Abstract
Acellular dermal matrix (ADM) is derived from natural skin by removing the entire epidermis and the cell components of dermis, but retaining the collagen components of dermis. It can be used as a therapeutic alternative to “gold standard” tissue grafts and has been widely used in many surgical fields, since it possesses affluent predominant physicochemical and biological characteristics that have attracted the attention of researchers. Herein, the basic science of biologics with a focus on ADMs is comprehensively described, the modification principles and technologies of ADM are discussed, and the characteristics of ADMs and the evidence behind their use for a variety of reconstructive and prosthetic purposes are reviewed. In addition, the advances in biomedical applications of ADMs and the common indications for use in reconstructing and repairing wounds, maintaining homeostasis in the filling of a tissue defect, guiding tissue regeneration, and delivering cells via grafts in surgical applications are thoroughly analyzed. This review expectedly promotes and inspires the emergence of natural raw collagen-based materials as an advanced substitute biomaterial to autologous tissue transplantation.
Graphical Abstract

Similar content being viewed by others
1 Introduction
Autografts have long been considered as the “gold standard” material for repairing damaged tissue grafts, since they can achieve good therapeutic effects. Nevertheless, the use of autografts comes with some disadvantages including a second surgical site for donor tissue, delayed healing and potential morbidity in the donor site, post-operative bleeding, and discomfort for the patients. To this end, researchers have long sought better alternatives to autografts. As early as 1985, Hcek et al. [1] used for the first time allogeneic dermis as autologous skin transplantation carrier, and transplanted it into animal models and scab wounds of burn patients, achieving certain effects in wound repair and scar hyperplasia inhibition. Nevertheless, subsequent studies demonstrated that the presence of cellular components in allogeneic tissues can lead to immune rejection of allogeneic skin grafts [2], which gave rise to the research on cell-free allogeneic tissue materials. In the 1990s, Compton et al. [3] successfully prepared acellular dermal matrix (ADM) and applied it to the treatment of burn wounds, achieving good clinical effects. In addition, Livesey et al. [4] applied allogeneic ADM with removed epidermis and cells to repair burn wounds. Initially, ADM was limited to burn medicine/surgery. However, ADM materials have several advantages including good cell affinity, biocompatibility, certain mechanical properties, and an ability to induce cell growth and proliferation. Therefore, they have been widely used in plastic surgery and wound care, as well as in surgical subspecialties including oral, maxillofacial, urogenital, and general/oncology surgery [5].
More specifically, ADM, including allogenic and xenogeneic ADM depending on the source, is prepared from skin, where the entire epidermis and cell components of dermis are removed, while the collagen components and basic tissue structure of dermis are retained [6]. Traditionally, ADM materials are made from a variety of terrestrial animal tissues, such as porcine hide and human skin. In general, the cellular immunity that the cells present in the transplanted tissue may trigger an immune response, leading to the rejection of the skin transplantation. After removing the cellular components with strong immunogenicity, ADMs with collagen as the main component have very low antigenicity and can almost cause no rejection reaction of the host. However, some researchers consider that using terrestrial animal tissues may risk the spread of zoonotic viruses, especially in the current epidemic situation. In addition, residual xenoantigens, such as galactose-α-1 3-galactose (α-Gal) epitopes, may induce immune responses due to the inappropriate process of ADMs. Consequently, fish skin-derived ADMs and collagen have been developed since they do not contain α-Gal antigen and have a low risk for prion and/or virus infection, and they are economical and sustainable source of collagen [7]. However, the main component of fish-derived ADMs is generally less thermally stable and more readily degradable compared to terrestrial animal-derived collagen [8]. Legal aspects and costs affect the choices of ADM, and human ADM remains the most researched type. Porcine ADM (pADM) has been shown to share significant similarities with human ADM regarding the histological structure and biocompatibility and has the advantages of greater availability and lower cost [9].
As a natural extracellular matrix (ECM), ADM has increasingly attracted the attention of biomedical researchers due to its natural abundance, high biocompatibility, and excellence in a variety of biomedical applications [10, 11]. Its three-dimensional (3D) interconnected network structure has a channel diameter of 100–200 μm formed by the collagen bundles that remain after the removal of the epidermis, dermal fibroblasts, and epidermal appendages, and is beneficial for cell growth. It is also worth noting that ADM forms a soft framework structure that can contribute to the toughness and ductility of the scaffold, further enabling the scaffold to undergo deformation and absorb energy after it yields soft tissues, which is not seen in pure extracted collagen.
2 ADM modification
Natural skin tissue has several characteristics that are compatible with the human body, such as adaptive mechanical strength and hydrophilic properties. ADM can be regarded as a collagen scaffold with a 3D network structure, which remains after the natural skin tissue undergoes a series of physical, chemical, and biochemical processes to remove the epidermis and intradermal cells and other components. Therefore, the physicochemical properties of ADM are not exactly the same as those of natural tissues. In particular, ADM tends to have weaker mechanical properties and lower resistance to enzymatic degradation than natural tissues [12, 13]. However, actual application scenarios often require transplant substitutes such as ADM to have similar properties to natural tissues. Thus, in order to better meet the application requirements, the ADM needs to be modified in order to avoid the adverse effects of the decellularization process on its physicochemical properties, or further improve its performance so that it can be applicable to different application scenarios (such as body surface coverage, repair, filling, and in vivo application). Furthermore, the removal of cellular components does not guarantee that the immunogenicity of ADM is completely removed. A certain degree of modification is beneficial to reduce the immune response induced by the material [4]. Consequently, it is necessary to modify the ADM properly.
2.1 Modification method and principle
The modification of ADM can be generally divided into physical methods and chemical methods. Physical modification refers to the use of physical technologies to treat ADM to achieve the desired modification effect. Physical methods commonly include thermal dehydrogenation, ultra-violet (UV) irradiation, drilling, and freeze drying. More specifically, thermal dehydrogenation is the formation of amide bonds or crosslinking through severe dehydration of collagen molecules at high temperature [14]. UV irradiation forms bonds between the nuclei of aromatic residues, such as tyrosine and phenylalanine. Therefore, UV irradiation offers the potential to link protein molecules, and enables the bonding within polypeptide chains [15]. Drilling concerns the artificial formation of aperture structures on the surface or inside the ADM by mechanical or laser means [16]. Freeze drying is a method of drying ADM, which can also be used as a method for micro-adjusting the pore structure of ADM. In general, the degree of physical modification is weak and therefore is not usually used alone.
Chemical methods refer to the use of chemical modifiers to modify the ADM, and usually include the use of materials such as glutaraldehyde (GA), carbodiimide (EDC)/N-Hydroxysuccinimide (NHS), epoxy compounds, nanomaterials, and dialdehyde polysaccharide. The main component of ADM is collagen, which contains many reactive groups such as amino, carboxyl, and hydroxyl groups on its side chains [17, 18]. Studies have shown that the ε-amino group of collagen lysine side chains is the most reactive group in collagen amino acid side chain residues under alkaline conditions [19]. Therefore, ADM can be modified by many chemical modifiers that react with the corresponding amino groups, such as GA, dialdehyde polysaccharide, epoxy compounds, and plant polyphenols. The most classic example is the crosslinking reaction between GA and collagen. It is known that the reaction of amino groups with aldehyde groups is completed in two steps, i.e., nucleophilic addition and β-elimination reaction. First, nucleophilic addition is produced by the lone pair electron attack on the carbonyl group in the amino group, which is followed by proton transfer, and finally, a stable Schiff base is formed by dehydration [20]. Dialdehyde polysaccharides can also form Schiff bases with amino groups on collagen [21]. The presence of charge polarization and epoxy ring tension in the epoxy group on the epoxy compound endows the epoxy group with high reactivity, promoting the reaction with groups containing active hydrogen, such as amino, carboxyl, and sulfhydryl groups. In general, epoxy compounds mainly react with the amino groups on collagen under alkaline and neutral conditions [22]. In addition, among the chemical reactions of collagen carboxyl groups, the most classic is the crosslinking of collagen under the action of EDC/NHS [23]. The principle is that EDC couples first with the carboxyl group to form an O-isoyl urea structure, then combines with NHS to form an intermediate, and finally is attacked by nucleophilic primary ammonia to form amide cross-links; that is, isomeric peptide bonds are formed between adjacent molecules, and the activated intermediate (water-soluble urea derivative) can be removed and cleaned [24, 25]. The enzymes involved in the natural self-crosslinking process of collagen can also be used to modify ADM [26]. Transglutaminase mainly catalyzes the acyl transfer reaction between the γ-carboxamide group (acyl donor) of the glutamine residues and the ε-amino group (acyl acceptor) of the lysine residues in the collagen peptide chains, resulting in the inter- and intra-molecular formation of ε-(γ-glutamyl)-lysine covalent bonds [27, 28]. Lysyl oxidase can catalyze the oxidative deamination of ε-amino lysine residues to produce aldehyde groups, which then react with the amino groups to form Schiff bases to achieve crosslinking [29, 30]. Moreover, plant polyphenols possess the general chemical properties of phenolic compounds and have the ability to bind to proteins; thus, a large number of hydrogen bonds are formed in collagen, realizing the desired modification effect [31]. A combination diagram of several common modifiers with active sites on ADM is illustrated in Fig. 1.
