Abstract
Background
The demand for designing a new technology that can emphasize the complete removal of heavy metals increased as a result of the industrial revolution. Bioremediation was found to have a potent impact on the degradation of organic and inorganic environmental pollutants.
Main body
Bioremediation is a multidisciplinary technology that possesses safe, efficient, and low-cost characteristics. Also, one of the important features of bioremediation technology is the in-situ treatment which reduces the possibility of transmitting the contaminants to another site. The application of genetic engineering, to engineer a microorganism to acquire the ability to remove different types of heavy metals at a time or to generate a transgenic plant, is considered one of the new promising bioremediation approaches.
Short conclusion
Removal of heavy metal pollution still represents a big challenge for ecologists that’s why this review shed some light on bioremediation technology; its importance, mechanism of action, and prospects.
Similar content being viewed by others
Background
The world accelerated industrial revolution and the uses of natural resources during metal mining and industry have a great impact on the environment due to heavy metal pollution. Today, one of the most destructive effects facing the world is the contamination with heavy metals, which reaches the air, soil, and water (Asha and Sandeep 2013; Raghunandan et al. 2014, 2018). Although trace concentrations of some metals have a vital effect on the health of living organisms, high levels of heavy metals represent toxic effects too (Ahemad 2019; Ahuti 2015). Also, heavy metals can hardly be degraded in the soil, so their complete detoxification represents a challenge to scientists. Despite the efforts spent to tackle the environmental pollution issue, the world still suffers from the hazardous effects of heavy metals, and so a new technology should be discovered to contain the disaster of heavy metal contamination, one of which is the bioremediation (Raghunandan et al. 2014, 2018).
Several methods have been accomplished to remediate heavy metals pollution, among them Physico-chemical (conventional) methods such as ion exchange, redox, electrochemical techniques, membrane filtration, and precipitation (Nissim et al. 2018; Qasem et al. 2021). The disadvantages of the conventional methods are the inability of these methods to detoxify heavy metals permanently (Sun et al. 2020), in addition to the cost-effectiveness and the hazardous by-products produced by the elimination process. However, the conventional method is considered effective for large areas contaminated with small amounts of heavy metals and for highly polluted local areas (Huët and Puchooa 2017). Consequently, building a new technology that emphasizes the complete removal of heavy metals represents a challenge for scientists. Interestingly, microbial remediation of heavy metal has a far-reaching progressive prospect among the decontamination methods. Microorganisms especially soil microbes can tolerate high levels of heavy metals, some microorganisms need certain types of metals as a micronutrient (i.e., Fe3+ is essentially utilized by all bacteria while Fe2+ is significant for anaerobic bacteria) to perform their metabolic activities (Ahemad 2019). The bioremediation process could be conducted Ex-situ by transferring the contaminated area to be treated or even in situ by delivering the biological source to the polluted land (Shannon and Unterman 1993; Naz et al. 2005). Most microorganisms follow two common mechanisms in the bioremediation process; metal sequestering or immobilization and enhancement of solubility properties of the metal, other organisms oxidize or reduce the heavy metals to a less toxic form (Donald 2013). The bioremediation process also could be accomplished in aerobic and anaerobic environments; however, the aerobic environment was found to be more efficient and faster than anaerobic conditions.
