Abstract
Mismanagement of various wastes especially waste water produced by tanning processes has caused serious environmental problems and ultimately impaired human health. Constant efforts have been making to alleviate the pollution of tannery wastewater (TWW), yet terminal treatment still takes dominance. In this review, research on TWW treatment from 2000 to 2021 was summarized, and main methods such as coagulation and flocculation, adsorption, biological treatment, membrane filtration, advanced oxidation process were briefly discussed. More detailed introduction was given to the method of electrochemical treatment since it has excellent performance such as environmental friendliness and high efficiency, hence attracting more and more research attention in recent years. In view of the harsh physi-chemical conditions of TWW, integrated or combined treatment methods are accordingly recommended with better performance and multi-function, however comprehensive studies on optimization of methods combination and cost-effectiveness are needed. The certain issues that the residue Cr in treatment sludge and high salinity in effluent still remain were put forward in this work and potential solutions were provided. Moreover, this review proposed the perspective that realizing multi-function, recycling, and intensification should be the developing direction for future TWW treatment. This review is expected to provide a general guide for researchers who aspire to ameliorate TWW pollution problems and understand various methods utilized in this field.
Graphical abstract

Similar content being viewed by others
1 Introduction
Tannery industry plays important roles in many countries including China, Italy, India, Brazil, etc., where relatively complete tannery industry chains have been developed, and has been contributing the important parts for the economic growth and employment. However, the inappropriate management of waste produced in tannery industry also brings challenges to the environment, and increasing attention has been raised on alleviating the pollution from tannery industry.
Tannery industry is particularly known as the high-water consumption industry with large discharge of wastewater. The wastewater from tannery industry usually contains hairs, proteins, acids, alkalis, chromium salts, sulphides, chlorides, tannins, solvents, dyes, auxiliaries, and many others compounds coming from the incomplete chemical immobilization into leather products in the multi-step tanning process that converts raw hide/skin of animals to commercial commodities [1]. Studies aiming at evaluations of toxicity, genotoxicity and environmental risk of tannery effluent were carried out by many researchers, and the negative impact of tannery wastewater (TWW) without proper treatment discharged to the environment has been confirmed [2,3,4,5]. Therefore, TWW usually charactered with severe physio-chemical conditions is detrimental to environment as well as human bodies, and effective decontamination is urgently required. With the increasingly stringent requirements for environmental protection, many efforts for reducing the pollution in wastewater from tannery industry have been conducted [6]. In spite of the investigations concerning the recycling and reusing of tanning agent [7], alternative carrier medium [8], enzymatic unhairing process [9], efficient management [10], and other cleaner production methods are booming [11,12,13,14], the terminal treatments of TWW still cannot be avoided for most areas in the present situation [15].
In this paper, we analyzed the articles related to the terminal treatment of TWW from year 2000 to 2021, focusing more on the last decade, and summarized the comprehensive methods for TWW treatment, in the hope that the work would provide researchers with a quick understanding of current progresses in this field. Term words “tannery”, “leather”, “wastewater” and “treatment” were adopted via searching Web of Science core collection v5.35 (http://apps.webofknowledge.com/) to find the related studies. Second or tertiary treatments for TWW decontamination were identified and discussed, including adsorption, flocculation and coagulation, biological treatment, membrane filtration, advanced oxidation processes found in literatures, especially the promising electrochemical treatments of increasing concern (Fig. 1). It should be noted that some methods such as alkaline precipitation [16], wet air oxidation [17], bioleaching [18], solar evaporation [19], ion exchange [20] etc. were also reported for TWW treatment, but were not included in our studies, due to the limited references. Possible problems behind the present TWW treatment methods were proposed with promising solutions provided. Moreover, we also introduced our points of view that what should TWW treatment in the future focus on.
2 Characteristics of tannery wastewater
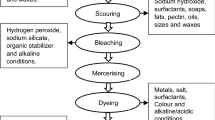
The entire leather manufacturing processes can be basically divided into three parts: beamhouse stage, tanning stage, and post-tanning and finishing stage illustrated in Fig. 2 [1]. Figure 2 also demonstrates the pollution profile of main leather processing steps, and the conventional wet-end processes usually accounts for nearly 90% of the total pollution load in a tannery [21].
As a large quantity of chemicals (e.g., acids, alkalis, chromium salts, tannins, sulfates, phenolics, surfactants, dyes, auxiliaries, sulphonated oils and biocide etc.) used in the tanning processes to convert the semi-soluble protein “collagen” presenting in raw hide/skins into highly durable commercial leather products are usually incompletely immobilized by the hide/skins, the massive effluent or wastewater of tanning processes is featured with serious physio-chemical conditions, i.e., a basic dark brown colored waste with high contents of COD, BOD, TDS, chromium, phenolics, high pH and pungent odor [15, 22]. Most tanneries have their own TWW treatment plants or deliver TWW to a sewage treatment plant nearby through sewerage pipes, however, high concentration of BOD, COD, TDS, sulfate and phenolics are detected above the prescribed limits due to the limited effective treatment methods [23]. The direct discharge standards of effluent from TWW treatment plant in different countries were listed in Table 1, from which we can see that the required quality parameters of treated water are not so high that most treatment plants could not meet. However, treated effluent characterized with high pollutants content in several studies were still reported. For examples, TSS content ranging from 250 to 35,200 mg/L, BOD content ranging from 250 to 2960 mg/L, total Cr content ranging from 4.5 to 15 mg/L were still detected in some TWW treatments [24,25,26,27]. The fact should be highly noticed that there still exist some plants without enough safety or efficiency in handling TWW, leading to the urgent need for advanced technologies with better performance worldwide.
3 Treatment for tannery wastewater
3.1 Flocculation and coagulation
Flocculation and coagulation are generally utilized as pre or post methods for TWW treatment. Conventional inorganic flocculants or coagulants such as aluminium, silicon, calcium, iron-based compounds were widely adopted to reduce COD, TSS, colority and the concentration of many pollutants before further TWW treatment [32,33,34,35]. Organic compounds such as proteins, polymers were also developed for coagulation or flocculation [36, 37]. It is noteworthy that electrocoagulation (EC) process has been raising interest for TWW treatment, in which aluminium and iron electrodes were most extensively selected [38,39,40]. The mechanism for EC includes the anode dissolution and water splitting, contributing the formation of hydroxide coagulants under an applied electric filed [41, 42]. When ion exchange membranes were installed, electrodialysis (ED) could be induced to precipitate and segregate ions such as NH4+, SO42−, Cr (III), etc. from TWW towards anodic and cathodic zones [43]. Combination of EC and ED for treatment of TWW with better performance would be expected [44]. Though it looks facile and have the effect of removing both organic and inorganic pollutants, flocculation and coagulation is not suitable to serve as the main process for TWW treatment. The reason is clear: the harsh quality of TWW usually requires massive addition of flocculants or coagulants to obtain a relatively satisfactory decontamination performance, however this would make the treatment cost increase a lot. Furthermore, the treatment sludge is supposed to characterized with high concentration and high toxicity, and might cause pollution transfer without proper disposal methods.
3.2 Biological treatment
Bio-degradation of contaminants is the most widely used method for wastewater treatment nowadays. One typical biological treatment for TWW could be seen in Fig. 3. Mechanism of biological treatment by diverse microbes is complicated, including but not limited to adsorption, degradation, coagulation and detoxification of pollutants in wastewater. The bio-processes such as active sludge, biofilm, anaerobic hydrolysis acidification, biological filter etc. have been extensively applied in TWW treatment plants, since these biological treatment processes not only cost-effective but also featured with desirable functions such as denitrification and dephosphorization.
Due to high salinity with toxic substances presented, TWW often has adverse effects on growth and physiological activity of common micro-organisms, eventually impeding the efficiency of biological treatments [45, 46]. For this reason, researchers identified and cultured microbes featured with high tolerance for salinity and heavy metals. Some qualified active bacteria, archaea, fungi were elucidated in studies [47,48,49,50,51]. Examples of microbial species’ isolation from high salinity and heavy metal conditions to their inoculation in real wastewater treatment practice were not scarce, and the paper that reviewed some progresses in decontamination by halophilic microorganisms in saline wastewater could be referenced [52]. In TWW treatment, research mostly concentrated on the overall detoxification efficiency of diverse microbes. A study compared detoxification efficiency of four fungal strains immobilized on nylon mesh, and after 120 h, removal performance with 82.52% COD, 86.19% color, 99.92% Total Cr, 95.91% Total Pb were observed [53]. Other studies with similar comparisons potentially offered more options of effective micro-organisms for TWW biological treatment [54,55,56,57]. Some investigations demonstrated that COD, Total Cr, color could be largely removed together with reduced biotoxicity in aeration lagoons of common effluent treatment plants (CETPs) during the treatment of industrial TWW, providing the ability to address harsh wastewater [26, 51]. With the purpose of decrement of microbe’s acclimation time in CETPs, the special active sludge or bio-film dedicated for treating TWW were cultured so that the quality of effluent got considerable improvement [58,59,60,61,62]. Since TWW often contains abundant nutrient substance, anaerobic digestion became an alternative way for pollutant removal together with bio-gas production. Technologies such as up-flow anaerobic sludge blanket reactors (UASBs), membrane bioreactors, non-aerated biofilm, and packed bed reactor were thereby exploited for denitrification, dephosphorization, anammox, detoxification, and bio-gas production through cultivation of tolerant microbial species, despite the fact that TWW generally has an inhibition effect on enzymatic reactions for anaerobic digestion [63,64,65,66,67,68]. Combined oxic-anoxic biological treatments or facultative ponds were also developed for a better and multi-target pollutants removal, where the optimization of oxic-anoxic treatment process’s configuration played an important role for full scale application [60, 69,70,71].
Another biological treatment widely utilized for TWW treatment is constructed wetlands (CWs), which is characterized with energy saving, handy operation and environmentally sound [72]. CWs are actually some small ecosystems consisting of plants, microorganisms, aquatic animals, where physical, chemical, biological decontamination processes co-exist [73]. For a certain CW fed with TWW, tolerant plants as well as micro-organisms, and packing stuffs should be paid the same attention as CW’s types such as vertical flow, horizontal subsurface flow, surface flow, etc. [74]. A pilot-scale constructed wetland planted with specially choosing phragmites in Venezuela showed high COD (82%) and NH4+-N removals (96%), and almost complete Cr removal in the outflow [75]. Hybrid constructed wetland systems, i.e., horizontal subsurface flow combined with free water surface flow or subsurface vertical flow combined with horizontal flow and vertical flow were also under careful inspection, which exhibited the excellent properties for denitrification, dephosphorization, and detoxification [76, 77]. Varied from the conventional CWs with plants growing on gravel, sand, porous soil, etc., a novel floating treatment wetlands (FTWs), inoculated with selected bacteria, was also designed, to achieve satisfying amelioration of effluent quality [78]. More studies about designing CWs for TWW treatment have been conducted, and all these CWs system exhibited promising prospects for multiple target contaminants removal [79,80,81,82,83]. Practice experience such as how to select plants, substrate, operation load, etc. was summarized in one substantial work [84].
Biological treatments have been the most extensively adopted methods for TWW treatment until now, still the problems such as inconvenient isolation and acclimation of tolerant species, time consuming, and non-biodegradable pollutants also baffled their applications. In addition, the sudden changes in TWW volume are another great challenge for the operation of biological treatments. One another issue also gradually emerged nowadays: construction of a biological treatment plant that has TWW wastewater treatment scale usually requires a certain land occupation, however this would be more and more difficult because limit of land exploitation in the future would become stricter.
3.3 Membrane filtration
Technologies of microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RO) have been actively developed in the past two decades. A simplified membrane process is illustrated in Fig. 4. Polluted water permeates membranes under propulsive forces such as pressure, flow, concentration gradient, etc. with various contaminants rejected simultaneously. Membrane filtration has been a hot spot domain for TWW treatment.