These physical methods and chemical reactions are the cornerstones of the modification process as a whole. During the whole modification process, different types of modification take place under the action of these "cornerstones", which can be divided into physical structure modification, crosslinking, and masking. Physical structure changes generally refer to the microstructure changes caused by the modification of ADM. Crosslinking refers to the formation of links or bridges between active groups in ADM, as well as the reaction between two or more functional groups in the modifier and active sites in ADM, requiring the modifier to be a double or multiple functional group reagents [32]. Furthermore, it also refers to the reaction of active groups in ADM to form links induced by physical or chemical actions. In masking, only one functional group in the modifier reacts with the active groups on ADM [32, 33]. The modifier may be a single, double, or multiple functional reagents. A schematic drawing of the crosslinking and masking modifications is depicted in Fig. 2. For example, epoxy compounds of mono-functional groups can form only epoxidation and react with amine compounds without forming bridges or crosslinks, which is the so-called masking. Only multifunctional epoxy compounds can induce bridging or crosslinking between collagen chains. The Comparisons of different modification technologies are listed in Table.1.
Based on the classification of the above-mentioned modification types, it can be known that different types of modification have different effects. In general, the effects of physical structure modification concern the changes in performance caused by the changes of the physical structure of ADM. Compared to physical structure modification, crosslinking and masking have more apparent effects on the physicochemical properties and biocompatibility of ADM. The crosslinking structure of ADM has a key effect on its physicochemical properties, and the appropriate crosslinking method can effectively regulate the structural stability, mechanical properties, and degradation resistance of ADM materials [34]. Moreover, the chemical groups and molecular structures on the surface of ADM materials also play a key role in their performance, and masking is an important method that can be used to regulate the surface properties of ADM materials [11]. The specific effects of modification on ADM are analyzed in the following sections.
2.2 Effects of modification on physicochemical properties
The chemical bonds, hydrogen bonds, and van der Waals forces within collagen are crucial for ADM ability to maintain the physicochemical properties. In general, the modifications can directly affect the physiochemical properties of ADM. Among them, crosslinking can significantly improve the mechanical properties and degradation resistance. This is because crosslinking can generate intramolecular, intermolecular, and interfibrous crisscross links in the ADM, and can form a linear crosslinked network structure, which can greatly improve the physicochemical properties of ADM, such as the mechanical properties, stability, and degradation resistance. Ma et al. [35] used EDC, oxidized chitosan oligosaccharide (OCOS), and GA to crosslink with the ADM of basa fish. Their results revealed that the three crosslinking agents could improve the mechanical properties, thermal stability, and enzyme degradation resistance of the materials, with GA having the most apparent effect, followed by EDC. The thermal shrinkage temperatures of the GA, EDC, and OCOS crosslinked samples were increased by 20.52, 18.27 and 8.04 °C, respectively, compared to those of the un-crosslinked samples. This is due to that GA has the smallest molecular weight and the strongest reactivity, which can produce the highest crosslinking degree. Nevertheless, GA can cause cytotoxicity and calcification after implantation, which is detrimental to its application. On the other hand, OCOS contributes more to the improvement of material hydrophilicity, since OCOS crosslinking can introduce polar groups that increase hydrophilicity. EDC modification can improve the stability of materials to a certain extent. However, it should be mentioned that, due to the limited active sites available for reaction in ADM, the distance between each point may not be appropriate; thus, the crosslinking effect of EDC is limited, and it needs to be combined with other crosslinking methods to enhance its modification effect. For example, Hu et al. [36] combined thermal dehydrogenation and EDC crosslinking on porcine ADM, and the designed reaction mechanism is shown in Fig. 3. They reported that the thermal denaturation temperature increased from 65.2 °C before modification to 88.3 °C, and the seven-day degradation rate in collagenase solution decreased from 80% to about 29%. Sung et al. [37] compared the enzyme degradation performance of collagen modified by epoxy compounds. They found that the anti-enzyme ability of single-epoxide epoxy compound-treated collagen was similar to that of un-crosslinked collagen, while that of double and multi-epoxide treatments was improved. These differences can be attributed to the different effects of masking and crosslinking. Fu et al. [38] demonstrated the feasibility of incorporating reduced graphene oxide (RGO) nanoparticles to ADM in order to improve the physicochemical characteristics of natural scaffold materials and develop a highly efficient local transplantation system for mesenchymal stem cells (MSCs) in diabetic wound healing. In addition, some physical modifications play a role as well. For instance, Lin et al. [39] studied the changes in the biomechanical properties of laser microporous ADM through stress–strain and stress-relaxation tests on samples with different spacing between pores, and determined that ADM with a spacing of 350 μm had good biomechanical properties.

Adapted from ref. [36], copyright 2013, with permission from the Elsevier)
The diagarm of the mechanism of synergistic effect of EDC and dehydrothermal crosslinking on porcine ADM (PADM). (
2.3 Effect of modification on microstructure
The removal of cells and cellular components from the dermis leaves behind a natural mixture of structural and functional proteins that constitute the ECM, such as collagen, fibronectin, laminin, and vimentin. Consequently, an in vitro environment analogous to the in vivo microenvironment is provided, where cells can adhere, proliferate, and exert their paracrine function [40]. Modifications can change the microstructure of ADM to a certain degree, with physical structure modifications having a great effect on ADM microstructure. Drilling can directly transform the ADM structure and adjust its pore size and porosity, thus improving its permeability. In practical application, drilling can increase the contact area between ADM and cells, providing favorable conditions for cell adhesion and migration into the ADM [41]. Laser micropore ADM grafting in combination with split thickness autografting can improve wound healing, and implantation studies have reported vascular infiltration and slow cell growth [42]. However, collagen and elastic fibers in ADM may suffer some adverse effects during laser drilling. Freeze drying can make the ADM structure looser, and facilitate the growth of cells, as well as the preservation of materials. Furthermore, crosslinking also affects the microstructure. Study has shown that the modification of ADM by oxidized chitosan oligosaccharide may lead to the shortening of the distance between collagen fibers [13]. In addition, chemical crosslinking can stabilize the structure of ADM, which is beneficial to maintaining the pore size and porosity to a certain extent.
2.4 Effect of modification on biocompatibility
The main component of native ADM is collagen, which has good biocompatibility, and there are many sites on collagen that promote cell adhesion, such as GxxʹGExʺ cell-adhesive motifs [43]. In general, modification has a significant effect on ADM biocompatibility, especially the role of chemical modifiers, since chemical modification often involves the change of many groups and possible residues. Modifiers such as GA and formaldehyde are known to adversely affect ADM biocompatibility and their use has been restricted [44]. Epoxy compounds are another category of commonly used chemical modifiers in the field of collagen modification. Materials treated by epoxy compounds have good biological and physical properties; thus, epoxy compounds are considered as acceptable ADM modifiers [45]. Moreover, crosslinking using EDC/NHS is frequently used for collagen-based material stabilization. This process forms ‘zero-length’ amide bonds between carboxylate groups (e.g., on aspartic acid or glutamic acid residues) and adjacent primary amines (e.g., on lysine residues). A major advantage of this treatment is its non-toxicity, since neither EDC nor NHS are incorporated into the material and can be easily removed by washing, making EDC/NHS a widely used crosslinking method for ADM modification [46, 47]. Nevertheless, it is worth mentioning that, although EDC/NHS-mediated ADM self-crosslinking is non-toxic due to the removal of exogenous substances, it may adversely affect the cell adhesion of the material. Studies have indicated that EDC crosslinking requires a large amount of carboxylic acid groups, but the GxxʹGExʺ cell adhesion motif in collagen contains a key carboxylic acid group [43]. These motifs bind to the cell surface integrins, which are a major heterodimer somatic adhesion receptor. The carboxylic acid base group on the E residue of the GxxʹGExʺ motif is essential for the cellular adhesion on collagen [48]. The free carboxylic acid group in the integrin binding motif is the same as the group chemically modified by EDC crosslinking; thus, the carboxylic acid group consumed by crosslinking will have an adverse effect on cell adhesion. Therefore, several studies have combined EDC with other crosslinking methods to reduce carboxyl consumption. As it can be observed in Fig. 4, the integrin-binding GxxʹGExʺ motifs in collagen I have the differential integrin specificity, each with amino acid substitutions at the x position [49]. In recent years, to improve the biocompatibility of modified ADM, many new modifiers have emerged, such as dialdehyde chitosan, dialdehyde sodium alginate, and epoxy chitosan, which have achieved good results. The obtained crosslinked ADM has good biocompatibility and the ability to promote cell adhesion. Zhu et al. [50] modified an acellular porcine dermal matrix with sodium dialdehyde alginate with different oxidation degrees, and the results revealed that, after modification with sodium dialdehyde alginate with appropriate oxidation degrees, the material had good biocompatibility and was conducive to cell adhesion and proliferation.

