al. have reported that estrogen promotes DNA and protein synthesis in endometrial cells through mTOR signaling [32].
Size, number, and location of fibroids have been suggested to negatively influence fertility [33]. In the present study, we included 24 infertile women, each with a single non-cavity distorting intramural fibroid. The mean (± SEM) size of fibroids was 5.15 ± 0.30 cm (range 3.30–7.50 cm). No significant difference was observed in gene expression of mTOR, TSC1, and TSC2 in infertile women with intramural fibroid located in the posterior wall as compared to infertile women with intramural fibroid located in the anterior wall. We performed correlation and regression analysis to see the association between fibroid size and immunohistochemical expression of selected markers of the mTOR pathway (viz., p-mTOR, p-TSC2, and p-S6) in the infertile group; however, no significant association was seen (unpublished observation). Our results are supported by the findings of Surrey et al. who observed that neither size nor volume of IM leiomyoma correlated with decrease in implantation rates in women undergoing IVF-ET [34]. The presence of small intramural fibroids has been reported previously to be associated with a significant reduction in pregnancy rates in IVF/ICSI cycles [35]. Small and single intramural fibroid that does not have direct contact with the endometrium has also been reported to change receptivity [36].
In a recent study, Pier et al. have reported decreased expression of implantation marker, viz., leukemia inhibitory factor in infertile patients with non-cavity distorting intramural leiomyoma compared to fertile controls. Similar to our study, they had recruited patients with fibroid size > 3 cm in size. The authors suggested that large fibroids 3 cm or greater exert local changes that downregulated LIF expression [37]. Pertinently, reports are available in literature where IM fibroids have been suggested to affect endometrial receptivity through a specific and selective molecular mechanism of action, and that paracrine signaling from fibroids to the endometrium may contribute to fibroid-related infertility [9, 12]. In view of the abovementioned studies and our findings, it may be suggested that irrespective of size and location, non-cavity distorting intramural fibroids may affect endometrial function by deregulating mTOR pathway. Studies on the evaluation of the status of mTOR and its downstream targets in midsecretory endometrium following surgical/nonsurgical treatment of non-cavity distorting leiomyoma are needed to give further insight on the role of mTOR pathway during the window of implantation.
To summarize, loss of TSC2 function and enhanced expression of activated mTOR and its downstream target indicates heightened mTOR signaling in infertile women with non-cavity distorting intramural leiomyomas. In light of results of our previous study, demonstrating aberrant Akt activation during window of implantation in infertile women with non-cavity distorting uterine leiomyomas, it might be proposed that PI3K/Akt regulated activation of mTOR pathway may contribute to impaired endometrial receptivity leading to compromised fertility in these women. The present findings provide avenues for future investigation of mTOR pathway as a nonsurgical alternative for treatment of infertility in these patients.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- mTOR:
-
Mammalian target of rapamycin
- GE:
-
Glandular epithelium
- ART:
-
Assisted reproductive technologies
- PCOS:
-
Polycystic ovarian syndrome
- IVF/ICSI/ET:
-
In vitro fertilization/intracytoplasmic sperm injection/embryo transfer
- PI3K:
-
Phosphatidylinositol 3-kinase
- Akt/PKB:
-
Protein kinase B
- TSC1:
-
Hamartin
- TSC2:
-
Tuberin
- S6K1:
-
Ribosomal S6 kinase
- 4E-BP1:
-
4E-binding protein 1
- Phospho-S6:
-
Phosphorylated S6 ribosomal protein
- TCTP:
-
Translationally controlled tumor protein
References
Makker A, Singh MM (2006) Endometrial receptivity in relation to fertility, infertility and antifertility: clinical assessment using structural, biochemical and molecular markers. Med Res Rev 26:699–746. https://doi.org/10.1002/med.20061
Sun B, Yeh J (2022) Non-invasive and mechanism-based molecular assessment of endometrial receptivity during the window of implantation: current concepts and future prospective testing directions. Front Reprod Health 4(Article 863173):1–17. https://doi.org/10.3389/frph.2022.863173