Main text
Definition of heavy metals
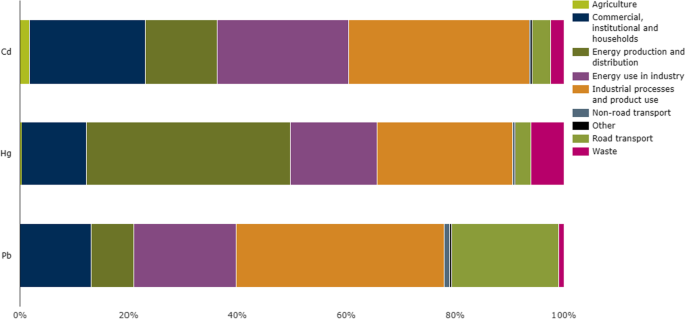
These can be defined as the elements that have a density higher than 5 g/cm3, also the metals or metalloids which have an atomic mass greater than 4000 kg m−3 or 5 times larger than water are considered heavy metals (Paschoalini and Bazzoli 2021). A lot of elements fall into this class however, only a few metals (arsenic (As), cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), lead (Pb), mercury (Hg), nickel (Ni), tin (Sn), vanadium (V) and zinc (Zn).) commonly existed in the contaminated air, water, and soil. These metals could be found in many forms; insoluble such as carbonate, oxides, silicate, and sulfides, or soluble such as salt forms (Arfala et al. 2018), also, heavy metals when persisted in their ionic state (e.g., Cd+2, Pb+2, Hg+2, As+3) represent the most toxic form as they combined with bio-molecules and for a complex harder to be dissociated (Duruibe et al. 2007). Recently, researchers paid great attention to studying the diffusion phenomenon and mobility through soil layers and in aquifers (Cuevas et al. 2012). According to the European Environment Agency reports, industrial process and product use, energy production and distribution, and energy use in industry are the most contributed sectors in the emission of Cd, Hg, and Pb as represented in Fig. 1. However, road transportation, commercial, institutional, and households have a significant contribution in Pb emission (EEA 2019).
Effect of different life sectors on the emission of Cd, Hg, and Pb in the environment (EEA, 2019)
Effect of heavy metals on living organisms
Heavy metals with trace concentrations are considered micronutrients that are essential and have nutritional value for some metabolic processes of living cells (Ray and Ray 2009), however, elevated levels may have an adverse impact on the health of aquatic and terrestrial living organisms and the environment as they cause dangerous morbidity and mortality (Wang et al. 2006; Ray and Ray 2009). Heavy metals could be transported to the living cells through the air, water, and food chains and consequently, they alter the physical and chemical properties of the transported material. Heavy metal pollution affects the ecosystem balance by reducing the microbial population of the soil which participates in decomposing the organic material used in crops growing, and so they indirectly affect the food chain of other living organisms, thereby, the world health organization (WHO) and the United States Environmental Protection Agency (USEPA) assigned the acceptable limit for different heavy metals in water as represented in Table 1. Some metals can destruct living cells directly such as mercury, cadmium, lead, and chromium others have indirect effects such as zinc a corrosive material, and arsenic which pollute catalysts (Hogan 2010).
Effect of heavy metals on human
Heavy metals exert their effects by interfering with the function of the organs, however, some of these metals are useful at low concentrations such as arsenic, copper, nickel, iron, etc. (Ray and Ray 2009), however, at a high concentration, these metals become cytotoxic as well as carcinogenic for the cells, especially after long term exposure (Jaishankar et al. 2014; Valko et al. 2016).
Malfunction of human organs is the predominant phenomenon of infected bodies, Zinc for example causes severe gastrointestinal, kidney, brain, respiratory, and heart damage (Hrynkiewicz and Baum 2014). Cadmium has the same effect in addition to hypophosphatemia and causes damage to the central nervous system (Hrynkiewicz and Baum 2014). Arsenic and mercury damage the liver, the heart, and the central nervous system and cause hypophosphatemia and cancer (Tamele and Vázquez Loureiro 2020). Lead which is commonly introduced to the environment in different forms such as mining, lead smelting, ceramic and glass industries, ammunition, storage battery, and tetraethyl-lead manufacturing (Held and Don 2000) has a destructive effect on the liver, the heart, and the central nervous system and cause hypophosphatemia, cancer, and anemia (Koning et al. 2001; Iranzo et al. 2001; Hrynkiewicz and Baum 2014). A disastrous disease has already emerged due to heavy metal pollution such as “Itai Itai” in Japan as a result of Cd pollution (Gautam et al. 2015), “Arsenecosis” in Bangladesh due to As, and “Minimata” in Japan due to Hg (Volesky 1990).