Ceramic membranes are very commonly used in TWW treatment, and efforts to explore cheaper and more efficient ceramic membrane materials have been taking all the time. Boehmite [85], natural clay [86], pozzolan [87], perlite [88, 89] as raw materials for manufacturing and utilization of membranes, in TWW treatment have been reported. Organic membranes were also another kind of materials under inspection. Velu et al. devised the polyethersulfone ultrafiltration membrane that achieved a 80–90% reduction in BOD and COD in TWW treatment [90]. Religa et al. investigated the properties of membranes on Cr recycling as well as pollutants removal, and polyamide/polysulfone membranes were thus suggested for treating TWW [91]. Membranes carrying adsorbents for pollutants removal showed outstanding performance in several studies. One versatile layered double hydroxides (LDHs)/polyacrylonitrile (PAN) membranes were smartly weaved, which achieved more than 99% Cr (III) removal in synthetic TWW [92]. Researchers also combined nano-filtration and RO processes, where about 78% permeate recovery with low TDS was achieved in one pilot plant, and the water recovered from the membrane system was successfully reused for tannery process [93]. Higher selectivity and lower operating pressure are ideal characteristics for membrane fabrication, a review briefly introduced the novel “loose nanofiltration” appearing to satisfy this. This kind of membrane is supposed to possess high permeation of salts and small organic molecules, which therefore could be used for resource separation / recovery and may be potential for highly saline TWW treatment and valuable substances recycling [94]. Membrane process integrated in some steps of tanning cycle instead of together treatment of mixed TWW was put forward, especially when valuable resources needed to be recycled and the quality of effluent in each tanning step was under strict control [95]. As we can see, fouling is really troublesome for membrane filtration especially when TWW with aggressive conditions being treated. Therefore, methods for alleviating the foulants on membrane have been considered for years. For examples, TWW pretreatment via coagulation [96], electrocoagulation [97], and other approaches before filtration were studied. Membrane bioreactor (MBR) basically consisted of membranes and active sludge, where treatment performance could be augmented through additional microbial activities, has also been applied to TWW treatment [64, 98, 99].
Those encouraging studies on membrane filtration for TWW treatment really fascinate researchers, while limitation of membrane processes cannot be recklessly ignored. The membrane fouling as well as aging problem aside, the processes for preparing membranes are always complicated in order to obtain homogeneous, stable and functional properties. What’s more, the capital cost spent on equipment to provide sufficient propulsive forces also was concerned. Given this, exploitation of membrane filtration to ameliorate huge amounts water and sustain high pollutants removal efficiency coupled with high permeate flux in one TWW treatment plant seems to be difficult. Accordingly, combination with other methods or employment of membrane filtration as tertiary process seem to be more practical.
3.4 Advanced oxidation processes
Advanced oxidation processes (AOPs) have been an active research hotspot in recent years due to their excellent capability for the removal of reluctant pollutants, under the stricter discharge standards of waste effluents. Basically, this kind of method usually utilizes various ways to produce much strong oxidant species such as O3, peroxy radical, hydroxyl radical, sulfate radical, etc. to attack recalcitrant organic pollutants in wastewater so that the effluents after treatment could meet the required quality. Due to the feature of tannery, many persistent contaminants in the TWW such as tanning agents, polycyclic compounds, and metal complexes, are hard to be totally degraded by conventional methods without AOPs adopted [100].
Generally, in the light of the specific ways to produce reactive oxidant species (ROS), AOPs used in wastewater treatment could be categorized (Fig. 5) to O3-based AOPs (single O3, O3/H2O2 and O3/catalysis), UV-based AOPs (UV/H2O2, UV/PDS or PMS and UV/Cl2), electrochemical AOPs (introduced in the later section), catalytic AOPs (Fenton reactions, photo-Fenton and photo-TiO2), and physical AOPs (ultrasound or micro-wave excitation and plasma) [101]. Among all these techniques, Fenton [102,103,104], O3 [105,106,107], and photo (or UV) catalytic AOPs [108,109,110] were found to be utilized for TWW tertiary treatment and obtained pretty good performance in some studies. Exploration for simultaneous degradation of refractory pollutants and segregation of Cr seems more promising when AOPs as methods to address TWW. In one UV-based AOP research, enhanced chromium fixation with high COD removal was achieved at the same time, due to H2O photoionization-generated electrons as well as hydroxide ions helping the formation of more hydroxyl radicals and occurrence of co-precipitation effect [111]. Many laboratory studies showed strong evidence that AOPs could efficiently degrade some refractory, non-biodegradable, and toxic organic pollutants, and effectively improve the water quality of effluent compared to conventional biological treatment. Nevertheless, some issues and disputes did arise in practice or scale-up utilization of AOPs. For example, a study compared different AOPs treatment (TiO2/UV, O3, Fenton and H2O2/UV) for enhancing biodegradability of real TWW, however, the related results showed the failure of these selected AOPs for pre-treatment of TWW [112].
When choose AOPs for practical TWW treatment, some important factors should be carefully considered. O3, Fenton, and other catalytic AOPs usually need the extra reagent cost, and the favorable reaction conditions for AOPs in TWW need to be extra regulated in advance to obtain desirable performance, which will further increase the treatment cost. Besides, ROS produced via AOPs treatment are supposed to be quenched in a very short time, while mass transfer between contaminants and ROS are always limited especially when reactions occurred in large reactors. Furthermore, some heavy metal like Cr might be oxidized to higher valence, and not all recalcitrant organic pollutants could be transformed to non-hazardous forms, which may make the conventional AOPs unreliable. In the view of the above reasons, AOPs may not be one preferable solution for some large CETPs especially their throughput is always huge. Nevertheless, some small plants special for TWW treatment built nearby tanneries could consider this kind of method as pre or post treatment processes.
3.5 Electrochemical treatment
Electrochemical treatment of wastewater has been an active research spot in recent years due to its prominent performance in removal of a range of organic and inorganic contaminants. In fact, electrochemical treatment of wastewater has already been used as pre or post treatment process, however the high energy consuming and facilities cost inhibited its scale-up application. Given that TWW usually contains high salinity and much metal ions that could improve the conductivity of the wastewater to be treated, further promotion of electrochemical treatment is to be expected. In this section, technique of electro-oxidation, cathode reduction, microbial fuel cell for TWW treatment will be discussed, and detailed introduction of electro-oxidation are given.
3.5.1 Electro-oxidation
Organic contaminants are usually removed via electro-oxidation (EO) during electrochemical treatment processes of wastewater. EO could be basically classified to three categories [113]: (1) One is direct electron transfer (DET) of organic pollutants adsorbed on anodes. The DET process could occur in most EO systems, but generally could not serve as the final mineralization approach hence considered negligible impact on pollutants’ degradation. Nevertheless, some special contaminants such as perfluorinated compounds were proved to be efficiently decomposed only when DET as the trigger step [114, 115]. (2) Mediated oxidation (MO), which depends on anodic reactive oxidant species (ROS) catalytic formation to decompose contaminants. The process can be described via several reactions under electric field:
It is worth noting that the generated ROS include not only common.OH, HClO, but also some other reactive substances such as carbonate radical, phosphate radical, superoxide radical, hydrogen peroxide, etc., and even PMS/PDS were also found in electro-oxidation system [116]. However, most electrochemical studies in TWW treatment nowadays concentrate more interest on.OH, HClO, H2O2. (3) The last one is Electro-Fenton (EF), photo-electro-Fenton (PEF) or another combined electro-Fenton, e.g., ultrasound-electro-Fenton. Differentiated from conventional Fenton process, EF, PEF and other combined EF systems can work without the extra addition of H2O2. In one typical EF system, O2 is diffused to a cathode commonly called as gas diffusion cathode (GDC, porous films electrodes often utilized) and H2O2 is generated through the reaction:
The generated H2O2 could be activated for.OH production via diverse approaches, e.g., addition of Fe materials, photo-catalysis, ultrasonic assistance, etc. A typical electrochemical reactor for wastewater treatment could be shown in Fig. 6.
Studies have demonstrated the superior decontamination performance of EO processes for TWW treatment. Encouraging results that COD, TOC, NH3-N, color, pathogen etc. were efficiently removed mainly via electro-generated.OH and active chlorine were reported [117,118,119,120]. With the purpose of boosting ROS formation and strengthening EO decontamination performance, assisting methods were also carefully used including sonar-enhanced [121], UV-enhanced [122]. Typically, the effluent quality after EO treatment would obtain satisfying improvement, and its bio-degradability would be suitable for natural bio-degradation. The commercial scale reuse of TWW after EO treatment was investigated by an experiment, and the feasibility of treated TWW for leather manufacture was confirmed [123]. Though most papers preferred to elaborate the dominant roles of.OH and active chlorine to decompose refractory pollutant molecules in TWW, it is noteworthy that sulfate ions included in TWW could be also converted to sulfate radicals [124]. Usually sulfate ions are harmful to biological treatment processes, while researchers have found that electro-generated sulfate radicals originated from the high-level sulfate ions contributed to the improved contaminants abatement in EO processes [125,126,127]. Scholars may take a page from these covers when facing the treatment requirement of TWW containing high sulfate ions.
When applying EO processes for wastewater treatment, the impact of some factors such as pH, electrolyte type, reactor configuration, etc. should be considered. However, among which current density (or applied voltage) and electrode (materials, shape, size, etc.) are the most significant factors influencing pollutants abatement performance of EO. Higher current density (or applied voltage) within specific range generally benefits EO process, yet serious consideration of energy cost and electrode service life should be taken into. What’s more, it is apparent that water splitting would occur and occupy the center stage of various electrochemical reactions under higher current density (or applied voltage), which, will adversely decreases EO efficiency. Preliminary quantitative analysis of energy consumption in EO processes is feasible when indexes such as the mineralization current efficiency (MCE) [128], the electrical energy required to destruct the target contaminants by one order magnitude (EEO) [129], the specific energy cost per unit mass of TOC removed (ECTOC) [130], were introduced to calculate efficiency of current or voltage used in amelioration of wastewater:
where F is the Faraday constant (96,487 C mol−1), V is the solution volume, I is the current applied, Δ(TOC) is the TOC removal, 4.32 × 107 is a conversion factor to homogenize units and m is the number of carbon atoms of target contaminants. The number of electrons exchanged per each contaminant molecule for complete mineralization was taken as n.
where U is the voltage, J is the current density, A is the electrode surface area, t is the reaction time, V is the total volume of the reactor, and C0 and Ct are the concentrations of pollutants at the beginning and at time t, respectively.
The meaning of U, I, t, V, and Δ(TOC) is the same as the aforementioned Eqs. (7), (8). When Δ(TOC) is placed by Δ(COD), this equation could also calculate ECCOD.