Makker A, Goel MM (2013) Uterine leiomyomas: effects on architectural, cellular and molecular determinants of endometrial receptivity. Reprod Sci 20:631–638. https://doi.org/10.1177/1933719112459221
Navarro A, Bariani MV, Yang Q, Al-Hendy A (2021) Understanding the impact of uterine fibroids on human endometrium function. Front Cell Develop Biol 9(Article 633180):1–16. https://doi.org/10.3389/fcell.2021.633180
Eldar-Geva T, Meagher S, Healy DL, McLachlan V, Breheny S, Wood C (1998) Effect of intramural, subserosal and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril 70:687–691. https://doi.org/10.1016/s0015-0282(98)00265-9
Guven S, Kart C, Unsal MA, Odaci E (2013) Intramural leiomyoma without endometrial cavity distortion may negatively affect the ICSI-ET outcome. Reprod Biol Endocrinol 11:102–110. https://doi.org/10.1186/1477-7827-11-102
Christopoulos G, Vlismas A, Salim R, Islam R, Trew G, Lavery S (2017) Fibroids that do not distort the uterine cavity and IVF success rates: an observational study using matching criteria. BJOG 124:615–621. https://doi.org/10.1111/1471-0528.14362
Wang X, Chen L, Wang H, Li Q, Qi H (2018) The impact of noncavity-distorting intramural fibroids on the efficacy of in vitro fertilization-embryo transfer: an updated meta-analysis. BioMed Res Int Article ID 8924703:1–13. https://doi.org/10.1155/2018/8924703
Rackow BW, Taylor HS (2010) Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril 93:2027–2034. https://doi.org/10.1016/j.fertnstert.2008.03.029
Pier BD, Bates GW (2015) Potential causes of subfertility in patients with intramural fibroids. Fertil Res Pract 1:12–20. https://doi.org/10.1186/s40738-015-0005-2
Makker A, Goel MM, Nigam D, Bhatia V, Mahdi AA, Das V, Pandey A (2017) Endometrial expression of homeobox genes and cell adhesion molecules in infertile women with intramural fibroids during window of implantation. Reprod Sci 24:435–444. https://doi.org/10.1177/1933719116657196
Unlu C, CelikO CN, Otlu B (2016) Expression of endometrial receptivity genes increase after myomectomy of intramural leiomyomas not distorting the endometrial cavity. Reprod Sci 23:31–41. https://doi.org/10.1177/1933719115612929
Guo Z, Yu Q (2019) Role of mTOR signaling in female reproduction. Front Endo 10(Article 692):1–13. https://doi.org/10.3389/fendo.2019.00692
Ekizceli G, Inan S, Oktem G, Onur E, Ozbilgin K (2017) Assessment of mTOR pathway molecules during implantation in rats. Biotech Histochem 92:450–458. https://doi.org/10.1080/10520295.2017.1350749
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124:471–484. https://doi.org/10.1016/j.cell.2006.01.016
Chen X, He J, Ding Y, Zeng L, Gao R, Cheng S, Liu X, Wang Y (2009) The role of mTOR in mouse uterus during embryo implantation. Reproduction 138:351–356. https://doi.org/10.1530/REP-09-0090
Niknafs B, Shokrzadeh N, Alivand MR, Shariati MBR (2022) The effect of dexamethasone on uterine receptivity, mediated by the ERK1/2-mTOR pathway, and the implantation window: an experimental study. Int J Reprod Biomed 20:47–58. https://doi.org/10.18502/ijrm.v20i1.10408
Makker A, Goel MM, Nigam D, Mahdi AA, Das V, Agarwal A, Pandey A, Gautam A (2018) Aberrant Akt activation during implantation window in infertile women with intramural uterine fibroids. Reprod Sci 25:1243–1253. https://doi.org/10.1177/1933719117737844
Noyes RW, Hertig AT, Rock J (1975) Dating the endometrial biopsy. Am J Obstet Gynecol 122:262–263. https://doi.org/10.1016/s0002-9378(16)33500-1
Sati L, Soygur B, Celik-Ozenci C (2016) Expression of mammalian target of rapamycin and downstream targets in normal and gestational diabetic human term placenta. Reprod Sci 23(3):324–332. https://doi.org/10.1177/1933719115602765
Roos S, Jansson N, Palmberg I, Saljo K, Powell TL, Jansson T (2007) Mammalian target of rapamycin in the human placenta regulates leucine transport and is downregulated in restricted fetal growth. J Physiol 582:449–459. https://doi.org/10.1113/jphysiol.2007.129676
Li S, Chen X, Ding Y, Liu X, Wang Y, He J (2011) Expression of translationally controlled tumor protein (TCTP) in the uterus of mice of early pregnancy and its possible significance during embryo implantation. Hum Reprod 26:2972–2980. https://doi.org/10.1093/humrep/der275
Zeng X, Huang Z, Mao X, Wang J, Wu G (2012) N-carbamylglutamate enhances pregnancy outcome in rats through activation of the PI3K/PKB/mTOR signaling pathway. PLoS One 7:e41192. https://doi.org/10.1371/journal.pone.0041192