Adapted from ref. [49], copyright 2019, with permission from the Elsevier)
Schematic representation showing the potential modification of integrin α1 (blue lines) and α2 (pink lines) binding sites on collagen I by EDC (red) or UV (green). The collagen α1(I) chain and binding sites are depicted in orange and the α2(I) chain and binding sites are in blue. + + + + , + + and + above the integrin I-domain indicate strong, moderate and weak collagen binding, respectively. (
2.5 Effect of modification on functionality
The most basic properties that determine if ADM can be used as tissue repair material include resistance to enzyme degradation, porosity, surface properties, and mechanical properties. However, in many application scenarios, ADM is required to have a variety of functions to better meet the service requirements.
ADM materials are often used to promote wound healing. In general, the wound surface is warm and moist, which make it very conducive to bacterial reproduction. Therefore, it is of high importance to inhibit the growth and reproduction of bacteria. Modification of ADM materials to improve their antibacterial performance has been one of the research hotspots in this field for a long time. ADM materials are often endowed with antimicrobial properties by being combined with antimicrobial agents. Commonly used antibacterial agents include antibiotics, chemical antibacterial agents (e.g., quaternary ammonium salt), metal and nano-metal materials, and natural antibacterial agents (e.g., chitosan). Cai et al. [51] developed an ADM hydrogel combined with vancomycin, which is an antimicrobial agent used to induce hemostasis, expedite antimicrobial activity, and promote tissue repair. In vivo antibacterial and hemostatic experiments on a rat model indicated that a vancomycin-loaded ADM hydrogel could promote the healing of infected tissue and the effective control of hemorrhage. Chen et al. [52] crosslinked ADM with oxidized chitosan oligosaccharide and prepared silver nano-particles in situ with aldehyde groups that were not fully reacted (Fig. 5). The obtained ADM material exhibited good antibacterial effects against Escherichia coli and Staphylococcus aureus and achieved a good repair effect on full-thickness skin defects of a rat model. Moreover, chitosan and its derivatives have antibacterial and good biological properties; thus, they have been widely used in the field of collagen modification. Liu et al. [53] prepared dialdehyde chitosan with obvious antibacterial effects on Escherichia coli and Staphylococcus aureus, which was successfully used to crosslink collagen. Chen et al. [54] prepared oxidized quaternary ammonium chitosan salt and applied it to the modification of ADM, exhibiting good physicochemical and antibacterial properties.

Adapted from ref. [52], copyright 2019, with permission from the Elsevier)
Schematic outline of the process of in situ synthesis of silver nano particles on oxidized chitosan oligosaccharide cross-linked porcine ADM scaffold. (
In clinical use, the effect of tissue repair is closely related to the degree of material vascularization. Only vascularization can provide sufficient oxygen and nutrients to tissues, improving the chances of survival, and can ensure their functional status after survival. Studies have demonstrated that, when dermal substitutes are used for skin repair, the slow vascularization after transplantation can lead to poor epidermal cell activity, easy peeling and necrosis of the epidermal layer, and low survival rate of the transplant [55]. In terms of structure, the thickness, porosity, and pore size of ADM materials affect vascularization. It is generally believed that, the thinner the tissue, the higher its permeability, and the easier the vascularization. Zhang et al. reported that aged ADM is more porous than young ADM, has superior recellularization, revascularization, and immunogenicity properties, and thus, it may be a more favorable tissue engineering scaffold [56]. Pruitt et al. [57]. suggested that endothelial cells, lymphocytes, and growth factors of surrounding tissues can successfully penetrate into the ADM when the pore size is greater than 80 μm, thus achieving vascularization. Appropriate porosity and pore size are conducive to the infiltration of tissue fluids and cells; thus, the appropriate modification of the ADM pore size is conducive to its vascularization. Chai et al. used a laser punch to generate uniformly distributed micropores on porcine ADM, which facilitated the passage of tissue fluids and the penetration of newly formed microvessels. Therefore, ADM can be used advantageously in promoting the wound healing rate and reducing the contraction rate. However, the heat produced by the laser beam can cause certain damage at the periphery of the micropores [42]. In terms of ADM composition, substances that can promote vascularization include growth factors [58], stem cells [59], endothelial cells [60], and exosomes [61]. Song et al. [62] constructed an ADM modified with vascular endothelial growth factor (VEGF)-loaded multi-walled carbon nanotubes (MWNTs), which were able to carry VEGF to cells or tissues. The composite material with 3% MWNTs exhibited lower cytotoxicity and an appropriate release performance, which prompted faster vascularization of the ADM than other scaffolds. Zhu et al. [40] indicated that ADM can provide a suitable microenvironment for adipose-derived stem cells (ADSCs), and in vivo implanted ratADSC-pADM exhibited improved vascularization and mitigated inflammatory response compared to untreated pADM. Van Eps et al. [63] demonstrated that the amniotic fluid allograft treatment of ADM caused enhanced vascularization and cellularization, which was reflected by increased histomorphometric scores at 14 days.
Calcification is a commonly met problem when ADM materials are used as implant material. The deposition of calcium phosphate in biological tissues is called calcification. For instance, GA is a commonly used crosslinking agent for collagenous biomaterials which contains two active aldehyde groups. Although it can stabilize and modify structures with high-phosphorus content on the biomaterials, these may become calcification sites when exposed to ECMs with high calcium content [64]. In order to prepare ideal anti-calcification biomaterials, there are certain research directions that are followed, including blockage of residual aldehyde groups, use of non-aldehyde crosslinking agents, use of ion competition inhibition, and locally-controlled drug delivery.
Natural and artificial ADM scaffolds with spatially organized collagen fibers can provide a suitable architecture and environment for cell attachment and proliferation. To expand their biomedical applications, researchers have also aimed at the functional modification of ADM materials in order to be used for controlled drug delivery. Chu et al. [65] used polyethylene glycol (PEG) and graphene oxide (GO) to prepare a novel nanocarrier, namely PEGylated GO(GO-PEG), with high solubility and superior biocompatibility for biological applications as a drug carrier. Subsequently, they synthesized GO-PEG-mediated quercetin (GO-PEG/Que) as a precursor, and then assembled it with ADM to produce an ADM-GO-PEG/Que hybrid scaffold. The latter could enhance the hydrophilicity and controllability of the delivery of hydrophobic drugs and serve as a powerful drug delivery system for developing stem cell-based therapies, tissue engineering, and regenerative medicine.
3 Characteristics and application mechanism of ADM
An ideal dermal replacement should include properties such as tensile structural strength, contain natural substrates such as proteoglycans and laminin that promote wound healing, produce a noto-minimal immune response, and have the ability to integrate into native tissue. Most of these characteristics are included in ADMs. More specifically, advantages of dermal substitutes include low immunogenicity after application, temporary or permanent coverage of the skin and soft tissue, elasticity and pliability to contour any tissue defects, minimal application time in the operation room, and minimal patient morbidity, since no harvest sites are required [66].