As ROS formation were commonly anodic catalyzed, anodes used in EO processes were categorized into two main types, i.e. active electrodes (e.g. Pt, IrO2, RuO2, etc.) and non-active electrodes (e.g. BDD, PbO2, SnO2, etc.) [113]. Non-active electrodes were proved to produce more ROS instead of side-reaction like oxygen generation with no contribution to wastewater treatment. This was also interpreted as higher overpotential for O2 evolution of non-active anodes [116]. The complete underlying principles for more ROS generation and better pollutants degradation capacity of non-active anodes are still unclear. It was assumed that main oxidant specie.OH produced in anodic zone have a weaker adsorption to anode’s surface were to explain this phenomena [131]. A study compared removal efficiency of various pollutants in TWW using Ti based electrodes with coating materials, in which the authors believed that EO processes could serve as complementary means for ammonia elimination with lower energy consumption [132]. A team investigated the role of electrode materials in raw TWW treatment, where DSA® Ti/RuO2, Ti/IrO2 and Ti/BDD electrodes were utilized in a continuous flow system, and the better performance of non-active electrode Ti/BDD was confirmed [133]. The similar results that high pollutant removal efficiency, low energy consumption, and high electrode stability of Ti/BDD electrode were again elaborated in one post-treatment experiment of TWW [134]. Three dimensional electrodes as well as rotating disk electrodes were utilized recently notwithstanding the investigation of plate electrodes in EO for TWW treatment still dominated. Cylindrical graphite electrodes which are less expensive were used as both anode and cathode for tannery saline wastewater treatment in an experiment, and promising result was obtained that treated effluent reused for pickling process without adverse effect [123]. One interesting study was carried out by a team from India: a three-phase, three-dimensional fluidized type electrochemical reactor was designed with particle electrodes, and a desirable removal of COD and chromium from tannery industrial wastewater was observed [135]. A novel electrochemical reactor with a rotating stainless-steel cathode and stationary Ti/TiRuO2 anode rings was developed to treat TWW in one 2017’s study, where electrodes are positioned vertically and parallel to each other with a gap of 5 mm between them, and up to 91% TOC removal was achieved after 2 h [136]. The state of the art of electrode design is filter like electrode or reactive electrochemical membrane (REM), which could greatly improve mass transfer and pollutants removal behaviors. This kind of electrode is basically fabricated by conductive filter membrane, and relevant reports could be found in some studies [137,138,139]. To the best of our knowledge, more and more novel and efficient electrodes have been developed and adopted for decontaminating noxious saline wastewater including landfill leachate, reverse osmosis concentrate, etc. [140, 141]. And these studies may shed light on the application of these novel electrodes for TWW treatment.
3.5.2 Electrochemical reduction and microbial fuel cells
Aside from anode’s EO process for wastewater treatment, reduction reactions on cathode could be utilized for metal ions (Mn+) precipitation and recovery from saline water [142]. Under electric field, cations could migrate to cathode:
The mechanism could be also adopted for Cr containing wastewater treatment. Sheet electrodes of steel, copper, and lead materials were investigated in one study for Cr cathode deposition, 99% recover of total chromium was achieved within 2 h mainly in the form of Cr(OH)3 from TWW sample [143]. A cost-effective electrochemical system employed with graphite and aluminum as anode and cathode was observed with 96.5% removal efficacy of total chromium in one real TWW treatment experiment [144]. Since segregation and recovery of chromium always has a priority in case of TWW treatment, combination of anode oxidation and cathode reduction may provide a new direction for simultaneous removal of recalcitrant organic pollutants and Cr (III).
Microbial fuel cells (MFC) were also introduced to treat TWW as one novel method by some researchers. In one typical MFC system, specific microorganisms living in anodic zone and usually forming biofilm on anode, will produce electrons by degradation of organic substances, then these produced electrons will transfer from anode to cathode through external circuit. Accordingly, this kind of electrochemical system could decompose organic pollutants and generate electric energy. When using pretreated TWW as substrate for MFC processes, 90%, 84% and 96% removal of COD, BOD5, and sulfate, was respectively obtained with a current density of 11.2 A/m2 [145]. In another study, mineralization of humic acid and reduction of ferrocyanide in anodic compartment and cathodic compartment, were observed respectively, when soak liquor effluent from a tannery was used as substrate [146]. There were more studies about MFC employed in TWW treatment for both pollutants abatement and electric energy generation [147, 148]. However, as mentioned in the former section, isolation and acclimation of tolerant microbe species is the key factor that may limit the scale-up exploitation of this technique. What's more, the biological fitness for microbe growth, and electrodes materials employed in MFC are necessary for determination of MFC performance, distinguished with normal EO system which puts more emphasis on current efficiency of electrodes.
The main defect of limiting the practical applications of electrochemical treatments is the relatively high cost of the electrode and the possible risk of harmful byproducts, i.e. absorbable organic halogen (AOX) generated in high chloride content of TWW [149]. In addition, the investment on electrochemical supporting equipment and power supply should also be balanced when large-scale industrial application was considered in TWW treatment. Despite these above, utilization of electrochemical treatment as pre or post treatment method in TWW still seems promising due to its environmental friendliness, good versatility and high efficiency. Compared to the inhibition effect on conventional biological treatment, the high salinity nature of TWW appears to be more helpful for electrochemical processes on the contrary. The in-situ generated ROS combined DET without no or little agents addition in electrochemical reactors could quickly decompose most recalcitrant pollutants and impose limited impact on effluent.
3.6 Adsorption
Adsorption has been widely utilized in wastewater treatment due to its comparatively low cost and good flexibility. As to TWW treatment, utilization as post-method after biological or other treatment is more reasonable since terrible physicochemical properties of raw TWW influent would make most adsorbents ineffective. Activated carbon is always the most common adsorbent for TWW treatment, and the exploration of low-cost materials for activated carbon manufacture has been in processes. A study recycling waste rubber tires and another study utilizing agriculture wastes as raw materials to manufacture activated carbon for Cr (III) removal in synthetic TWW are representative examples [150, 151]. Raw clay minerals and their modified counterparts served as cost-effective and broad-resource adsorbents for pollutants removal have been subjected to investigations in some studies, and satisfying results were achieved [152,153,154]. Considering that a large quantity of solid waste is produced from tannery procedures, recycling these wastes for adsorbents have been proposed [155,156,157]. In general, the processes of conversion tannery waste to adsorbents were not so complicated, mainly including agent leaching, pyrolysis, drying, milling, sieving etc., whereas the way to ensure consistent adsorption ability remains unsolved. Besides these inorganic materials, bio-sorbents that made of biomass have attracted much attention. Processing barks, fruits peelings, reeds, biochar, etc. into bio-sorbents have been extensively reported [158,159,160,161,162]. Further efforts have been taken to introduce some microbes such bacteria, fungi, etc. for removal of pollutants in TWW, and these microbes themselves with their secretions showed good properties of adsorption in many research [163,164,165]. Utilization of micro-algae for wastewater treatment has also been proposed as a promising method for TWW treatment, recent studies provided a promising sight into the adsorption of the toxic metals and other pollutants in diluted tannery effluent by micro-algae [166,167,168]. More researchers focused on the segregation of Cr in TWW, they usually exploited various methods, e.g., changing pore size distribution, loading active substances, etc., to modify conventional adsorbents eventually creating more active sites or surface functional groups to enhance adsorption abilities. Generally, Cr (III) constitutes the vast majority for the total Cr in TWW, still Cr (VI) was assumed to possibly emerge in some studies, and researchers have made efforts to remove the total Cr using adsorption [169, 170].
3.7 Integrated methods
The methods we discussed in Sects. 3.1 to 3.6 have their own advantages and disadvantages when utilized for TWW treatment, and a brief qualitative comparison of all the treatment methods was demonstrated in Table 2. Though more efforts have been taking to improve these methods’ efficacy by researchers, complete detoxification of recalcitrant organic and inorganic pollutants in TWW using one single method is presumably expensive and unreliable. Based on the pros and cons of each method, the appropriate combination of different treatment methods could lead to multi-effective performance and save cost. Hence, increasing researchers have combined diverse techniques for a better performance of TWW treatment recently. Selected studies using integrated or combined methods to treat real or synthetic TWW, from year 2010 to 2021, were therefore summarized in Table 3.*In above table: Concentrations are in mg/L except pH and color, TWW tannery wastewater, Cr total chromium, TS total solids, TDS total dissolved solids, TSS total suspended solids, TN total nitrogen, TP total phosphorus, TOC total organic carbon, DOC dissolved organic carbon, EC electrocoagulation, MF microfiltration, NF nanofiltration, RO reverse osmosis, EO electrooxidation, ASP active sludge process, UASB upflow anaerobic sludge bed, BAF biological aerated filter, MBR membrane bioreactor, MBBR moving bed biofilm reactor, OD oxidation ditch, CW constructed wetland, SBR sequential batch reactor, PAC powdered active carbon, GAC granular active carbon,“Unknown” means that original data was not found
No doubts that most integrated or combined methods for TWW treatment better reduce pollution and greatly improve the quality of effluent, however, it is still necessary to carefully consider optimal implementation of wastewater treatment alternatives when multiple objectives or criteria and hierarchy processes are required in practical running of TWW treatment plants [182]. One study used analytical hierarchy process (AHP) and grey relation analysis (GRA) for optimal selection of treatment alternatives in a full scale tannery effluent treatment plant is in progress [183]. More investigations are needed in the reality that stricter environmental regulations have been implemented for a considerate number of CETPs.
4 Problems to be solved and potential solutions
Even though these treatment methods discussed before could greatly ameliorate the quality of effluent from a tannery hence greatly reduce its harmful environmental impact, there still remains some problems baffling TWW treatment plants. The most concerned and controversial problems are Cr pollution and high salinity.
The content of Cr in effluent of TWW treatment plants is reduced as much as possible, however the real threat comes from the treatment by-products through various advanced segregation methods. By-products after wastewater treatment, including Cr-containing sludge, foulants, adsorbents, etc. are extremely hazardous yet usually in poor management. To save cost, these by-products are always transferred to landfill treatment, which might cause serious pollution transfer. Compared to conventional landfill, recycling or reuse of these by-products is a rather desirable way as Cr has much value for industrial production. Nevertheless, it should be acknowledged that the facile and cost-effective recycling or reusing methods seems hard to find, even worse is that these methods are highly dependent on the adopted methods in TWW treatments. Rather than the laborious trials to eliminate the Cr pollution in wastewater treatment processes, we believe that reducing discharge at sources is more reasonable and cost-effective. Therefore, cleaner production strategy in tanning processes has been proposed as one more promising way as it could reduce even eradicate Cr discharge to effluent so that a series of tough problems concerning Cr disposal in subsequent wastewater treatment processes could be solved. Cleaner production strategy such as enhancing Cr uptake in tanning process or “Cr exhaustion” [184], novel Cr tanning agent carriers [185], Cr-free syntans [186], non-chrome metal tanning agents and chrome free organic tanning agents [187,188,189], etc. are good examples, and these low-risk strategies could relieve the pressure from Cr segregation in wastewater treatment processes even eliminate Cr pollution from root cause.
High salinity is another annoying problem in TWW treatment since it not only undermines treatment performance of most methods but also hard to be totally removed. Efforts have been conducted to acclimate various active halophilic species to biological treatment of TWW, and membrane filtration as well as electro-chemical treatment are also promising methods to deal with saline wastewater, which have been discussed in the former sections. However, the fact that the treated effluent still contains high salinity shadows the effect of these approaches, leading to post-treatment or desalination on high agenda. Approaches of desalination could be basically divided to six domains: distillation, reverse osmosis, membrane filtration, evaporation, electrodialysis, electro-deionization [190]. Though commonly high cost, forementioned desalination approaches could be considered at present situation since the quality of treated TWW is not that harsh due to the pretreatment, and total desalination operation cost may not be as expensive as expected. An example of TWW post-treatment for desalination is shown in Fig. 7. One investigation that combined MBR and RO units exhibited another good case, in which bio-fouling or scaling of RO process as well as salt content of effluent were largely reduced [191]. A crucial issue that how to handle saline reject or concentrate should be taken into account too, especially for widely used membrane filtration and electrodialysis, because of their relatively lower cost and facile operation. One possible sustainable perspective for future is that application of combined processes, i.e. combination of desalination system and integrated agricultural systems using saline reject or concentrate as nutrients [192]. With respect to our topic, TWW treatment, combined method such as membrane filtration integrated with CWs may be one kind of good solution. In that composite system, main processes such as biological, membrane, electrochemical treatment count for toxic substances removal, and the treated effluent is used for feeding livings in CWs, where deeper clarification and desalination could be simultaneously achieved.