Zeng X, Mao X, Huang Z, Wang F, Wu G, Qiao S (2013) Arginine enhances embryo implantation in rats through PI3K/PKB/mTOR/NO signaling pathway during early pregnancy. Reproduction 145:1–7. https://doi.org/10.1530/REP-12-0254
Li R, Wu J, Wang Y, Liu X, Chen X, Tong C, Ding Y, Su Y, Chen W, Zhang C, Gao R (2017) Mice endometrium receptivity in early pregnancy is impaired by maternal hyperinsulinemia. Mol Med Rep 15:2503–2510. https://doi.org/10.3892/mmr.2017.6322
HesamShariati MB, Seghinsara AM, Shokrzadeh N, Niknafs B (2019) The effect of fludrocortisone on the uterine receptivity partially mediated by ERK1/2-mTOR pathway. J Cell Physiol 234:20098–20110. https://doi.org/10.1002/jcp.28609
Daikoku T, Cha J, Sun X, Tranguch S, Xie H, Fujita T, Hirota Y, Lydon J, DeMayo F, Maxson R, Dey SK (2011) Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell 21:1014–1102. https://doi.org/10.1016/j.devcel.2011.09.010
Tanaka Y, Park JH, Tanwar PS, Kaneko-Tarui T, Mittal S, Lee H-J, Teixeira JM (2012) Deletion of Tuberous Sclerosis 1 in somatic cells of the murine reproductive tract causes female infertility. Endocrinology 153:404–416. https://doi.org/10.1210/en.2011-1191
Adhikari D, Zheng W, Shen Y, Gorre N, Ha ̈ma ̈la ̈inen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K (2010) Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet 19:397–410. https://doi.org/10.1093/hmg/ddp483
Lu KH, Wu W, Dave B, Slomovitz BM, Burke TW, Munsell MF, Broaddus RR, Walker CL (2008) Loss of Tuberous Sclerosis Complex-2 function and activation of mammalian target of rapamycin signaling in endometrial carcinoma. Clin Cancer Res 14:2543–2550. https://doi.org/10.1158/1078-0432.CCR-07-0321
Baek MO, Song HI, Han JS, Yoon MS (2018) Differential regulation of mTORC1 and mTORC2 is critical for 8-Br-cAMP-induced decidualization. Exp Mol Med 50:1–11. https://doi.org/10.1038/s12276-018-0165-3
Wang Y, Zhu L, Kuokkanen S, Pollard JW (2015) Activation of protein synthesis in mouse uterine epithelial cells by estradiol 17β is mediated by a PKC-ERK1/2-mTOR signaling pathway. PNAS 112:E1382-1391. https://doi.org/10.1073/pnas.1418973112
Somigliana E, Vercellini P, Daguati R, Pasin R, De Giorgi O, Crosigniani PG (2007) Fibroids and female reproduction: a critical analysis of the evidence. Hum Reprod Update 13:465–476. https://doi.org/10.1093/humupd/dmm013
Surrey ES, Lietz AK, Schoolcraft WB (2001) Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertil Steril 75:405–410. https://doi.org/10.1016/s0015-0282(00)01714-3
Khalaf Y, Ross C, EI-Toukhy T, Hart R, Seed P, Braude P, (2006) The effect of small intramural uterine fibroids on the cumulative outcome of assisted conception. Hum Reprod 21:2640–2644. https://doi.org/10.1093/humrep/del218
Celik O, Koc O, Yurci A, Ersahin A, Celik N, Tanilir F et al (2022) Receptivity-based uterine fibroid surgery: an updated systematic review of the evidence. Clin Exp Obstet Gynecol 49:114–126. https://doi.org/10.31083/j.ceog4905114
Pier B, Crellin C, Katre A, Conner MG, Novak L, Young SL, Arend R (2020) Large, non-cavity distorting intramural leiomyomas decrease inhibitory factor in the secretory phase endometrium. Reprod Sci 27:569–574. https://doi.org/10.1007/s43032-019-00056-x
Acknowledgements
The authors thank Ms. Nidhi Verma and Mr. Kamlesh Sharma for their technical support. The valuable inputs by Dr. Geetanjali Mishra and Dr. Abnish Gautam are acknowledged. We thank all women who participated in the study.
Funding
Department of Biotechnology, Government of India, New Delhi (Grant No. BT/PR5242/MED/30/803/2012).
Author information
Authors and Affiliations
Contributions
AM contributed to the conceptualization, design, experimental works, analysis of results, and drafting of the manuscript; MMG contributed to analysis of results and review and editing of the manuscript; DN contributed to experimental work; IM contributed to analysis of results; and AP contributed to the study material and review and editing of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of King George Medical University, Lucknow, India (ref. code: 70th ECM IIB/P6), for use of human tissue for research and was based on informed patient consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makker, A., Goel, M.M., Nigam, D. et al. mTOR signaling and endometrial receptivity in infertile women with intramural uterine leiomyomas. Middle East Fertil Soc J 28, 13 (2023). https://doi.org/10.1186/s43043-023-00138-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-023-00138-6