It is well known that ADM materials have many conscience interactions with the host during their application. The engineered 3D reticulate structure allows ADMs to function as a “sustainer” to promote epidermis regeneration and revascularization, and thus reduce the risk of infection. In addition, multiple studies have confirmed that ADM materials are capable of recellularization, revascularization, and active remodeling. A study supports the use of human ADM as a hospitable scaffold for hosting cell infiltration and remodeling. Regardless of the patient age or time after implantation, all grafts have shown evidence of cellular infiltration, neovascularization, and active remodeling. The results have also suggested that there is a relationship between implant duration and the extent of recellularization, as well as that human ADM materials can be successfully incorporated into host tissue when used in superior capsule reconstruction [67]. For example, Chu et al. [68] grafted MSCs onto an ADM scaffold to treat diabetic cutaneous wounds, and found that the MSC-ADM treatment significantly increased angiogenesis and rapidly completed the re-epithelialization of newly formed skin on diabetic mice. Furthermore, type-I collagen fiber degradation in ADM and new type-I collagen fiber synthesis in diabetic wound sites treated with MSC-ADM during the first 7 days of wound healing were intravitally and dynamically monitored by second harmonic generation imaging. Moreover, a research revealed that porcine ADM can effectively promote wound healing and scar formation through epidermal stem cells (ESCs), and this process is related to the alteration of the internal miRNA levels [69]. In addition, the regulatory function of porcine ADM treatment on miRNAs in ESCs has been investigated. For instance, Chen et al. [70] reported that the treatment of porcine ADM reduced the levels of miR-124-3p.1 and miR-139-5p in wounds. In particular, MiR-124-3p.1 and miR-139-5p inhibited the expression of JAG1 and Notch1, respectively, by directly targeting the miRNAs in ESCs. That is to say, the porcine ADM induced the down-regulation of miR-124-3p.1/139-5p in wounds and the up-regulation of JAG1/Notch1 in ESCs, thus promoting the cutaneous wound healing.
4 Biomedical applications of ADM
ADMs have good biocompatibility and biodegradability, similar to other protein materials, natural polyesters, and polymers [71,72,73], ADM materials have been widely used to promote wound healing and reconstruct damaged human tissues due to their excellent biocompatibility and tissue regeneration properties. In general, ADM materials can be directly used as wound coverings, soft tissue repair materials, tissue filling materials, etc. in the fields of skin wound healing, otolaryngology, stomatology, abdominal repair, plastic surgery, and so on. The common clinical indications of ADMs are showed in Table2. In recent years, several novel applications of ADMs have been developed, and ADM materials can also be used as the raw material for the construction of tissue repair materials. Currently, there is extensive commercialization and successful application of ADM products, such as Alloderm™ (LifeCell), AlloMax™ (CR Bard Davol), FlexHD™ (Ethicon), Permacol™ (Covidien), SurgiMend™ (TEI bioscience), Strattice™ (LifeCell), and XenMatrix™ (CR Bard Davol).
4.1 Applications in skin wound repair and dermatology
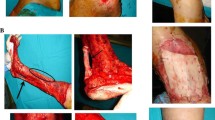
ADM materials have been used in soft tissue reconstructions since 1995, and were initially applied for the treatment of burns. Until now, several efforts have been made to realize skin regeneration in the healing of deep burn wounds. Relevant studies have demonstrated that ADM derived from allogeneic or heterogeneous skin as a natural promising dermal scaffold can be applied in clinical tissue repair. They have reported that ADMs can provide a proper scaffold for cell colonization, which can be favorably combined with wound site edges. ADM materials have been shown to promote cell migration, proliferation, and vascularization, and therefore accelerate wound healing [74]. Until now, many commercial ADM dressings have been successfully applied in clinical practice with good therapeutic effects and have become a routine treatment for burns and other skin wounds. Figure 6 demonstrates the processes and effects of a human ADM (hADM) used to repair a post-traumatic deep wound at a plantar site with satisfactory results [75].

Adapted from ref. [75], copyright 2021, with permission from the Wiley)
(A) A 43-year-old patient presented to the first-aid with a post-traumatic deep wound at plantar site; (B) a rotation flap was realized at first. Then the patient was admitted to the ambulatory setting: a surgical and enzymatic debridment was realized in ambulatory setting of the residual deep defect; (C) a double-layer grafting was realized: a thick hADM graft (800 μm) (ie, deep dermis) was positioned in the bottom of the wound bed, along with an overlying deep-frozen thick patch (600 μm), fixed with steril strips, and covered with a paraffin gauze and a povidoneiodine gauze; (D) longitudinal incisions were performed over the grafts before application; the dressing was changed every 7 days. After 3 weeks, the previous hADMs grafts were completely integrated into the wound bed, and a new thin hADM meshed 3:1 was applied with steril strips; (E) covered by hyaluron gauzes changed every 72 h; (F) Final closure was achieved after further 4 weeks. (
Autografting is often impossible in patients with large burns, and is often associated with donor-site complications in terms of aesthetic results and possible infections. Therefore, ADM materials represent a precious source of dermis, necessary to restore the lost barrier function. Chen et al. reported that, after using DermACELL®, a lower degree of contractures and cicatricial hyperplasia commonly seen in deep burns was observed [76].
In addition, diabetic foot ulcer (DFU) is a chronic, refractory disease in need of multidisciplinary endeavor, and ADM materials have been adopted to address this annoying issue. A systematic review and meta-analysis showed that [77], compared with the standard of care, ADMs may accelerate the healing speed of uninfected, non-ischemic, and full-thickness DFU. ADMs have exhibited superiority compared to the standard of care alone, while generating no additional complications. Furthermore, the Integra® Dermal Regeneration Template was used in a clinical study to treat DFU, and the analysis demonstrated that the key biomarkers of the wound healing process were activated at a gene-expression level, while the synthesis of collagen I, collagen III, and elastin was also promoted and modulated within the 28-day observation period [78].
Moreover, an ADM scaffold with a uniformly aligned 3D nanofibrous structure was used for the targeted delivery of MSCs in order to repair full-thickness cutaneous defects, which is another significant application aspect. In the study by Qi [79], denatured ADM seeded with bone marrow MSCs (BM-MSCs) was implanted into murine cutaneous wounds, and promoted wound healing in terms of angiogenesis, re-epithelialization, and skin appendage regeneration. In a case report [80], ADM combined with amniotic stem cells was used to treat skin burns, and the patient was discharged from the hospital on the 12th day after surgery with complete wound healing. Moreover, micronized ADM has also been used as a cell culture substrate to expand human fibroblasts and as a cell transplantation vehicle for skin regeneration [81].
4.2 Applications in otorhinolaryngology and maxilla-facial surgery
In the field of otorhinolaryngology and head and neck surgery, ADMs have been mainly used to repair tympanic membrane perforation, nasal septum perforation, skull base defects, and head and face soft tissue defects [82]. In addition, ADM allografts are safe and effective adjuncts that can be used in the closure of oronasal fistulae in hard palates greater than 15 mm in diameter [83]. Moreover, ADMs have also been commonly utilized as a viable alternative for soft tissue augmentation in facial reconstruction. A large number of patients with cutaneous malignancies in areas involving the midface, nasal sidewall, and nasal alae are commonly left with a significant defect, which concerns the concomitant loss of volume following wide local excision. In addition to providing a scaffold for wound healing, ADMs, such as AlloDerm, have been shown to effectively restore the volume after full-thickness excisions in the midface and nasal alae [66].
4.3 Applications in stomatology
After their successful application in the field of burn wounds, ADMs have gradually gained popularity in the repair of oral soft tissues, including clinical soft tissue defects caused by tumors, mucosal diseases, trauma, fistula repair, and pre-denture surgery. Over the years, autogenous soft-tissue grafts, i.e., subepithelial connective tissue graft (sCTGs) have been considered as the gold standard for peri-implant soft tissue augmentation. Panwar et al. concluded that the immediate implant placement with soft tissue augmentation, both with ADMs and sCTGs, is a safe, viable, and predictable method. Although the aforementioned study achieved favorable results in the sCTG group compared to the ADM group, the use of ADMs can still be considered, especially when the patient is not consenting for the use of a sCTG or there is not plenty palatal tissue and in the case of patients with coagulopathies. Moreover, a 36-case research on the application of ADMs in the reconstruction of oral mucosal defects demonstrated that ADM grafts were safe and effective [84]. Furthermore, several studies has used ADMs for root coverage treatments and soft tissue augmentation. It has been demonstrated that ADMs can enhance keratinized tissue, especially while treating challenging cases which include thinner palatal tissues, multiple teeth recession, restricted period of treatment, and individuals with a decreased pain threshold [85]. As a barrier membrane, ADM has also been used for various purposes including the increase of the attached gingiva [86] and guided bone regeneration [87]. In a clinical case, acellular dermal matrix was used for soft tissue augmentation in a maxillary dental implant lacking buccal bone, and good results were obtained [88] (Fig. 7).