Simplified diagram of wastewater treatment and desalination chain (biological treatment + RO), adapted from [193]
5 TWW treatment for the future
The challenges for TWW treatment nowadays lie not only in noxious pollutants removal, but also in balance of cost-effectiveness. As mentioned before one single method we discussed in former sections could not achieve TWW totally decontamination as well as cost saving, integrated or combined methods should be carefully recommended in wastewater treatment plants. For instance, AOPs treatment combined with active sludge process could enhance the bio-degradability of wastewater, which might reduce the operation time and pollution load in aeration tank therefore increasing treatment capacity in one sense. Electrocoagulation combined with electrooxidation could simultaneously degrade organic pollutants and precipitate inorganic pollutants, which might be used for TWW treatment steps simplification. In addition, resources recycling would be considered in the future TWW treatment plants since the concept of “Carbon Neutrality” has become a general trend worldwide. Specific to wastewater treatment field, not only the optimization as well as upgradation of treatment processes, but also valuable resources recycling as well as reusing are supposed to be extensively advocated. The considerable amount of recycled water, salts, metal, etc. separated from TWW could bring economic income hence contributing to treatment plants’ long-term self-sustaining operation. What’s more, as the requirement of land use intensification prevails in many countries, wastewater treatment plant should be constructed as compact as possible in the future. This means that step by step treatment processes of space division would be less and less popular, especially when land use permission gets stricter. The physical and chemical treatment methods such as membrane filtration, electrochemical oxidation, etc. should be therefore well developed and play a more important role in the future, since the hourly treatment capacity of these methods could be enough large compared to conventional biological treatment so that the land occupation of treatment plants could be greatly reduced.
To summarize, TWW treatment in the future should aim at multi-function, recycling, and intensification. In the light of this direction, we herein proposed one possible future TWW treatment plan as shown in Fig. 8. In this plan, treatment flow was simplified to pre-treatment, major treatment, and advanced treatment processes, which conforms to the concept of intensive and integrated wastewater treatment requirement. The pre-treatment step is designed for separation of large matter or sand from TWW, where some parameters such as pH, temperature, etc. could be simultaneously regulated. The major treatment step should have an excellent ability to degrade or detoxify pollutants fast as well as safely, and valuable resources recycling could be also achieved in this step. The advanced treatment step is utilized for deep mineralization, disinfection, and sterilization. The whole treatment processes put high requirements on the ability of fast decontamination, therefore more advanced and greener chemical or physical treatment methods should be applied in the future. The treated effluent is supposed to have enough high quality, and it could be reused for filling the demand from domestic and industrial water. To reduce the impact on the surrounding environment, underground or semi-underground construction of TWW treatment plant is recommended too, at least there should be enough green belt covered in the zone of treatment plant. This plan is just one ideal case, however realizing multi-function, recycling, and intensification in future wastewater treatment should always be the target of TWW.
6 Conclusions
This work presented an updated review of TWW treatment research from year 2000 to 2021. Main methods for TWW treatment could be divided into coagulation and flocculation, adsorption, biological treatment, membrane filtration, advanced oxidation process, and electrochemical treatment. These methods summarized from references are generally served as second or tertiary treatment approaches for TWW. Though higher efficiency has been pursuing and some encouraging results have been achieved, complete decontamination of TWW via just one single method is inefficient and unreliable. Recently, research related to integrated or combined methods for TWW treatment continues to spring up, which appears to be more promising, since the enhanced and multi-functional performances were constantly observed. Based on the harsh nature of TWW, this review recommends integrated methods for TWW treatment to realize desirable decontamination efficacy, however optimization for methods combination and cost effectiveness should be further conducted in the future. It is admitted that Cr by-products and high salinity might be the huge defect of the most presented TWW treatment methods, while these issues could be partly resolved by combining additional treatment processes, and the promising cleaner production of Cr tanning processes is shedding light on the problems as well. In the future, the TWW treatment should focus more on multi-function, recycling, and intensification, the proposed plan that emphasizes on forementioned concepts in Sect. 5 might be one case. In conclusion, most TWW treatment methods at this stage have their own cons and pros, yet they could not serve as TWW treatment method independently. We believe the combination of various advanced methods capable of resources recycling simultaneously for TWW treatment would be the promising research direction in future.
Availability of data and materials
Available.
Abbreviations
- TWW:
-
Tannery wastewater
- COD:
-
Chemical oxygen demand
- BOD:
-
Biochemical oxygen demand
- TOC:
-
Total organic carbo
- TDS:
-
Total dissolved solids
- DBPs:
-
Dibutyl phthalates
- EC:
-
Electrocoagulation
- ED:
-
Electrodialysis
- CETPs:
-
Common effluent treatment plants
- CWs:
-
Constructed wetlands
- UASBs:
-
Up-flow anaerobic sludge blanket reactors
- MF:
-
Microfiltration
- UF:
-
Ultrafiltration
- NF:
-
Nanofiltration
- RO:
-
Reverse osmosis
- LDHs:
-
Layered double hydroxides
- PAN:
-
Polyacrylonitrile
- AOPs:
-
Advanced oxidation processes
- ROS:
-
Reactive oxidant species
- PDS:
-
Peroxydisulfate
- PMS:
-
Peroxomonosulfate
- EO:
-
Electro-oxidation
- DET:
-
Direct electron transfer
- MO:
-
Mediated oxidation
- EF:
-
Electro-Fenton
- PEF:
-
Photo-electro-Fenton
- MCE:
-
Mineralization current efficiency
- EEO:
-
Electric energy per one order magnitude
- ECTOC :
-
Specific energy cost per unit mass of TOC removed
- MFC:
-
Microbial fuel cells
- AOX:
-
Absorbable organic halogen
References
Lofrano G, Meric S, Zengin GE, Orhon D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: a review. Sci Total Environ. 2013;461:265–81. https://doi.org/10.1016/j.scitotenv.2013.05.004.
Tigini V, Giansanti P, Mangiavillano A, Pannocchia A, Varese GC. Evaluation of toxicity, genotoxicity and environmental risk of simulated textile and tannery wastewaters with a battery of biotests. Ecotoxicol Environ Saf. 2011;74(4):866–73. https://doi.org/10.1016/j.ecoenv.2010.12.001.
Montalvao MF, De Souza JM, Guimaraes ATB, De Menezes IPP, Castro ALD, Rodrigues ASD, Malafaia G. The genotoxicity and cytotoxicity of tannery effluent in bullfrog (Lithobates catesbeianus). Chemosphere. 2017;183:491–502. https://doi.org/10.1016/j.chemosphere.2017.05.080.
Kanagaraj G, Elango L. Hydrogeochemical processes and impact of tanning industries on groundwater quality in Ambur, Vellore district, Tamil Nadu, India. Environ Sci Pollut Res. 2016;23(23):24364–83. https://doi.org/10.1007/s11356-016-7639-4.
Arias-Barreiro CR, Nishizaki H, Okubo K, Aoyama I, Mori IC. Ecotoxicological characterization of tannery wastewater in Dhaka, Bangladesh. J Environ Biol. 2010;31(4):471–5.
Zhao C, Chen W. A review for tannery wastewater treatment: some thoughts under stricter discharge requirements. Environ Sci Pollut Res Int. 2019;26(25):26102–11. https://doi.org/10.1007/s11356-019-05699-6.
Zhang CX, Xia FM, Long JJ, Peng BY. An integrated technology to minimize the pollution of chromium in wet-end process of leather manufacture. J Clean Prod. 2017;154:276–83. https://doi.org/10.1016/j.jclepro.2017.03.216.
Sathish M, Madhan B, Sreeram KJ, Rao JR, Nair BU. Alternative carrier medium for sustainable leather manufacturing—a review and perspective. J Clean Prod. 2016;112:49–58. https://doi.org/10.1016/j.jclepro.2015.06.118.
Dettmer A, Cavalli E, Ayub MAZ, Gutterres M. Environmentally friendly hide unhairing: enzymatic hide processing for the replacement of sodium sulfide and delimig. J Clean Prod. 2013;47:11–8. https://doi.org/10.1016/j.jclepro.2012.04.024.
Maqbool A, Ali S, Rizwan M, Ishaque W, Rasool N, Rehman MZU, Bashir A, Abid M, Wu LH. Management of tannery wastewater for improving growth attributes and reducing chromium uptake in spinach through citric acid application. Environ Sci Pollut Res. 2018;25(11):10848–56. https://doi.org/10.1007/s11356-018-1352-4.
Wang YN, Zeng YH, Chai XW, Liao XP, He Q, Shi B. Ammonia nitrogen in tannery wastewater: distribution, origin and prevention. J Am Leather Chem Assoc. 2012;107(2):40–50.
Marsal A, Cuadros S, Olle L, Bacardit A, Manich AM, Font J. Formaldehyde scavengers for cleaner production: a case study focused on the leather industry. J Clean Prod. 2018;186:45–56. https://doi.org/10.1016/j.jclepro.2018.03.109.
Sundar VJ, Muralidharan C, Mandal AB. A novel chrome tanning process for minimization of total dissolved solids and chromium in effluents. J Clean Prod. 2013;59:239–44. https://doi.org/10.1016/j.jclepro.2013.07.002.
Rivela B, Moreira MT, Bornhardt C, Mendez R, Feijoo G. Life Cycle assessment as a tool for the environmental improvement of the tannery industry in developing countries. Environ Sci Technol. 2004;38(6):1901–9. https://doi.org/10.1021/es034316t.
Cooman K, Gajardo M, Nieto J, Bornhardt C, Vidal G. Tannery wastewater characterization and toxicity effects on Daphnia spp. Environ Toxicol. 2003;18(1):45–51. https://doi.org/10.1002/tox.10094.
Wang DD, Ye YX, Liu H, Ma HR, Zhang WM. Effect of alkaline precipitation on Cr species of Cr(III)-bearing complexes typically used in the tannery industry. Chemosphere. 2018;193:42–9. https://doi.org/10.1016/j.chemosphere.2017.11.006.
Tripathi PK, Rao NN, Chauhan C, Pophali GR, Kashyap SM, Lokhande SK, Gan LH. Treatment of refractory nano-filtration reject from a tannery using Pd-catalyzed wet air oxidation. J Hazard Mater. 2013;261:63–71. https://doi.org/10.1016/j.jhazmat.2013.07.002.
Zeng J, Gou M, Tang YQ, Li GY, Sun ZY, Kida KJ. Effective bioleaching of chromium in tannery sludge with an enriched sulfur-oxidizing bacterial community. Biores Technol. 2016;218:859–66. https://doi.org/10.1016/j.biortech.2016.07.051.
Srithar K, Mani A. Open fibre reinforced plastic (FRP) flat plate collector (FPC) and spray network systems for augmenting the evaporation rate of tannery effluent (soak liquor). Sol Energy. 2007;81(12):1492–500. https://doi.org/10.1016/j.solener.2007.02.004.
Sahu SK, Meshram P, Pandey BD, Kumar V, Mankhand TR. Removal of chromium(III) by cation exchange resin, Indion 790 for tannery waste treatment. Hydrometallurgy. 2009;99(3–4):170–4. https://doi.org/10.1016/j.hydromet.2009.08.002.
Dixit S, Yadav A, Dwivedi PD, Das M. Toxic hazards of leather industry and technologies to combat threat: a review. J Clean Prod. 2015;87:39–49. https://doi.org/10.1016/j.jclepro.2014.10.017.
Saxena G, Chandra R, Bharagava R N. Environmental pollution, toxicity profile and treatment approaches for tannery wastewater and its chemical pollutants. In: Reviews of environmental contamination and toxicology, vol 240. P. DeVoogt, Editor. 2017. p. 31–69.
Yadav A, Raj A, Purchase D, Ferreira LFR, Saratale GD, Bharagava RN. Phytotoxicity, cytotoxicity and genotoxicity evaluation of organic and inorganic pollutants rich tannery wastewater from a Common Effluent Treatment Plant (CETP) in Unnao district, India using Vigna radiata and Allium cepa. Chemosphere. 2019;224:324–32. https://doi.org/10.1016/j.chemosphere.2019.02.124.
Bharagava RN, Saxena G, Mulla SI, Patel DK. Characterization and identification of recalcitrant organic pollutants (rops) in tannery wastewater and its phytotoxicity evaluation for environmental safety. Arch Environ Contam Toxicol. 2018;75(2):259–72. https://doi.org/10.1007/s00244-017-0490-x.