Effect of heavy metals on plants
Physiological dysfunction and malnutrition are the most important disorders that affect plant growth due to excessive concentration of heavy metal pollution, also the disturbance in the ecological balance between plants and microorganisms has a great impact on crops. Malfunctions of the vital physiological processes such as Photosynthesis, and respiration may lead to the degradation of the major organelles following plant death (Glombitza and Reichel 2013). As a consequence of the excessive intake of heavy metals by plants, human and animal health will be affected (Babak et al. 2013).
Toxicity of heavy metals to the microorganisms
Heavy metals also have a great impact on the growth of microorganisms depending on the type and concentration of the polluted source. Different mechanisms were found to be involved in the toxicity of heavy metals such as dysfunction of enzymatic reactions, production of reactive oxygen species (ROS) which function as soluble electron carries, induction of oxidative damage that may cause changes in DNA and protein formation (Gauthier et al. 2014; Hildebrandt et al. 2007). Also, heavy metal toxicity affects the transcription and translation of DNA by charging the phosphate group negatively using electrostatic interaction which may cause mutagenesis (Genchi et al. 2020), causing acute hurt to the cell membrane and cytoplasmic molecules. Hence, exposure to heavy metals can affect both morphological, biochemical, and physiological properties (Frimmel 2003; Fashola et al. 2016).
Principles of the bioremediation process
Bioremediation can be defined as the use of biological diversity, directly or indirectly, to convert toxic pollutants into a harmless form (Asha and Sandeep 2013), so bioremediation is a holistic approach that includes plant, fungi, bacteria, actinomycetes, and algae all of them could be used as a biological agent to detoxify heavy metals. Two different strategies are utilized to remediate toxic pollutants; in-situ, where the process of decontamination occurred at the contaminated place itself by bringing the biological agent to the site of contamination or promoting the indigenous organisms to deal with contaminants by facilitating the suitable condition for their propagation. The second one is ex-situ, by which the contaminated place is transferred away to another site to be processed (Kumar et al. 2011a, b; Kumar et al. 2016; Raghunandan et al. 2014, 2018). There are many mechanisms by which the organism can manipulate the detoxification process, however, the utilization of the toxic metal by the microorganism as a source of nutrition is the main concept (Sun et al. 2020). So, microbial bioremediation is considered a multidisciplinary field that required more research and investigations.
Types of bioremediations
Bioremediation is classified, according to the site at which the bioremediation process occurred, into two different strategies:
In-situ bioremediation
This strategy corresponded with treating the polluted surfaces where they are located, this strategy depends on detoxifying the dissolved and sorbed pollutants directly by the microorganism, it can be applied in groundwater, unsaturated and saturated soils, also it is considered an efficient method to remediate organic chemicals in contrary to ex-situ strategy (Brar et al. 2006), also in-situ bioremediation expanded to treat inorganic and toxic metals. Moreover, the application of microorganisms that have a chemotactic ability to facilitate moving into the contaminated areas and hence the degradation of harmful compounds will be safer (Kulshreshtha et al. 2014). Furthermore, stimulating the reduction of heavy metals at the place minimizes the chance of contaminant transportation downgradient. A challenging issue facing in-situ bioremediation is the selection of one organism or a consortium of organisms that has the potential ability to detoxify the targeted metals. In lab-scale, it was found that Fe3+ and sulfate-reducing microorganisms have the enzymatic ability to biodegrade some heavy metals such as U(VI), Tc (VIII), Cr (VI), and Co (III) (Gorby et al. 1998; Tebo and Obraztsova 1998; Lloyd et al. 2000). Also, species of Geobacteraceae were found to be a dominant group during the stimulation process for reducing Fe3+, also, the members of this group were detected in the stimulation process to reduce U(VI) of contaminated Aquifer. So, the Geobacteraceae group was considered to play an important role in stabilizing contaminants and reducing metals within subsurface environments (He et al. 2019). The following are some techniques used for “in-situ” bioremediation:
Biosparging
Biosparging system is Constructed by injecting the air through a pipe below the water table which enhances the growth of indigenous microbes due to elevated oxygen concentration (Jain et al. 2012). Also, it differs from bioventing in mixing the soil and the groundwater by injecting the air in the saturated area, which allows the movement of volatile organic compounds upward to the unsaturated area this process is affected by the biodegradability of the contaminants and soil characteristics. This system possesses low construction cost and flexibility in adapting the design (Atlas and Philp 2005).