Adapted from ref. [88], copyright 2020, with permission from the Wiley)
Acellular dermal matrix used for soft tissue augmentation in a maxillary dental implant lacking buccal bone. (A) Clinical scenario before bone augmentation; (B) 6 months after guided bone regeneration; (C and D) soft tissue augmentation by using an acellular dermal matrix; (E) flap closure; (F) 5 year recall showing the stability of the obtained soft tissue volume. (
4.4 Applications in abdominal/chest wall repair
The integrity of the soft tissue of abdominal or chest walls might be lost after trauma, herniation, or tumor resection. It remains a significant challenge for surgeons to repair or reconstruct such forms of soft tissue defects. Due to its unique structure and excellent biological properties, such as the ability to support vascular ingrowth and incorporate native tissues, ADM is nowadays commonly used to repair tissue defects in cases when the available autogenous musculofascial tissue is not adequate for tension-free closure [89]. This approach has significantly improved the success rates of such operations and has reduced the incidence of wound complications [90, 91]. After analyzing patients who had at least 36 months of postoperative follow-up, a report revealed that ADMs can provide durable long-term outcomes in complex abdominal wall reconstruction with hernia recurrence rates similar to those for synthetic meshes, and achieve lower infection and mesh explantation rates than those reported for synthetic meshes [92]. Moreover, relevant studies have shown that ADM can reduce complications for patients with multiple comorbidities and for those with abdominal wall reconstructions in contaminated wound environments [93, 94]. In addition, based on a similar principle of ADM application in abdominal wall reconstruction, ADM has also exhibited good application outcomes in chest wall repair. A study investigated 146 patients [95 with synthetic mesh (65.1%) and 51 with ADM (34.9%)] with oncologic defects who underwent resection and chest wall reconstruction. The study concluded that the ADM patients experienced fewer surgical-site complications than the synthetic mesh ones. Therefore, surgeons should consider selectively using ADMs for chest wall reconstruction, particularly in patients with higher risk for surgical-site complications [95]. A clinical study has shown a complicated abdominal hernia repair with Strattice®, a non-crosslinked porcine acellular dermal matrix [96] (Fig. 8).

Adapted from ref. [96], copyright 2017, with permission from the Elsevier)
Case: abdominal reconstruction of gastroschisis, complicated by infection and failure of previous repairs. (a) Repair breakdown; (b) mesh removal and debridement; (c) right component separation; (d) left component separation; (e) Strattice® inlay; (f) primary fascial closure; (g) wound closure; (h) 5-month follow-up without evidence of hernia recurrence. Impending extrusion of permanent fascial sutures is evidence, however. (
4.5 Applications in plastic surgery
Since plastic surgery often involves the reformation, filling, or restoration of skin and other soft tissues, ADM materials have also been widely used in plastic surgery due to their unique advantages in soft tissue repair. A report from the American Society of Plastic Surgeons states that ADM was used in > 60% of all breast reconstructions performed in the USA in 2018 [97]. Immediate direct-to-implant breast reconstruction with ADM is the method of choice for many plastic surgeons and patients. Among the reported benefits of ADM are the reduced risk of implant exposure and migration, a more satisfying definition of the inframammary fold, and reduction of the postoperative pain through the increase of the implant-pocket volume and the elimination of the need for coverage from surrounding muscles or fascia [98]. A study reported the acceptable rates of reoperations and implant loss of ADM after investigating the complications, reconstructive failure, and possible risk factors in direct-to-implant breast reconstruction with ADM (primarily Strattice™) [97]. Moreover, breast reconstruction using ADM for the complete coverage of implants has been shown to reduce such complications and improve the aesthetic outcomes [99]. In addition, ADMs have also been used as a common filler in a variety of areas such as nose, vaginal, penile girth, and urethral plastic surgery.
4.6 Applications as raw material
While ADM has many excellent biological characteristics, its form is limited to the inherent mode and its design cannot be further improved. In order to better expand its application range based on its excellent biological characteristics, researchers have focused on its application as a raw material for the construction of biological materials. In recent years, ADM has also been used to develop ECM hydrogels, as a substrate for 3D printing, as a raw material for electrospinning, as well as to extract collagen and collagen fibers. Jin et al. fabricated ADM and gelatin methacrylamide (GelMA) bioinks, and their results demonstrated that ADM preserved the main ECM components of the skin and GelMA had tunable mechanical properties. Based on these bioinks, a 3D bioprinting functional skin model was developed, which could not only promote in vitro cell viability and proliferation, but also accelerate wound healing and re-epithelization, stimulate dermal ECM secretion and angiogenesis, improve the wound healing quality in a moist microenvironment, and support epidermis reconstruction [100]. For an abdominal-wall repair application, Yang, et al. mixed a modified solution of ADM powder and silk fibroin (SF) and constructed an antiadhesion isolation layer on the abdominal cavity sides of polypropylene (PP) meshes through electrostatic spinning. The results in rat models demonstrated that the ADM/SF-PP meshes could effectively reduce the inflammatory response at the contact surface between the meshes and the abdominal organs, and quickly promote the regeneration of the abdominal surface tissue, preventing and reducing abdominal adhesion and supporting the restoration of the abdominal wall [101]. Liu et al. prepared an advanced collagen aggregate from porcine ADM, and demonstrated that it could serve as a better alternative source of collagen for further application in the food and biomedicine industries [102].
5 Conclusions
ADM is derived from the natural ECM and has inherent biocompatibility, suitable mechanical properties, and controllable non-toxic degradability. As an alternative to “gold standard” tissue grafts, ADM scaffolds can be favorably used in the reconstruction and repair of wounds, and they can maintain the homeostasis in the filling of tissue defects, guide tissue regeneration, and deliver cells via grafts in surgical applications. Nevertheless, there is still a lot of work to be done before ADMs can be extensively accepted by doctors and patients. The long-term immunomodulatory molecular interaction mechanisms and cascade reactions between ADMs and the body need to be further clarified. The full conversion process of ADMs in humans needs also to be better defined. In addition, functional ADMs need to be further developed and industrialized in order to achieve better application results than ordinary ADMs. The use of certain technologies developed in recent years [103,104,105,106] in combination with ADMs may also improve its performance and broaden its range of applications. It is strongly believed that in the near future, with the development of ADM research, perfect repair materials can be obtained able to reproduce the effects of autologous tissue transplantation.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
06 May 2023
A Correction to this paper has been published: https://doi.org/10.1186/s42825-023-00121-x
References
Heck EL, Bergstresser PR, Baxter CR. Composite skin graft: frozen dermal allografts support the engraftment and expansion of autologous epidermis. J Trauma Acute Care Surg. 1985;25(2):106–12.
Sedmak DD, Orosz CG. The role of vascular endothelial cells in transplantation. Arch Pathol Lab Med. 1991;115(3):260–5.
Compton CC, Hickerson W, Nadire K, Press W. Acceleration of skin regeneration from cultured epithelial autografts by transplantation to homograft dermis. J Burn Care Rehabil. 1993;14(6):653–62.
Livesey SA, Herndon DN, Hollyoak MA, Atkinson YH, Nag A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation. 1995;60(1):1–9.
Kirsner RS, Bohn G, Driver VR, Mills JL Sr, Nanney LB, Williams ML, Wu SC. Human acellular dermal wound matrix: evidence and experience. Int Wound J. 2015;12(6):646–54.
Parmaksiz M, Dogan A, Odabas S, Elcin AE, Elcin YM. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed Mater. 2016;11(2):022003.
Xu N, Peng XL, Li HR, Liu JX, Cheng JS, Qi XY, Ye SJ, Gong HL, Zhao XH, Yu J, Xu G, Wei DX. Marine-derived collagen as biomaterials for human health. Front Nutr. 2021;8: 702108.
Li D, Sun WQ, Wang T, Gao Y, Wu J, Xie Z, Zhao J, He C, Zhu M, Zhang S, Wang P, Mo X. Evaluation of a novel tilapia-skin acellular dermis matrix rationally processed for enhanced wound healing. Mater Sci Eng C Mater Biol Appl. 2021;127: 112202.