Pathe PP, SureshKumar M, Kharwade KSN. Common effluent treatment plant (CEPT) for wastewater management from a cluster of small scale tanneries. Environ Technol. 2004;25(5):555–63. https://doi.org/10.1080/09593332608618562c.
Chandra R, Bharagava RN, Kapley A, Purohit HJ. Bacterial diversity, organic pollutants and their metabolites in two aeration lagoons of common effluent treatment plant (CETP) during the degradation and detoxification of tannery wastewater. Biores Technol. 2011;102(3):2333–41. https://doi.org/10.1016/j.biortech.2010.10.087.
Verma T, Ramteke PW, Garg SK. Quality assessment of treated tannery wastewater with special emphasis on pathogenic E-coli detection through serotyping. Environ Monit Assess. 2008;145(1–3):243–9. https://doi.org/10.1007/s10661-007-0033-4.
Zhou H, Tan Z, Li X. Assessment of wastewater pollution in pig leather industry in China. Water Environ J. 2012;26(4):521–9. https://doi.org/10.1111/j.1747-6593.2012.00312.x.
Khanh Tran T, Jyh Leu H, Quyet VuT, Tam Nguyen M, Anh Pham T, Kiefer R. Hydrogen production from the tannery wastewater treatment by using agriculture supports membrane/adsorbents electrochemical system. Int J Hydrogen Energy. 2020;45(6):3699–711. https://doi.org/10.1016/j.ijhydene.2019.05.040.
Alemu T, Mekonnen A, Leta S. Integrated tannery wastewater treatment for effluent reuse for irrigation: Encouraging water efficiency and sustainable development in developing countries. J Water Process Eng. 2019;30: 100514. https://doi.org/10.1016/j.jwpe.2017.10.014.
Ali Z, Malik RN, Qadir A. Heavy metals distribution and risk assessment in soils affected by tannery effluents. Chem Ecol. 2013;29(8):676–92. https://doi.org/10.1080/02757540.2013.810728.
Tolkou AK, Zouboulis AI. Synthesis and coagulation performance of composite poly-aluminum-ferric-silicate-chloride coagulants in water and wastewater. Desalin Water Treat. 2015;53(12):3309–18. https://doi.org/10.1080/19443994.2014.933614.
Ayoub GM, Hamzeh A, Semerjian L. Post treatment of tannery wastewater using lime/bittern coagulation and activated carbon adsorption. Desalination. 2011;273(2–3):359–65. https://doi.org/10.1016/j.desal.2011.01.045.
Puchana-Rosero MJ, Lima EC, Mella B, Da Costa D, Poll E, Gutterres M. A coagulation-flocculation process combined with adsorption using activated carbon obtained from sludge for dye removal from tannery wastewater. J Chil Chem Soc. 2018;63(1):3867–74. https://doi.org/10.4067/s0717-97072018000103867.
Song Z, Williams CJ, Edyvean RGJ. Treatment of tannery wastewater by chemical coagulation. Desalination. 2004;164(3):249–59. https://doi.org/10.1016/s0011-9164(04)00193-6.
Mageshkumar M, Karthikeyan R. Modelling the kinetics of coagulation process for tannery industry effluent treatment using Moringa oleifera seeds protein. Desalin Water Treat. 2016;57(32):14954–64. https://doi.org/10.1080/19443994.2015.1070294.
Zhu JF, Zhang GH, Li JG. Preparation of amphoteric polyacrylamide flocculant and its application in the treatment of tannery wastewater. J Appl Polym Sci. 2011;120(1):518–23. https://doi.org/10.1002/app.33175.
Manenti DR, Modenes AN, Soares PA, Boaventura RAR, Palacio SM, Borba FH, Espinoza-Quinones FR, Bergamasco R, Vilar VJP. Biodegradability and toxicity assessment of a real textile wastewater effluent treated by an optimized electrocoagulation process. Environ Technol. 2015;36(4):496–506. https://doi.org/10.1080/09593330.2014.952676.
Elabbas S, Ouazzani N, Mandi L, Berrekhis F, Perdicakis M, Pontvianne S, Pons MN, Lapicque F, Leclerc JP. Treatment of highly concentrated tannery wastewater using electrocoagulation: Influence of the quality of aluminium used for the electrode. J Hazard Mater. 2016;319:69–77. https://doi.org/10.1016/j.jhazmat.2015.12.067.
Deghles A, Kurt U. Treatment of raw tannery wastewater by electrocoagulation technique: optimization of effective parameters using Taguchi method. Desalin Water Treat. 2016;57(32):14798–809. https://doi.org/10.1080/19443994.2015.1074622.
Bazrafshan E, Mohammadi L, Ansari-Moghaddam A, Mahvi AH. Heavy metals removal from aqueous environments by electrocoagulation process—a systematic review. J Environ Health Sci Eng. 2015. https://doi.org/10.1186/s40201-015-0233-8.
Fernandes A, Pacheco MJ, Ciríaco L, Lopes A. Review on the electrochemical processes for the treatment of sanitary landfill leachates: Present and future. Appl Catal B. 2015;176–177:183–200. https://doi.org/10.1016/j.apcatb.2015.03.052.
Tamersit S, Bouhidel KE, Zidani Z. Investigation of electrodialysis anti-fouling configuration for desalting and treating tannery unhairing wastewater: feasibility of by-products recovery and water recycling. J Environ Manag. 2018;207:334–40. https://doi.org/10.1016/j.jenvman.2017.11.058.
Deghles A, Kurt U. Treatment of tannery wastewater by a hybrid electrocoagulation/electrodialysis process. Chem Eng Processing-Process Intensific. 2016;104:43–50. https://doi.org/10.1016/j.cep.2016.02.009.
Malaviya P, Singh A. Physicochemical technologies for remediation of chromium-containing waters and wastewaters. Crit Rev Environ Sci Technol. 2011;41(12):1111–72. https://doi.org/10.1080/10643380903392817.
Vidal G, Nieto J, Cooman K, Gajardo M, Bornhardt C. Unhairing effluents treated by an activated sludge system. J Hazard Mater. 2004;112(1–2):143–9. https://doi.org/10.1016/j.jhazmat.2004.04.004.
Wang Z, Zhang XX, Lu X, Liu B, Li Y, Long C, Li AM. Abundance and Diversity of bacterial nitrifiers and denitrifiers and their functional genes in tannery wastewater treatment plants revealed by high-throughput sequencing. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0113603.
Tripathi M, Vikram S, Jain RK, Garg SK. Isolation and growth characteristics of chromium(VI) and pentachlorophenol tolerant bacterial isolate from treated tannery effluent for its possible use in simultaneous bioremediation. Indian J Microbiol. 2011;51(1):61–9. https://doi.org/10.1007/s12088-011-0089-2.
Sul WJ, Kim IS, Ekpeghere KI, Song B, Kim BS, Kim HG, Kim JT, Koh SC. Metagenomic insight of nitrogen metabolism in a tannery wastewater treatment plant bioaugmented with the microbial consortium BM-S-1. J Environ Sci Health A Toxic Hazard Subst Environ Eng. 2016;51(13):1164–72. https://doi.org/10.1080/10934529.2016.1206387.
Sivaprakasam S, Dhandapani B, Mahadevan S. Optimization studies on production of a salt-tolerant protease from pseudomonas aeruginosa strain bc1 and its application on tannery saline wastewater treatment. Braz J Microbiol. 2011;42(4):1506–15. https://doi.org/10.1590/s1517-83822011000400038.
Bharagava RN, Yadav S, Chandra R. Antibiotic and heavy metal resistance properties of bacteria isolated from the aeration lagoons of common effluent treatment plant (CETP) of tannery industries (Unnao, India). Indian J Biotechnol. 2014;13(4):514–9.
Zhuang XL, Han Z, Bai ZH, Zhuang GQ, Shim HJ. Progress in decontamination by halophilic microorganisms in saline wastewater and soil. Environ Pollut. 2010;158(5):1119–26. https://doi.org/10.1016/j.envpol.2010.01.007.
Sharma S, Malaviya P. Bioremediation of tannery wastewater by chromium resistant novel fungal consortium. Ecol Eng. 2016;91:419–25. https://doi.org/10.1016/j.ecoleng.2016.03.005.
Paisio CE, Quevedo MR, Talano MA, Gonzalez PS, Agostini E. Application of two bacterial strains for wastewater bioremediation and assessment of phenolics biodegradation. Environ Technol. 2014;35(14):1802–10. https://doi.org/10.1080/09593330.2014.882994.
Okoduwa SIR, Igiri B, Udeh CB, Edenta C, Gauje B. Tannery effluent treatment by yeast species isolates from watermelon. Toxics. 2017. https://doi.org/10.3390/toxics5010006.
Baccar R, Blanquez P, Bouzid J, Feki M, Attiya H, Sarra M. Decolorization of a tannery dye: from fungal screening to bioreactor application. Biochem Eng J. 2011;56(3):184–9. https://doi.org/10.1016/j.bej.2011.06.006.
Sivaprakasam S, Mahadevan S, Sekar S, Rajakumar S. Biological treatment of tannery wastewater by using salt-tolerant bacterial strains. Microb Cell Fact. 2008. https://doi.org/10.1186/1475-2859-7-15.
Li D, Liang XH, Jin Y, Wu CD, Zhou RQ. Isolation and nitrogen removal characteristics of an aerobic heterotrophic nitrifying-denitrifying bacterium, Klebsiella sp. TN-10. Appl Biochem Biotechnol. 2019;188(2):540–54. https://doi.org/10.1007/s12010-018-02932-9.
Kalyanaraman C, Kameswari KSB, Varma VS, Tagra S, Rao JR. Studies on biodegradation of vegetable-based fat liquor-containing wastewater from tanneries. Clean Technol Environ Policy. 2013;15(4):633–42. https://doi.org/10.1007/s10098-012-0551-9.
Maharaja P, Mahesh M, Chitra C, Kalaivani D, Srividya R, Swarnalatha S, Sekaran G. Sequential oxic-anoxic bio reactor for the treatment of tannery saline wastewater using halophilic and filamentous bacteria. J Water Process Eng. 2017;18:47–57. https://doi.org/10.1016/j.jwpe.2017.03.011.
Fathima A, Rao JR, Unni NB. Trivalent chromium removal from tannery effluent using kaolin-supported bacterial biofilm of Bacillus sp isolated from chromium polluted soil. J Chem Technol Biotechnol. 2012;87(2):271–9. https://doi.org/10.1002/jctb.2710.
Lu J, Yan X, Ma YF, Tian CX, Ding JC. Impact of salinity on treatment of saline wastewater by sequencing batch biofilm reactor process. J Cent South Univ. 2014;21(5):1989–94. https://doi.org/10.1007/s11771-014-2147-5.
Xiao YY, Roberts DJ. A review of anaerobic treatment of saline wastewater. Environ Technol. 2010;31(8–9):1025–43. https://doi.org/10.1080/09593331003734202.
Umaiyakunjaram R, Shanmugam P. Study on submerged anaerobic membrane bioreactor (SAMBR) treating high suspended solids raw tannery wastewater for biogas production. Biores Technol. 2016;216:785–92. https://doi.org/10.1016/j.biortech.2016.06.017.
Ei-Sheikh MA, Saleh HI, Flora JR, Abdel-Ghany MR. Biological tannery wastewater treatment using two stage UASB reactors. Desalination. 2011;276(1–3):253–9. https://doi.org/10.1016/j.desal.2011.03.060.
Anjali G, Sabumon PC. Development of of simultaneous partial nitrification, anammox and denitrification (SNAD) in a non-aerated SBR. Int Biodet Biodegrad. 2017;119:43–55. https://doi.org/10.1016/j.ibiod.2016.10.047.