Bioventing
Bioventing is a system that stimulates the existing soil microorganisms to degrade the source of pollution via injecting a limited amount of oxygen that sustains microbial activity (Jain et al. 2012). Injection of air is conducted in the unsaturated area in addition to supplementing it with nutrients and moisture (Philp and Atlas 2005). Bioveting could be more efficient in anaerobic biodegradation, also mixing nitrogen with oxygen will increase the potency of chlorinating remediation (Mihopoulos et al. 2000, 2002; Shah et al. 2001).
Bioaugmentation
Bioaugmentation is the application of outsourcing microbial strains that naturally occurred or are genetically engineered to decontaminate polluted soil or water. Treatment usually utilizes a consortium of microorganisms that produce all the required enzymes and degradative pathways. Bioaugmentation is used to treat municipal wastewater, soil, and groundwater polluted with chlorinated ethenes which are degraded to nontoxic ethylene and chloride (Jain et al. 2012).
Intrinsic bioremediation
Intrinsic bioremediation is defined as the stimulation of naturally occurring organisms by providing nutritional materials and oxygen to remediate heavy metals without attribution of any engineering steps (Riseh et al. 2022).
Engineered bioremediation
Engineered bioremediation is the adaptation of physicochemical conditions to enhance the propagation of introduced microorganisms to accelerate the bioremediation process.
Advantage of in-situ bioremediation
-
Cost-effectiveness of in-situ bioremediation
-
It can be used to treat large contaminated areas which could reach inaccessible regions.
-
Treating a wide variety of wastes, it may be used the decontaminate organic and inorganic wastes.
-
In-situ bioremediation is faster than the pump-and-treat method.
Challenges facing in-situ bioremediations
-
Limitations in depending on indigenous microorganisms as their metabolic activity could be inhibited by high levels of heavy metals.
-
Some pollutants may be bio-transformed due to microbial metabolic activity to an intermediate which could be more toxic and mobile than the original form.
-
In-situ bioremediation could be inappropriate in treating some contaminants such as recalcitrant.
-
In-situ bioremediation is most suitable for low-level scenarios of pollution (Kulshreshtha et al. 2014).
Ex-situ bioremediation
The core concept of this strategy is to treat the contaminated site by the excavation of soil to enhance microbial degradation. Five techniques were used in this strategy.
Slurry-phase
This technique relies on excavating the contaminated soil and mixing it with water and transporting the mixture to a bioreactor, followed by stone and rubble removal. The amount of water depends on the pollutant's type and concentration, the soil's nature, and the biodegradation rate. This process is followed by the separation of the soil by filtration or centrifugation, the soil is dried and retransferred to its original location, and the fluids are submitted to a further treatment step (EPA 2003).
Solid-phase
This technique involves three steps: excavation of the soil, followed by putting the soil into piles, the soil may contain municipal, agricultural, and organic wastes, followed by stimulation of the biodegradation process by supplying oxygen through a network of pipes to enhance microbial respiration and subsequently microbial activity. Solid-phase bioremediation requires a large space and a long time to be completed (Hyman and Dupont 2001).
Landfarming
This technique relies on the stimulation of indigenous organisms spread over the surface by supplementing the excavated soil with suitable nutrients and minerals, the excavated soil should be periodically tilled to stimulate the biodegradation process.
Soil biopiles
This technique is almost similar to landfarming bioremediation except in using above-ground piles and perforated pipes to inject air through the soil (Verma 2022). Application of this technique is interestingly valuable because of its low cost and full control of nutritional feed, aeration, and temperature (Whelan et al. 2015), also it’s the technique of choice in treating contaminated sites of extreme environments and in treating low molecular weight compounds by limiting volatilization (Gomez and Sartaj 2014).