Ge L, Zheng S, Wei H. Comparison of histological structure and biocompatibility between human acellular dermal matrix (ADM) and porcine ADM. Burns. 2009;35(1):46–50.
Tang LL, Liu H, Wang YL, Xian CY, Su AH. Evaluation of the biocompatibility of acellular porcine dermis. Colloids Surf B Biointerfaces. 2007;57(2):215–8.
Hu Y, Dan W, Xiong S, Kang Y, Dhinakar A, Wu J, Gu Z. Development of collagen/polydopamine complexed matrix as mechanically enhanced and highly biocompatible semi-natural tissue engineering scaffold. Acta Biomater. 2017;47:135–48.
Zheng X, Chen Y, Dan N, Dan W, Li Z. Highly stable collagen scaffolds crosslinked with an epoxidized natural polysaccharide for wound healing. Int J Biol Macromol. 2021;182:1994–2002.
Chen Y, Dan N, Wang L, Liu X, Dan W. Study on the cross-linking effect of a natural derived oxidized chitosan oligosaccharide on the porcine acellular dermal matrix. RSC Adv. 2016;6(44):38052–63.
Keogh MB, O’Brien FJ, Daly JS. Substrate stiffness and contractile behaviour modulate the functional maturation of osteoblasts on a collagen-GAG scaffold. Acta Biomater. 2010;6(11):4305–13.
Davidenko N, Bax DV, Schuster CF, Farndale RW, Hamaia SW, Best SM, Cameron RE. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J Mater Sci Mater Med. 2016;27(1):14.
Zhang Y, Zeng Y, Xin G, Zou L, Ding Y, Duyin J. Biological function evaluation and effects of laser micro-pore burn-denatured acellular dermal matrix. Burns. 2018;44(2):350–8.
Liu X, Zheng C, Luo X, Wang X, Jiang H. Recent advances of collagen-based biomaterials: multi-hierarchical structure, modification and biomedical applications. Mater Sci Eng C Mater Biol Appl. 2019;99:1509–22.
Dan W, Chen Y, Dan N, Zheng X, Wang L, Yang C, Huang Y, Liu X, Hu Y. Multi-level collagen aggregates and their applications in biomedical applications. Int J Polym Anal Charact. 2019;24(8):667–83.
Tu R, Shen SH, Lin D, Hata C, Thyagarajan K, Noishiki Y, Quijano RC. Fixation of bioprosthetic tissues with monofunctional and multifunctional polyepoxy compounds. J Biomed Mater Res. 1994;28(6):677–84.
Olde Damink LHH, Dijkstra PJ, Van Luyn MJA, Van Wachem PB, Nieuwenhuis P, Feijen J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J Mater Sci - Mater Med. 1995;6(8):460–72.
Xu Y, Li L, Yu X, Gu Z, Zhang X. Feasibility study of a novel crosslinking reagent (alginate dialdehyde) for biological tissue fixation. Carbohyd Polym. 2012;87(2):1589–95.
Di Y, Heath RJ. Collagen stabilization and modification using a polyepoxide, triglycidyl isocyanurate. Polym Degrad Stab. 2009;94(10):1684–92.
Olde Damink LHH, Dijkstra PJ, van Luyn MJA, van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17(8):765–73.
Nakajima N, Ikada Y. Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjug Chem. 1995;6(1):123–30.
Davidenko N, Schuster CF, Bax DV, Raynal N, Farndale RW, Best SM, Cameron RE. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015;25:131–42.
Eyre DR, Weis M, Rai J. Analyses of lysine aldehyde cross-linking in collagen reveal that the mature cross-link histidinohydroxylysinonorleucine is an artifact. J Biol Chem. 2019;294(16):6578–90.
Nair M, Johal RK, Hamaia SW, Best SM, Cameron RE. Tunable bioactivity and mechanics of collagen-based tissue engineering constructs: a comparison of EDC-NHS, genipin and TG2 crosslinkers. Biomaterials. 2020;254: 120109.
Jiang H, Zheng M, Liu X, Zhang S, Wang X, Chen Y, Hou M, Zhu J. Feasibility study of tissue transglutaminase for self-catalytic cross-linking of self-assembled collagen fibril hydrogel and its promising application in wound healing promotion. ACS Omega. 2019;4(7):12606–15.
Athens AA, Makris EA, Hu JC. Induced collagen cross-links enhance cartilage integration. PLoS One. 2013;8(4):e60719.
Lei Y, Xia Y, Wang Y. The tropoelastin and lysyl oxidase treatments increased the content of insoluble elastin in bioprosthetic heart valves. J Biomater Appl. 2018;33(5):637–46.
Liu T, Shi L, Gu Z, Dan W, Dan N. A novel combined polyphenol-aldehyde crosslinking of collagen film—applications in biomedical materials. Int J Biol Macromol. 2017;101:889–95.
Sung HW, Hsu C-S, Lee Y-S, Lin D-S. Crosslinking characteristics of an epoxy-fixed porcine tendon: effects of pH, temperature, and fixative concentration. J Biomed Mater Res. 1996;31(4):511–8.
Chen Y, Dan N, Huang Y, Yang C, Dan W, Liang Y. Insights into the interactions between collagen and a naturally derived crosslinker, oxidized chitosan oligosaccharide. J Appl Polym Sci. 2019;137(12):48489.
Chen Y, Dan N, Dan W. A review on the modification and application of acellular dermal matrix. Mater Rev. 2018;32(13):186–94.
Ma P, Wang Y, Li B, Hou H. Cross-linking effects of carbodiimide, oxidized chitosan oligosaccharide and glutaraldehyde on acellular dermal matrix of basa fish (Pangasius bocourti). Int J Biol Macromol. 2020;164:677–86.
Hu Y, Liu L, Dan W, Dan N, Gu Z, Yu X. Synergistic effect of carbodiimide and dehydrothermal crosslinking on acellular dermal matrix. Int J Biol Macromol. 2013;55:221–30.
Sung H-W, Hsu C-S, Wang S-P, Hsu H-L. Degradation potential of biological tissues fixed with various fixatives an in vitro study. J Biomed Mater Res B Appl Biomater. 1997;35(2):147–55.
Fu J, Zhang Y, Chu J, Wang X, Yan W, Zhang Q, Liu H. Reduced graphene oxide incorporated acellular dermal composite scaffold enables efficient local delivery of mesenchymal stem cells for accelerating diabetic wound healing. ACS Biomater Sci Eng. 2019;5(8):4054–66.
Lin H, Li W, Yan X. The biomechanical test on the laser microcavity acellular dermal matrix. Chin J Aesthet Med. 2010;19(5):694–6.
Zhu Z, Yuan ZQ, Huang C, Jin R, Sun D, Yang J, Luo XS. Pre-culture of adipose-derived stem cells and heterologous acellular dermal matrix: paracrine functions promote post-implantation neovascularization and attenuate inflammatory response. Biomed Mater. 2019;14(3):035002.
Zhu Z, Yuan ZQ, Huang C, Jin R, Sun D, Yang J, Luo XS. Construction of a dermis-fat composite in vivo: optimizing heterogeneous acellular dermal matrix with in vitro pretreatment. Jf Tissue Eng Regen Med. 2020;14(2):215–28.
Chai JK, Liang LM, Yang HM, Feng R, Yin HN, Li FY, Sheng ZY. Preparation of laser micropore porcine acellular dermal matrix for skin graft: an experimental study. Burns. 2007;33(6):719–25.
Farndale RW, Lisman T, Bihan D, Hamaia S, Smerling CS, Pugh N, Konitsiotis A, Leitinger B, de Groot PG, Jarvis GE, Raynal N. Cell–collagen interactions: the use of peptide toolkits to investigate collagen–receptor interactions. Biochem Soc Trans. 2008;36(2):241–50.
Xu Y, Li L, Wang H, Yu X, Gu Z, Huang C, Peng H. In vitro cytocompatibility evaluation of alginate dialdehyde for biological tissue fixation. Carbohydr Polym. 2013;92(1):448–54.
Chen Y, Dan N, Dan W, Yu G. Supercritical CO 2 fluid-assisted cross-linking of porcine acellular dermal matrix by ethylene glycol diglycidyl ether. J CO2 Util. 2018;25:264–74.
Davidenko N, Campbell JJ, Thian ES, Watson CJ, Cameron RE. Collagen-hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010;6(10):3957–68.
Pieper JS, Hafmans T, Veerkamp JH, van Kuppevelt TH. Development of tailor-made collagen–glycosaminoglycan matrices: EDC/NHS crosslinking, and ultrastructural aspects. Biomaterials. 2000;21(6):581–93.
Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin α2β1. Cell. 2000;101(1):47–56.
Bax DV, Davidenko N, Hamaia SW, Farndale RW, Best SM, Cameron RE. Impact of UV- and carbodiimide-based crosslinking on the integrin-binding properties of collagen-based materials. Acta Biomater. 2019;100:280–91.
Zhu S, Gu Z, Hu Y, Dan W, Xiong S. Evaluation of alginate dialdehyde as a suitable crosslinker on modifying porcine acellular dermal matrix: the aggregation of collagenous fibers. J Appl Polym Sci. 2016;133(25):43550.
Cai D, Chen S, Wu B, Chen J, Tao D, Li Z, Dong Q, Zou Y, Chen Y, Bi C, Zu D, Lu L, Fang B. Construction of multifunctional porcine acellular dermal matrix hydrogel blended with vancomycin for hemorrhage control, antibacterial action, and tissue repair in infected trauma wounds. Mater Today Bio. 2021;12: 100127.
Chen Y, Dan N, Dan W, Liu X, Cong L. A novel antibacterial acellular porcine dermal matrix cross-linked with oxidized chitosan oligosaccharide and modified by in situ synthesis of silver nanoparticles for wound healing applications. Mater Sci Eng C Mater Biol Appl. 2019;94:1020–36.
Liu X, Dan N, Dan W, Gong J. Feasibility study of the natural derived chitosan dialdehyde for chemical modification of collagen. Int J Biol Macromol. 2016;82:989–97.
Chen Y, Dan N, Huang Y, Bai Z, Yang C, Dan W, Cong L. Functional chemical modification of a porcine acellular dermal matrix with a modified naturally derived polysaccharide crosslinker. J Appl Polym Sci. 2019;136(21):47633.
Wang X-C, Li C, Shan F, Wang W-T, Zhu X-G, Jiang D-Y. Experimental study on the recycling of denatured acellular dermal matrix after burn. Zhonghua Shao Shang Za Zhi. 2012;28(3):201–6.
Zhang AJ, Chen FF, Jiang T, Tao CB, Li XY, Jin PS, Li Q. Acellular dermal matrix from different ages for tissue engineering scaffold: aged prior to young. J Biomater Tissue Eng. 2016;6(9):706–12.
Pruitt BA, Jr, Levine NS, Characteristics and uses of biologic dressings and skin substitutes. Archiv Surg 1984;119(3):312–322.
Zhang XR, Liu DW, Guo GH, Peng Y. Construction of tissue engineered skin with VEGF-modified human mesenchymal stem cells and acellular dermal matrix. Mater Sci Forum. 2009;610–613:1298–301.
Doornaert M, Depypere B, Creytens D, Declercq H, Taminau J, Lemeire K, Monstrey S, Berx G, Blondeel P. Human decellularized dermal matrix seeded with adipose-derived stem cells enhances wound healing in a murine model: experimental study. Ann Med Surg (Lond). 2019;46:4–11.
Zhang X, Yang J, Li Y, Liu S, Long K, Zhao Q, Zhang Y, Deng Z, Jin Y. Functional neovascularization in tissue engineering with porcine acellular dermal matrix and human umbilical vein endothelial cells. Tissue Eng Part C Methods. 2011;17(4):423–33.
Xiao S, Xiao C, Miao Y, Wang J, Chen R, Fan Z, Hu Z. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Ther. 2021;12(1):255.
Song Z, Yang Z, Yang J, Liu Z, Peng Z, Tang R, Gu Y. Repair of abdominal wall defects in vitro and in vivo using VEGF sustained-release multi-walled carbon nanotubes (MWNT) composite scaffolds. PLoS One. 2013;8(5):e64358.
Van Eps JL, Boada C, Scherba JC, Zavlin D, Arrighetti N, Shi A, Wang X, Tasciotti E, Buell JF, Ellsworth WAT, Bonville DJ, Fernandez-Moure JS. Amniotic fluid allograft enhances the host response to ventral hernia repair using acellular dermal matrix. J Tissue Eng Regen Med. 2021:15:1092-1104.
Yang L, Huang X, Deng L, Ma X, Jiang H, Ning Q, Liang Z, Lei Y, Wang Y. Pre-mounted dry TAVI valve with improved endothelialization potential using REDV-loaded PEGMA hydrogel hybrid pericardium. J Mater Chem B. 2020;8(13):2689–701.
Chu J, Shi P, Yan W, Fu J, Yang Z, He C, Deng X, Liu H. PEGylated graphene oxide-mediated quercetin-modified collagen hybrid scaffold for enhancement of MSCs differentiation potential and diabetic wound healing. Nanoscale. 2018;10(20):9547–60.
Patel S, Ziai K, Lighthall JG, Walen SG. Biologics and acellular dermal matrices in head and neck reconstruction: a comprehensive review. Am J Otolaryngol. 2021;43(1):103233.
Lederman ES, McLean JB, Bormann KT, Guttmann D, Ortega KD, Miles JW, Hartzler RU, Dorfman AL, Softic D, Qin X. Histologic case series of human acellular dermal matrix in superior capsule reconstruction. J Shoulder Elbow Surg. 2021;30(9):2146–55.
Chu J, Shi PP, Deng XY, Jin Y, Liu H, Chen MS, Han X, Liu HP. Dynamic multiphoton imaging of acellular dermal matrix scaffolds seeded with mesenchymal stem cells in diabetic wound healing. J Biophoton. 2018;11(7):e201700336.
Wang P, Shu B, Xu Y, Zhu J, Liu J, Zhou Z, Chen L, Zhao J, Liu X, Qi S, Xiong K, Xie J. Basic fibroblast growth factor reduces scar by inhibiting the differentiation of epidermal stem cells to myofibroblasts via the Notch1/Jagged1 pathway. Stem Cell Res Ther. 2017;8(1):114.
Chen X, Yang R, Wang J, Ruan S, Lin Z, Xin Q, Yang R, Xie J. Porcine acellular dermal matrix accelerates wound healing through miR-124–3p.1 and miR-139–5p. Cytotherapy. 2020;22(9):494–502.
Rahman M, Peng XL, Zhao XH, Gong HL, Sun XD, Wu Q, Wei DX. 3D bioactive cell-free-scaffolds for in-vitro/in-vivo capture and directed osteoinduction of stem cells for bone tissue regeneration. Bioact Mater. 2021;6(11):4083–95.
Wei D, Qiao R, Dao J, Su J, Jiang C, Wang X, Gao M, Zhong J. Soybean Lecithin-mediated nanoporous PLGA microspheres with highly entrapped and controlled released BMP-2 as a stem cell platform. Small. 2018;14(22):e1800063.
Wei DX, Dao JW, Chen GQ. A micro-ark for cells: highly open porous polyhydroxyalkanoate microspheres as injectable scaffolds for tissue regeneration. Adv Mater. 2018;30(31):e1802273.
Hu Z, Zhu J, Cao X, Chen C, Li S, Guo D, Zhang J, Liu P, Shi F, Tang B. Composite skin grafting with human acellular dermal matrix scaffold for treatment of diabetic foot ulcers: a randomized controlled trial. J Am Coll Surg. 2016;222(6):1171–9.
Tognetti L, Pianigiani E, Ierardi F, Lorenzini G, Casella D, Liso FG, De Pascalis A, Cinotti E, Rubegni P. The use of human acellular dermal matrices in advanced wound healing and surgical procedures: state of the art. Dermatol Ther. 2021;34(4):e14987.
Chen S-G, Tzeng Y-S, Wang C-H. Treatment of severe burn with DermACELL(®), an acellular dermal matrix. Int J Burns Trauma. 2012;2(2):105–9.
Guo X, Mu D, Gao F. Efficacy and safety of acellular dermal matrix in diabetic foot ulcer treatment: a systematic review and meta-analysis. Int J Surg. 2017;40:1–7.
Campitiello F, Mancone M, Cammarota M, D’Agostino A, Ricci G, Stellavato A, Della Corte A, Pirozzi AVA, Scialla G, Schiraldi C, Canonico S. Acellular dermal matrix used in diabetic foot ulcers: clinical outcomes supported by biochemical and histological analyses. Int J Mol Sci. 2021;22(13):7085.