Anjali G, Sabumon PC. Development of enhanced SNAD process in a down-flow packed bed reactor for removal of higher concentrations of NH4-N and COD. J Environ Chem Eng. 2015;3(2):1009–17. https://doi.org/10.1016/j.jece.2015.02.022.
Song Z, Williams CJ, Edyvean RGJ. Tannery wastewater treatment using an upflow anaerobic fixed biofilm reactor (UAFBR). Environ Eng Sci. 2003;20(6):587–99. https://doi.org/10.1089/109287503770736104.
Desta AF, Assefa F, Leta S, Stomeo F, Wamalwa M, Njahira M, Appolinaire D. Microbial community structure and diversity in an integrated system of anaerobic-aerobic reactors and a constructed wetland for the treatment of tannery wastewater in Modjo, Ethiopia. PLoS ONE. 2014;9(12):1. https://doi.org/10.1371/journal.pone.0115576.
Sodhi V, Bansal A, Jha MK. Excess sludge disruption and pollutant removal from tannery effluent by upgraded activated sludge system. Biores Technol. 2018;263:613–24. https://doi.org/10.1016/j.biortech.2018.04.118.
Tadesse I, Isoaho SA, Green FB, Puhakka JA. Removal of organics and nutrients from tannery effluent by advanced integrated wastewater pond systems (R) technology. Water Sci Technol. 2003;48(2):307–14. https://doi.org/10.2166/wst.2003.0135.
Vymazal J. The use constructed wetlands with horizontal sub-surface flow for various types of wastewater. Ecol Eng. 2009;35(1):1–17. https://doi.org/10.1016/j.ecoleng.2008.08.016.
Vymazal J. Constructed wetlands for treatment of industrial wastewaters: a review. Ecol Eng. 2014;73:724–51. https://doi.org/10.1016/j.ecoleng.2014.09.034.
Sultana M-Y, Akratos CS, Vayenas DV, Pavlou S. Constructed wetlands in the treatment of agro-industrial wastewater: a review. Hemij Ind. 2015;69(2):127–42. https://doi.org/10.2298/hemind150121018s.
Ramirez S, Torrealba G, Lameda-Cuicas E, Molina-Quintero L, Stefanakis AI, Pire-Sierra MC. Investigation of pilot-scale constructed wetlands treating simulated pre-treated tannery wastewater under tropical climate. Chemosphere. 2019;234:496–504. https://doi.org/10.1016/j.chemosphere.2019.06.081.
Zapana JSP, Arán DS, Bocardo EF, Harguinteguy CA. Treatment of tannery wastewater in a pilot scale hybrid constructed wetland system in Arequipa, Peru. Int J Environ Sci Technol. 2020;17(11):4419–30. https://doi.org/10.1007/s13762-020-02797-8.
Saeed T, Afrin R, Al Muyeed A, Sun GZ. Treatment of tannery wastewater in a pilot-scale hybrid constructed wetland system in Bangladesh. Chemosphere. 2012;88(9):1065–73. https://doi.org/10.1016/j.chemosphere.2012.04.055.
Shahid MJ, Tahseen R, Siddique M, Ali S, Iqbal S, Afzal M. Remediation of polluted river water by floating treatment wetlands. Water Sci Technol Water Supply. 2019;19(3):967–77. https://doi.org/10.2166/ws.2018.154.
Kaseva ME, Mbuligwe SE. Potential of constructed wetland systems for treating tannery industrial wastewater. Water Sci Technol. 2010;61(4):1043–52. https://doi.org/10.2166/wst.2010.474.
Dotro G, Larsen D, Palazolo P. Treatment of chromium-bearing wastewaters with constructed wetlands. Water Environ J. 2011;25(2):241–9. https://doi.org/10.1111/j.1747-6593.2010.00216.x.
Dotro G, Castro S, Tujchneider O, Piovano N, Paris M, Faggi A, Palazolo P, Larsen D, Fitch M. Performance of pilot-scale constructed wetlands for secondary treatment of chromium-bearing tannery wastewaters. J Hazard Mater. 2012;239:142–51. https://doi.org/10.1016/j.jhazmat.2012.08.050.
Ashraf S, Afzal M, Naveed M, Shahid M, Zahir ZA. Endophytic bacteria enhance remediation of tannery effluent in constructed wetlands vegetated with Leptochloa fusca. Int J Phytorem. 2018;20(2):121–8. https://doi.org/10.1080/15226514.2017.1337072.
Calheiros CSC, Quiterio PVB, Silva G, Crispim LFC, Brix H, Moura SC, Castro PML. Use of constructed wetland systems with Arundo and Sarcocornia for polishing high salinity tannery wastewater. J Environ Manag. 2012;95(1):66–71. https://doi.org/10.1016/j.jenvman.2011.10.003.
Calheiros CSC, Rangel A, Castro PML. Constructed wetlands for tannery wastewater treatment in Portugal: ten years of experience. Int J Phytorem. 2014;16(9):859–70. https://doi.org/10.1080/15226514.2013.798622.
Ray M, Bhattacharya P, Das R, Sondhi K, Ghosh S, Sarkar S. Preparation and characterization of macroporous pure alumina capillary membrane using boehmite as binder for filtration application. J Porous Mater. 2015;22(4):1043–52. https://doi.org/10.1007/s10934-015-9978-9.
Mouiya M, Abourriche A, Bouazizi A, Benhammou A, El Hafiane Y, Abouliatim Y, Nibou L, Oumam M, Ouammou M, Smith A, Hannache H. Flat ceramic microfiltration membrane based on natural clay and Moroccan phosphate for desalination and industrial wastewater treatment. Desalination. 2018;427:42–50. https://doi.org/10.1016/j.desal.2017.11.005.
Beqqour D, Achiou B, Bouazizi A, Ouaddari H, Elomari H, Ouammou M, Bennazha J, Younssi SA. Enhancement of microfiltration performances of pozzolan membrane by incorporation of micronized phosphate and its application for industrial wastewater treatment. J Environ Chem Eng. 2019. https://doi.org/10.1016/j.jece.2019.102981.
Saja S, Bouazizi A, Achiou B, Ouammou M, Albizane A, Bennazha J, Younssi SA. Elaboration and characterization of low-cost ceramic membrane made from natural Moroccan perlite for treatment of industrial wastewater. J Environ Chem Eng. 2018;6(1):451–8. https://doi.org/10.1016/j.jece.2017.12.004.
Majouli A, Tahiri S, Younssi SA, Loukili H, Albizane A. Elaboration of new tubular ceramic membrane from local Moroccan Perlite for microfiltration process. Application to treatment of industrial wastewaters. Ceram Int. 2012;38(5):4295–303. https://doi.org/10.1016/j.ceramint.2012.02.010.
Velu S, Muruganandam L, Arthanareeswaran G. Preparation and performance studies on polyethersulfone ultrafiltration membranes modified with gelatin for treatment of tannery and distillery wastewater. Braz J Chem Eng. 2015;32(1):179–89. https://doi.org/10.1590/0104-6632.20150321s00002965.
Religa P, Kowalik A, Gierycz P. Effect of membrane properties on chromium(III) recirculation from concentrate salt mixture solution by nanofiltration. Desalination. 2011;274(1–3):164–70. https://doi.org/10.1016/j.desal.2011.02.006.
Gore CT, Omwoma S, Chen W, Song YF. Interweaved LDH/PAN nanocomposite films: application in the design of effective hexavalent chromium adsorption technology. Chem Eng J. 2016;284:794–801. https://doi.org/10.1016/j.cej.2015.09.056.
Suthanthararajan R, Ravindranath E, Chitra K, Umamaheswari B, Ramesh T, Rajamani S. Membrane application for recovery and reuse of water from treated tannery wastewater. Desalination. 2004;164(2):151–6. https://doi.org/10.1016/s0011-9164(04)00174-2.
Guo SW, Wan YH, Chen XR, Luo JQ. Loose nanofiltration membrane custom-tailored for resource recovery. Chem Eng J. 2021;409:24. https://doi.org/10.1016/j.cej.2020.127376.
Cassano A, Molinari R, Romano M, Drioli E. Treatment of aqueous effluents of the leather industry by membrane processes—a review. J Membr Sci. 2001;181(1):111–26. https://doi.org/10.1016/s0376-7388(00)00399-9.
Dasgupta J, Mondal D, Chakraborty S, Sikder J, Curcio S, Arafat HA. Nanofiltration based water reclamation from tannery effluent following coagulation pretreatment. Ecotoxicol Environ Saf. 2015;121:22–30. https://doi.org/10.1016/j.ecoenv.2015.07.006.
Keerthi R, Vinduja V, Balasubramanian N. Electrocoagulation-integrated hybrid membrane processes for the treatment of tannery wastewater. Environ Sci Pollut Res. 2013;20(10):7441–9. https://doi.org/10.1007/s11356-013-1766-y.
Lujan-Facundo M, Fernandez-Navarro J, Alonso-Molina JL, Amoros-Munoz I, Moreno Y, Mendoza-Roca JA, Pastor-Alcaniz L. The role of salinity on the changes of the biomass characteristics and on the performance of an OMBR treating tannery wastewater. Water Res. 2018;142:129–37. https://doi.org/10.1016/j.watres.2018.05.046.
Artiga P, Ficara E, Malpei F, Garrido JM, Mendez R. Treatment of two industrial wastewaters in a submerged membrane bioreactor. Desalination. 2005;179(1–3):161–9. https://doi.org/10.1016/j.desal.2004.11.064.
Korpe S, Rao PV. Application of advanced oxidation processes and cavitation techniques for treatment of tannery wastewater—a review. J Environ Chem Eng. 2021;9(3): 105234. https://doi.org/10.1016/j.jece.2021.105234.
Miklos DB, Remy C, Jekel M, Linden KG, Drewes JE, Hübner U. Evaluation of advanced oxidation processes for water and wastewater treatment—a critical review. Water Res. 2018;139:118–31. https://doi.org/10.1016/j.watres.2018.03.042.
Lofrano G, Meric S, Inglese M, Nikolau A, Belgiorno V. Fenton oxidation treatment of tannery wastewater and tanning agents: synthetic tannin and nonylphenol ethoxylate based degreasing agent. Desalin Water Treat. 2010;23(1–3):173–80. https://doi.org/10.5004/dwt.2010.1991.
Karthikeyan S, Priya ME, Boopathy R, Velan M, Mandal AB, Sekaran G. Heterocatalytic Fenton oxidation process for the treatment of tannery effluent: kinetic and thermodynamic studies. Environ Sci Pollut Res. 2012;19(5):1828–40. https://doi.org/10.1007/s11356-011-0691-1.
Vilardi G, Rodriguez-Rodriguez J, Ochando-Pulido JM, Verdone N, Martinez-Ferez A, Di Palma L. Large Laboratory-Plant application for the treatment of a Tannery wastewater by Fenton oxidation: Fe(II) and nZVI catalysts comparison and kinetic modelling. Process Saf Environ Prot. 2018;117:629–38. https://doi.org/10.1016/j.psep.2018.06.007.
Huang GD, Pan F, Fan GF, Liu GG. Application of heterogeneous catalytic ozonation as a tertiary treatment of effluent of biologically treated tannery wastewater. J Environ Sci Health A Toxic Hazard Subst Environ Eng. 2016;51(8):626–33. https://doi.org/10.1080/10934529.2016.1159863.
Preethi V, Parama Kalyani KS, Iyappan K, Srinivasakannan C, Balasubramaniam N, Vedaraman N. Ozonation of tannery effluent for removal of cod and color. J Hazard Mater. 2009;166(1):150–4. https://doi.org/10.1016/j.jhazmat.2008.11.035.
Sivagami K, Sakthivel KP, Nambi IM. Advanced oxidation processes for the treatment of tannery wastewater. J Environ Chem Eng. 2018;6(3):3656–63. https://doi.org/10.1016/j.jece.2017.06.004.
Lee KM, Lai CW, Ngai KS, Juan JC. Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res. 2016;88:428–48. https://doi.org/10.1016/j.watres.2015.09.045.
Schrank SG, José HJ, Moreira RFPM, Schröder HF. Applicability of Fenton and H2O2/UV reactions in the treatment of tannery wastewaters. Chemosphere. 2005;60(5):644–55. https://doi.org/10.1016/j.chemosphere.2005.01.033.
Karthikeyan S, Boopathy R, Sekaran G. In situ generation of hydroxyl radical by cobalt oxide supported porous carbon enhance removal of refractory organics in tannery dyeing wastewater. J Colloid Interface Sci. 2015;448:163–74. https://doi.org/10.1016/j.jcis.2015.01.066.
Moradi M, Moussavi G. Enhanced treatment of tannery wastewater using the electrocoagulation process combined with UVC/VUV photoreactor: parametric and mechanistic evaluation. Chem Eng J. 2019;358:1038–46. https://doi.org/10.1016/j.cej.2018.10.069.
Schrank SG, Jose HJ, Moreira R, Schroder HF. Comparison of different Advanced Oxidation Process to reduce toxicity and mineralisation of tannery wastewater. Water Sci Technol. 2004;50(5):329–34. https://doi.org/10.2166/wst.2004.0345.
Chaplin A, Brian P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ Sci Processes Impacts. 2014;16(6):1182. https://doi.org/10.1039/C3EM00679D.
Zhuo Q, Deng S, Yang B, Huang J, Yu G. Efficient electrochemical oxidation of perfluorooctanoate using a Ti/SnO2-Sb-Bi anode. Environ Sci Technol. 2011;45(7):2973–9. https://doi.org/10.1021/es1024542.
Carter KE, Farrell J. Oxidative destruction of perfluorooctane sulfonate using boron-doped diamond film electrodes. Environ Sci Technol. 2008;42(16):6111–5. https://doi.org/10.1021/es703273s.
Sires I, Brillas E, Oturan MA, Rodrigo MA, Panizza M. Electrochemical advanced oxidation processes: today and tomorrow. A review. Environ Sci Pollut Res Int. 2014;21(14):8336–67. https://doi.org/10.1007/s11356-014-2783-1.
Naumczyk JH, Kucharska MA. Electrochemical treatment of tannery wastewaterRaw, coagulated, and pretreated by AOPs. J Environ Sci Health A Toxic Hazard Subst Environ Eng. 2017;52(7):649–64. https://doi.org/10.1080/10934529.2017.1297140.
Ding J, Zhao QL, Zhang YS, Wei LL, Li W, Wang K. The eAND process: enabling simultaneous nitrogen-removal and disinfection for WWTP effluent. Water Res. 2015;74:122–31. https://doi.org/10.1016/j.watres.2015.02.005.
Cruz-Rizo A, Gutierrez-Granados S, Salazar R, Peralta-Hernandez JM. Application of electro-Fenton/BDD process for treating tannery wastewaters with industrial dyes. Sep Purif Technol. 2017;172:296–302. https://doi.org/10.1016/j.seppur.2016.08.029.
Szpyrkowicz L, Naumczyk J, Grandi FZ. Electrochemical treatment of tannery wastewater using TiPt and Ti_Pt_Ir electrodes. War Res. 2016;29(2):517–24. https://doi.org/10.1016/0043-1354(94)00176-8.
Shestakova M, Vinatoru M, Mason TJ, Iakovleva E, Sillanpaa M. Sonoelectrochemical degradation of formic acid using Ti/Ta2O5-SnO2 electrodes. J Mol Liq. 2016;223:388–94. https://doi.org/10.1016/j.molliq.2016.08.054.
Siqueira IR, Vanzella C, Bianchetti P, Rodrigues MAS, Stulp S. Anxiety-like behaviour in mice exposed to tannery wastewater: the effect of photoelectrooxidation treatment. Neurotoxicol Teratol. 2011;33(4):481–4. https://doi.org/10.1016/j.ntt.2011.05.008.
Sundarapandiyan S, Chandrasekar R, Ramanaiah B, Krishnan S, Saravanan P. Electrochemical oxidation and reuse of tannery saline wastewater. J Hazard Mater. 2010;180(1–3):197–203. https://doi.org/10.1016/j.jhazmat.2010.04.013.
Galiana-Aleixandre MV, Mendoza-Roca JA, Bes-Pia A. Reducing sulfates concentration in the tannery effluent by applying pollution prevention techniques and nanofiltration. J Clean Prod. 2011;19(1):91–8. https://doi.org/10.1016/j.jclepro.2010.09.006.
Shin Y-U, Yoo H-Y, Ahn Y-Y, Kim MS, Lee K, Yu S, Lee C, Cho K, Kim H-I, Lee J. Electrochemical oxidation of organics in sulfate solutions on boron-doped diamond electrode: multiple pathways for sulfate radical generation. Appl Catal B. 2019;254:156–65. https://doi.org/10.1016/j.apcatb.2019.04.060.
Niu T, Cai J, Shi P, Zhao G. Unique electrochemical system for in situ SO4− generation and pollutants degradation. Chem Eng J. 2020;386:6. https://doi.org/10.1016/j.cej.2019.123971.
Cai J, Zhou M, Pan Y, Du X, Lu X. Extremely efficient electrochemical degradation of organic pollutants with co-generation of hydroxyl and sulfate radicals on Blue-TiO2 nanotubes anode. Appl Catal B Environ. 2019;257:2. https://doi.org/10.1016/j.apcatb.2019.117902.
Thiam A, Sires I, Antonio Garrido J, Maria Rodriguez R, Brillas E. Effect of anions on electrochemical degradation of azo dye Carmoisine (Acid Red 14) using a BDD anode and air-diffusion cathode. Sep Purif Technol. 2015;140:43–52. https://doi.org/10.1016/j.seppur.2014.11.012.
Xie R, Meng X, Sun P, Niu J, Jiang W, Bottomley L, Li D, Chen Y, Crittenden J. Electrochemical oxidation of ofloxacin using a TiO2-based SnO2-Sb/polytetrafluoroethylene resin-PbO2 electrode: reaction kinetics and mass transfer impact. Appl Catal B Environ. 2017;203:515–25. https://doi.org/10.1016/j.apcatb.2016.10.057.
Martínez-Huitle CA, Brillas E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B. 2009;87(3):105–45. https://doi.org/10.1016/j.apcatb.2008.09.017.
Brillas E, Martínez-Huitle CA. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl Catal B Environ. 2015;166–167:603–43. https://doi.org/10.1016/j.apcatb.2014.11.016.
Szpyrkowicz L, Kaul SN, Neti RN, Satyanarayan S. Influence of anode material on electrochemical oxidation for the treatment of tannery wastewater. Water Res. 2005;39(8):1601–13. https://doi.org/10.1016/j.watres.2005.01.016.
Tien TT, Luu TL. Electrooxidation of tannery wastewater with continuous flow system: Role of electrode materials. Environ Eng Res. 2019;25(3):324–34. https://doi.org/10.4491/eer.2018.349.
Le Luu T, Stephane DDF, Minh NH, Canh ND, Thanh BX. Electrochemical oxidation as a post treatment for biologically tannery wastewater in batch reactor. Water Sci Technol. 2019;80(7):1326–37. https://doi.org/10.2166/wst.2019.380.
Pavithra KG, Kumar PS, Christopher FC, Saravanan A. Removal of toxic Cr(VI) ions from tannery industrial wastewater using a newly designed three-phase three-dimensional electrode reactor. J Phys Chem Solids. 2017;110:379–85. https://doi.org/10.1016/j.jpcs.2017.07.002.
Palani R, Abdulgani A, Balasubramanian N. Treatment of tannery effluent using a rotating disc electrochemical reactor. Water Environ Res. 2017;89(1):77–85. https://doi.org/10.2175/106143016X14609975746046.
Li J, Ma J, Dai R, Wang X, Chen M, Waite TD, Wang Z. Self-enhanced decomplexation of cu-organic complexes and cu recovery from wastewaters using an electrochemical membrane filtration system. Environ Sci Technol. 2021;55(1):655–64. https://doi.org/10.1021/acs.est.0c05554.
Li Z, Shen C, Liu Y, Ma C, Li F, Yang B, Huang M, Wang Z, Dong L, Wolfgang S. Carbon nanotube filter functionalized with iron oxychloride for flow-through electro-Fenton. Appl Catal Environ. 2020. https://doi.org/10.1016/j.apcatb.2019.118204.
Zaky AM, Chaplin BP. Mechanism of p-substituted phenol oxidation at a Ti4O7 reactive electrochemical membrane. Environ Sci Technol. 2014;48(10):5857–67. https://doi.org/10.1021/es5010472.
Chen M, Pan S, Zhang C, Wang C, Zhang W, Chen Z, Zhao X, Zhao Y. Electrochemical oxidation of reverse osmosis concentrates using enhanced TiO2-NTA/SnO2-Sb anodes with/without PbO2 layer. Chem Eng J. 2020;3:99. https://doi.org/10.1016/j.cej.2020.125756.
Yu H, Li Y, Zhao M, Dong H, Yu H, Zhan S, Zhang L. Energy-saving removal of methyl orange in high salinity wastewater by electrochemical oxidation via a novel Ti/SnO2-Sb anode—air diffusion cathode system. Catal Today. 2015;258:156–61. https://doi.org/10.1016/j.cattod.2015.04.030.
Lalia B S,Hashaikeh R. Electrochemical precipitation to reduce waste brine salinity. Desalination. 2021; 498. doi: https://doi.org/10.1016/j.desal.2020.114796
Sirajuddin R, Kakakhel L, Lutfullah G, Bhanger MI, Shah A, Niaz A. Electrolytic recovery of chromium salts from tannery wastewater. J Hazard Mater. 2007;148(3):560–5. https://doi.org/10.1016/j.jhazmat.2007.03.011.
Da Silva GS, Dos Santos FA, Roth G, Frankenberg CLC. Electroplating for chromium removal from tannery wastewater. Int J Environ Sci Technol. 2019;17(2):607–14. https://doi.org/10.1007/s13762-019-02494-1.
Elabed A, El Abed S, Ibnsouda S, Erable B. Sustainable approach for tannery wastewater treatment: bioelectricity generation in bioelectrochemical systems. Arab J Sci Eng. 2019;44(12):10057–66. https://doi.org/10.1007/s13369-019-04050-y.
Rajeswari S, Vidhya S, Krishnaraj RN, Saravanan P, Sundarapandiyan S, Maruthamuthu S, Ponmariappan S, Vijayan M. Utilization of soak liquor in microbial fuel cell. Fuel. 2016;181:148–56. https://doi.org/10.1016/j.fuel.2016.04.121.
Sawasdee V, Pisutpaisal N. Simultaneous pollution treatment and electricity generation of tannery wastewater in air-cathode single chamber MFC. Int J Hydrogen Energy. 2016;41(35):15632–7. https://doi.org/10.1016/j.ijhydene.2016.04.179.
Grattieri M, Minteer SD. Microbial fuel cells in saline and hypersaline environments: advancements, challenges and future perspectives. Bioelectrochemistry. 2018;120:127–37. https://doi.org/10.1016/j.bioelechem.2017.12.004.
Garcia-Segura S, Ocon JD, Chong MN. Electrochemical oxidation remediation of real wastewater effluents—a review. Process Saf Environ Prot. 2018;113:48–67. https://doi.org/10.1016/j.psep.2017.09.014.
Gupta VK, Ali I, Saleh TA, Siddiqui MN, Agarwal S. Chromium removal from water by activated carbon developed from waste rubber tires. Environ Sci Pollut Res. 2013;20(3):1261–8. https://doi.org/10.1007/s11356-012-0950-9.
Mohan D, Singh KP, Singh VK. Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. J Hazard Mater. 2006;135(1–3):280–95. https://doi.org/10.1016/j.jhazmat.2005.11.075.