Qi Y, Dong Z, Chu H, Zhao Q, Wang X, Jiao Y, Gong H, Pan Y, Jiang D. Denatured acellular dermal matrix seeded with bone marrow mesenchymal stem cells for wound healing in mice. Burns. 2019;45(7):1685–94.
Kitala D, Labus W, Klama-Baryla A, Kraut M, Maj M, Szapski M. Application of amniotic stem cells on an acellular dermal matrix scaffold in a burned patient: a case report. Transpl Proc. 2020;52(8):2563–9.
Zhang X, Deng Z, Wang H, Yang Z, Guo W, Li Y, Ma D, Yu C, Zhang Y, Jin Y. Expansion and delivery of human fibroblasts on micronized acellular dermal matrix for skin regeneration. Biomaterials. 2009;30(14):2666–74.
Zhou Y, Zhang Z, Chen H, Liu J, Lin R. Application of acellular dermal matrix to reconstruct the defects after hypopharyngeal carcinoma resection. Am J Otolaryngol. 2021;42(2):102847.
El-Kassaby M, Khalifah M, Abdelfattah S. Acellular dermal matrix allograft: an effective adjunct to oronasal fistula repair in patients with cleft palate. Int J Oral Maxillofacial Surg. 2013;42(10):1196.
Shi L-J, Wang Y, Yang C, Jiang W-W. Application of acellular dermal matrix in reconstruction of oral mucosal defects in 36 cases. J Oral Maxillofac Surg. 2012;70(11):e586-91.
Shaikh MS, Lone MA, Matabdin H, Lone MA, Soomro AH, Zafar MS. Regenerative potential of enamel matrix protein derivative and acellular dermal matrix for gingival recession: a systematic review and meta-analysis. Proteomes. 2021;9(11):9010011
Liu C, Su Y, Tan B, Ma P, Wu G, Li J, Geng W. Reconstruction of attached soft tissue around dental implants by acelluar dermal matrix grafts and resin splint. In Int J Clin Exp Med. 2014;7:4666–76.
Du M, Zhu T, Duan X, Ge S, Li N, Sun Q, Yang P. Acellular dermal matrix loading with bFGF achieves similar acceleration of bone regeneration to BMP-2 via differential effects on recruitment, proliferation and sustained osteodifferentiation of mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 1):62–70.
Tavelli L, McGuire MK, Zucchelli G, Rasperini G, Feinberg SE, Wang HL, Giannobile WV. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J Periodontol. 2020;91(1):17–25.
Gowda AU, Chang SM, Chopra K, Matthews JA, Sabino J, Stromberg JA, Zahiri HR, Pinczewski J, Holton LH, 3rd, Silverman RP, Singh DP, Porcine acellular dermal matrix (PADM) vascularises after exposure in open necrotic wounds seen after complex hernia repair. Int Wound J. 2016;13(5):972–6.
Tao Y, Cheng XB, Wang ZJ, Tan RW, Yu XQ, Zhai ZW, Han JG. The application possibility of acellular dermal matrix decorated with nano-silver in the reconstruction of contaminated abdominal wall. Mater Sci Eng C Mater Biol Appl. 2021;119: 111645.
Maxwell DW, Hart AM, Keifer OP Jr, Halani SH, Losken A. A comparison of acellular dermal matrices in abdominal wall reconstruction. Ann Plast Surg. 2019;82(4):435–40.
Garvey PB, Giordano SA, Baumann DP, Liu J, Butler CE. Long-term outcomes after abdominal wall reconstruction with acellular dermal matrix. J Am Coll Surg. 2017;224(3):341–50.
Hassan AM, Asaad M, Seitz AJ, Liu J, Butler CE. Effect of wound contamination on outcomes of abdominal wall reconstruction using acellular dermal matrix: 14-year experience with more than 700 patients. J Am Coll Surg. 2021;233:676-684.
Asaad M, Kapur SK, Baumann DP, Liu J, Butler CE. Acellular dermal matrix provides durable long-term outcomes in abdominal wall reconstruction: a study of patients with over 60 months of follow-up. Ann Surg. 2020. https://doi.org/10.1097/SLA.0000000000004454.
Giordano S, Garvey PB, Clemens MW, Baumann DP, Selber JC, Rice DC, Butler CE. Synthetic mesh versus acellular dermal matrix for oncologic chest wall reconstruction: a comparative analysis. Ann Surg Oncol. 2020;27(8):3009–17.
Siy RW, Pferdehirt RE, Izaddoost SA. Non-crosslinked porcine acellular dermal matrix in pediatric abdominal wall reconstruction: a case series. J Pediatr Surg. 2017;52(4):639–43.
Kalstrup J, Balslev Willert C, Brinch-Moller Weitemeyer M, Hougaard Chakera A, Holmich LR. Immediate direct-to-implant breast reconstruction with acellular dermal matrix: evaluation of complications and safety. Breast. 2021;60:192–8.
Loo YL, Haider S. The use of porcine acellular dermal matrix in single-stage, implant-based immediate breast reconstruction: a 2-center retrospective outcome study. Plast Reconstr Surg Glob Open. 2018;6(8):e1895.
Suh YC, Kim JK, Kim NR, Choi JS, Kim YJ, Lee JH, Jun YJ. A comparative study of pre- or subpectoral expander position with the fenestrated Acellular dermal matrix anterior coverage, on drainage volume and Seroma Formation after Non-Nipple-Sparing Mastectomy. J Plast Reconstr Aesthet Surg. 2021;74(9):2237–43.
Jin R, Cui Y, Chen H, Zhang Z, Weng T, Xia S, Yu M, Zhang W, Shao J, Yang M, Han C, Wang X. Three-dimensional bioprinting of a full-thickness functional skin model using acellular dermal matrix and gelatin methacrylamide bioink. Acta Biomater. 2021;131:248–61.
Yang D, Song Z, Lin Y, Dong W, Fu S, Yang J, Zhang P, Gu Y. Prevention of intestinal adhesion and regeneration of abdominal wall tissue with meshes containing an electrostatically spun acellular dermal matrix (ADM)/silk fibroin (SF) fiber composite polypropylene mesh. J Mech Behav Biomed Mater. 2020;112: 104087.
Liu X, Dan N, Dan W. Preparation and characterization of an advanced collagen aggregate from porcine acellular dermal matrix. Int J Biol Macromol. 2016;88:179–88.
Cai R, Xiang H, Yang D, Lin KT, Wu Y, Zhou R, Gu Z, Yan L, Zhao Y, Tan W. Plasmonic AuPt@CuS heterostructure with enhanced synergistic efficacy for radiophotothermal therapy. J Am Chem Soc. 2021;143(39):16113–27.
Ge J, Yu JH, Yang H, Yang D, Cai R. Human serum albumin templated MnO2 nanosheets as an efficient biomimetic oxidase for biomolecule sensing. J Mater Chem B. 2020;8(48):11090–5.
Yang Y, Zhu W, Cheng L, Cai R, Yi X, He J, Pan X, Yang L, Yang K, Liu Z, Tan W, Chen M. Tumor microenvironment (TME)-activatable circular aptamer-PEG as an effective hierarchical-targeting molecular medicine for photodynamic therapy. Biomaterials. 2020;246: 119971.
Liu Y, Li X, Shi Y, Wang Y, Zhao X, Gong X, Cai R, Song G, Chen M, Zhang X. Two-dimensional intermetallic PtBi/Pt core/shell nanoplates overcome tumor hypoxia for enhanced cancer therapy. Nanoscale. 2021;13(33):14245–53.
Acknowledgements
We would like to thank Yanping Huang from Center of Engineering Experimental Teaching, School of Chemical Engineering, Sichuan University and Ying Song from Central Laboratory, School of Public Administration, Sichuan University for the useful discussion.
Funding
This research was supported by the National Natural Science Foundation of China, No. 32101081; Young Talent Support Program Project of Shaanxi University Science and Technology Association, 20200424; the Fundamental Research Funds for the Central Universities, No. 20826041E4156; and the Opening Project of Key Laboratory of Leather Chemistry and Engineering, (Sichuan University), Ministry of Education, No. SCU2021D005.
Author information
Authors and Affiliations
Contributions
YW, and ND made substantial contributions to the conception and design of this review. YC wrote the manuscript. XL, ZL and WD made the corrections. XZ and XH participated in the design of figures in this review. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Liu, X., Zheng, X. et al. Advances on the modification and biomedical applications of acellular dermal matrices. J Leather Sci Eng 4, 19 (2022). https://doi.org/10.1186/s42825-022-00093-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-022-00093-4