Ayele L, Perez E, Mayoral A, Chebude Y, Diaz I. Synthesis of zeolite A using raw kaolin from Ethiopia and its application in removal of Cr(III) from tannery wastewater. J Chem Technol Biotechnol. 2018;93(1):146–54. https://doi.org/10.1002/jctb.5334.
Volzone C, Garrido LB. Use of modified hydroxy-aluminum bentonites for chromium(III) removal from solutions. J Environ Manag. 2008;88(4):1640–8. https://doi.org/10.1016/j.jenvman.2007.08.003.
Aljerf L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study. J Environ Manag. 2018;225:120–32. https://doi.org/10.1016/j.jenvman.2018.07.048.
Piccin JS, Gomes CS, Mella B, Gutterres M. Color removal from real leather dyeing effluent using tannery waste as an adsorbent. J Environ Chem Eng. 2016;4(1):1061–7. https://doi.org/10.1016/j.jece.2016.01.010.
Puchana-Rosero MJ, Adebayo MA, Lima EC, Machado FM, Thue PS, Vaghetti JCP, Umpierres CS, Gutterres M. Microwave-assisted activated carbon obtained from the sludge of tannery-treatment effluent plant for removal of leather dyes. Colloids Surf A Physicochem Eng Asp. 2016;504:105–15. https://doi.org/10.1016/j.colsurfa.2016.05.059.
Mella B, Puchana-Rosero MJ, Costa DES, Gutterres M. Utilization of tannery solid waste as an alternative biosorbent for acid dyes in wastewater treatment. J Mol Liq. 2017;242:137–45. https://doi.org/10.1016/j.molliq.2017.06.131.
Hashem MA, Momen MA, Hasan M, Nur-a-Tomal MS, Sheikh MHR. Chromium removal from tannery wastewater using Syzygium cumini bark adsorbent. Int J Environ Sci Technol. 2019;16(3):1395–404. https://doi.org/10.1007/s13762-018-1714-y.
Ugya AY, Hua X, Ma J. Biosorption of Cr3+ and Pb2+ from tannery wastewater using combined fruit waste. Appl Ecol Environ Res. 2019;17(2):1773–87. https://doi.org/10.15666/aeer/1702_17731787.
Sumathi KMS, Mahimairaja S. Evaluation of adsorption potential of reed bed substrates for chromium(III) removal from tannery effluent: a batch study. Res J Chem Environ. 2009;13(1):59–65.
Namasivayam C, Holl WH. Chromium(III) removal in tannery waste waters using Chinese Reed (Miscanthus Sinensis), a fast growing plant. Holz Als Roh-Und Werkstoff. 2004;62(1):74–80. https://doi.org/10.1007/s00107-003-0431-4.
Abul Hashem M, Hasan M, Momen MA, Payel S, Nur-a-Tomal MS. Water hyacinth biochar for trivalent chromium adsorption from tannery wastewater. Environ Sustain Indic. 2020. https://doi.org/10.1016/j.indic.2020.100022.
Tan TW, Cheng P. Biosorption of metal ions with Penicillium chrysogenum. Appl Biochem Biotechnol. 2003;104(2):119–28. https://doi.org/10.1385/abab:104:2:119.
Aravindhan R, Fathima A, Selvamurugan M, Rao JR, Balachandran UN. Adsorption, desorption, and kinetic study on Cr(III) removal from aqueous solution using Bacillus subtilis biomass. Clean Technol Environ Policy. 2012;14(4):727–35. https://doi.org/10.1007/s10098-011-0440-7.
Natarajan R, Manivasagan R. Treatment of tannery effluent by passive uptake-parametric studies and kinetic modeling. Environ Sci Pollut Res Int. 2018;25(6):5071–5. https://doi.org/10.1007/s11356-017-9456-9.
Pena ADCC, Bertoldi CF, Fontoura JTD, Trierweiler LF, Gutterres M. Consortium of microalgae for tannery effluent treatment. Braz Arch Biol Technol. 2019. https://doi.org/10.1590/1678-4324-2019170518.
Saranya D, Shanthakumar S. Green microalgae for combined sewage and tannery effluent treatment: performance and lipid accumulation potential. J Environ Manag. 2019;241:167–78. https://doi.org/10.1016/j.jenvman.2019.04.031.
Ajayan KV, Selvaraju M, Unnikannan P, Sruthi P. Phycoremediation of tannery wastewater using microalgae scenedesmus species. Int J Phytorem. 2015;17(10):907–16. https://doi.org/10.1080/15226514.2014.989313.
Ji M, Su X, Zhao YX, Qi WF, Wang Y, Chen GY, Zhang ZY. Effective adsorption of Cr(VI) on mesoporous Fe-functionalized Akadama clay: optimization, selectivity, and mechanism. Appl Surf Sci. 2015;344:128–36. https://doi.org/10.1016/j.apsusc.2015.03.006.
Pakshirajan K, et al. Cr(III) and Cr(VI) removal from aqueous solutions by cheaply available fruit waste and algal biomass. Appl Biochem Biotechnol. 2013;170(3):498–513. https://doi.org/10.1007/s12010-013-0202-6.
Suganthi KV, Mahalakshmi M, Balasubramanian N. Development of hybrid membrane bioreactor for tannery effluent treatment. Desalination. 2013;309:231–6. https://doi.org/10.1016/j.desal.2012.10.014.
Vyrides I, Stuckey DC. Chromium removal mechanisms and bacterial community in an integrated membrane bioreactor system. Environ Eng Sci. 2011;28(9):661–70. https://doi.org/10.1089/ees.2010.0431.
Thankappan R, Nguyen TV, Srinivasan SV, Vigneswaran S, Kandasamy J, Loganathan P. Removal of leather tanning agent syntan from aqueous solution using Fenton oxidation followed by GAC adsorption. J Ind Eng Chem. 2015;21:483–8. https://doi.org/10.1016/j.jiec.2014.03.008.
Srinivasan SV, Mary GPS, Kalyanaraman C, Sureshkumar PS, Balakameswari KS, Suthanthararajan R, Ravindranath E. Combined advanced oxidation and biological treatment of tannery effluent. Clean Technol Environ Policy. 2012;14(2):251–6. https://doi.org/10.1007/s10098-011-0393-x.
Saxena S, Rajoriya S, Saharan VK, George S. An advanced pretreatment strategy involving hydrodynamic and acoustic cavitation along with alum coagulation for the mineralization and biodegradability enhancement of tannery waste effluent. Ultrason Sonochem. 2018;44:299–309. https://doi.org/10.1016/j.ultsonch.2018.02.035.
Saranya D, Shanthakumar S. An integrated approach for tannery effluent treatment with ozonation and phycoremediation: a feasibility study. Environ Res. 2020;183: 109163. https://doi.org/10.1016/j.envres.2020.109163.
Prabhavathy C, De S. Treatment of fatliquoring effluent from a tannery using membrane separation process: experimental and modeling. J Hazard Mater. 2010;176(1–3):434–43. https://doi.org/10.1016/j.jhazmat.2009.11.048.
Modenes AN, Espinoza-Quinones FR, Borba FH, Manenti DR. Performance evaluation of an integrated photo-Fenton—electrocoagulation process applied to pollutant removal from tannery effluent in batch system. Chem Eng J. 2012;197:1–9. https://doi.org/10.1016/j.cej.2012.05.015.
Liu WH, Zhang CG, Gao PF, Liu H, Song YQ, Yang JF. Advanced treatment of tannery wastewater using the combination of UASB, SBR, electrochemical oxidation and BAF. J Chem Technol Biotechnol. 2017;92(3):578–87. https://doi.org/10.1002/jctb.5037.
Azarian G, Miri M, Nematollahi D. Combined electrocoagulation/electrooxidation process for the COD removal and recovery of tannery industry wastewater. Environ Prog Sustain Energy. 2018;37(2):637–44. https://doi.org/10.1002/ep.12711.
Alemu T, Mekonnen A, Leta S. Integrated tannery wastewater treatment for effluent reuse for irrigation: encouraging water efficiency and sustainable development in developing countries. J Water Process Eng. 2019. https://doi.org/10.1016/j.jwpe.2017.10.014.
Dogruel S, Genceli EA, Babuna FG, Orhon D. An investigation on the optimal location of ozonation within biological treatment for a tannery wastewater. J Chem Technol Biotechnol. 2006;81(12):1877–85. https://doi.org/10.1002/jctb.1620.
Pophali GR, Chelani AB, Dhodapkar RS. Optimal selection of full scale tannery effluent treatment alternative using integrated AHP and GRA approach. Expert Syst Appl. 2011;38(9):10889–95. https://doi.org/10.1016/j.eswa.2011.02.129.
Aravindhan R, Saravanabhavan S, Thanikaivelan P, Rao JR, Nair BU. A chemo-enzymatic pathway leads towards zero discharge tanning. J Clean Prod. 2007;15(13):1217–27. https://doi.org/10.1016/j.jclepro.2006.07.010.
Hu J, Deng W. Application of supercritical carbon dioxide for leather processing. J Clean Prod. 2016;113:931–46. https://doi.org/10.1016/j.jclepro.2015.10.104.
Yu L, Qiang X, Cui L, Chen B, Wang X, Wu X. Preparation of a syntan containing active chlorine groups for chrome-free tanned leather. J Clean Prod. 2020;270: 122351. https://doi.org/10.1016/j.jclepro.2020.122351.
Ding W, Yi Y, Wang Y-N, Zhou J, Shi B. Preparation of a highly effective organic tanning agent with wide molecular weight distribution from bio-renewable sodium alginate. ChemistrySelect. 2018;3(43):12330–5. https://doi.org/10.1002/slct.201802540.
Ding W, Yi Y, Wang Y-N, Zhou J, Shi B. Peroxide-periodate co-modification of carboxymethylcellulose to prepare polysaccharide-based tanning agent with high solid content. Carbohydr Polym. 2019. https://doi.org/10.1016/j.carbpol.2019.115169.
Huang W, Song Y, Yu Y, Wang Y-N, Shi B. Interaction between retanning agents and wet white tanned by a novel bimetal complex tanning agent. J Leather Sci Eng. 2020;2(1):8. https://doi.org/10.1186/s42825-020-00023-2.
Jones E, Qadir M, Van Vliet MTH, Smakhtin V, Kang SM. The state of desalination and brine production: a global outlook. Sci Total Environ. 2019;657:1343–56. https://doi.org/10.1016/j.scitotenv.2018.12.076.
Scholz WG, Rouge P, Bodalo A, Leitz U. Desalination of mixed tannery effluent with membrane bioreactor and reverse osmosis treatment. Environ Sci Technol. 2005;39(21):8505–11. https://doi.org/10.1021/es050330p.
Giwa A, Dufour V, Al Marzooqi F, Al Kaabi M, Hasan SW. Brine management methods: recent innovations and current status. Desalination. 2017;407:1–23. https://doi.org/10.1016/j.desal.2016.12.008.
Lefebvre O, Moletta R. Treatment of organic pollution in industrial saline wastewater: a literature review. Water Res. 2006;40(20):3671–82. https://doi.org/10.1016/j.watres.2006.08.027.
Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (51878422, 42177060), Science & Technology Bureau of Chengdu (2017-GH02-00010-HZ) and Innovation Spark Project in Sichuan University (2082604401254) for the financial support.
Funding
National Natural Science Foundation of China (51878422, 42177060), Science & Technology Bureau of Chengdu (2017-GH02-00010-HZ) and Innovation Spark Project in Sichuan University (2082604401254).
Author information
Authors and Affiliations
Contributions
JZ conceived, designed and wrote this manuscript, QW and YT revised the manuscript, HG and JZ wrote and revised the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, J., Wu, Q., Tang, Y. et al. Tannery wastewater treatment: conventional and promising processes, an updated 20-year review. J Leather Sci Eng 4, 10 (2022). https://doi.org/10.1186/s42825-022-00082-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-022-00082-7