Composting bioremediation
Composting bioremediation is quite similar to landfarming bioremediation in excavating the contaminated soil to the surface and stimulating the indigenous microorganisms through feeding of nutrients and injecting air but differs in supplementing the soil with a bulk of additives such as corncobs, straw, and hay, this additive helps in oxygen distribution through the soil, maintaining the moisture content constant and turning frequency, however, application of composting process for biodegradation of volatile pollutants is not favorable because of the periodic turning during the process (Hobson et al. 2005).
Advantage of ex-situ bioremediation
-
Adequate control of the biodegradation process.
-
Suitability to detoxify a wide variety of contaminants.
-
Reduction of time required to complete the treatment process.
Challenges facing ex-situ bioremediation
-
Limitation of ex-situ bioremediation to biodegrade chlorinated hydrocarbons.
-
Some types of soils required further processing such as non-permeable soils.
Bioremediation mechanism of action
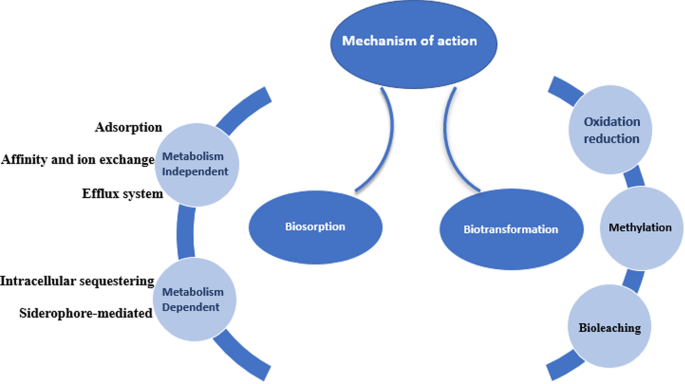
Due to the ubiquitous nature of microorganisms, they play a crucial role in the bioremediation of heavy metals, they can interact with heavy metals using different mechanisms to survive the toxicity of the metals. The two main concepts by which the organism can deal with contaminants are using the contaminant as a source of nutrition and protecting the organism itself (defense mechanism) from the toxic effect (Alvarez et al. 2017). As illustrated in Fig. 2, the microorganism reacts with the environmental contaminants using direct or indirect mechanisms some of which are biosorption and biotransformation (Tang et al. 2021).
Biosorption
It’s a mechanism by which the organism binds with the metal to form a complex that possesses a nontoxic feature. Certain criteria should be considered and investigated to achieve a potential biosorption mechanism; nature of the biosorbent, sorption capacity, kinetics of sorption, regeneration ability of the sorbent, percentage of metal recovery, cost-effectiveness of biosorption process, and separation flexibility of the biosorbent-metal complex (Bae et al. 2001, 2002, 2003). Two main categories are involved in the bioremediation process using the biosorption mechanism.
Metabolism-independent biosorption
This type of biosorption depends on the physical and chemical properties of the cell whether it was a live cell or a dead cell, this category involves the following:
Adsorption, also called extracellular sequestration, relies on the affinity between cellular components of the periplasm and the metal ion. Extracellular polymeric substance (EPS) associated with bacterial cell wall plays a significant role in metal adsorption. EPS is composed of polysaccharides, mucopolysaccharides, and proteins. It also contains a lot of functional groups (hydroxyl, carboxyl, amine, and phosphoric groups) that facilitate heavy metal sequestering (Guine et al. 2006).
Affinity and ion exchange by which the biosorbent (cellular component) binds with the metal ion, Cunninghamella were found to have a promising binding ability to heavy metals released textile wastewater (Tigini et al. 2010), also Saccharomyces cerevisiae can degrade Cd(II) and Zn(II) using the ion-exchange method.
Efflux system as a type of extracellular sequestration is one of the most important methods by which the organism can defend against the toxic effect of heavy metals by forming an outer protective material and ejecting the metal ion out of the cytoplasm to the periplasmic region (Dixit et al. 2015). Ma et al. (2016) reported that transformation and efflux are the basic methods usually used in bacterial resistance to heavy metals.
Metabolism-dependent biosorption
This mechanism is associated with the metabolic activity of a viable microorganism contrary to metabolism-independent biosorption.
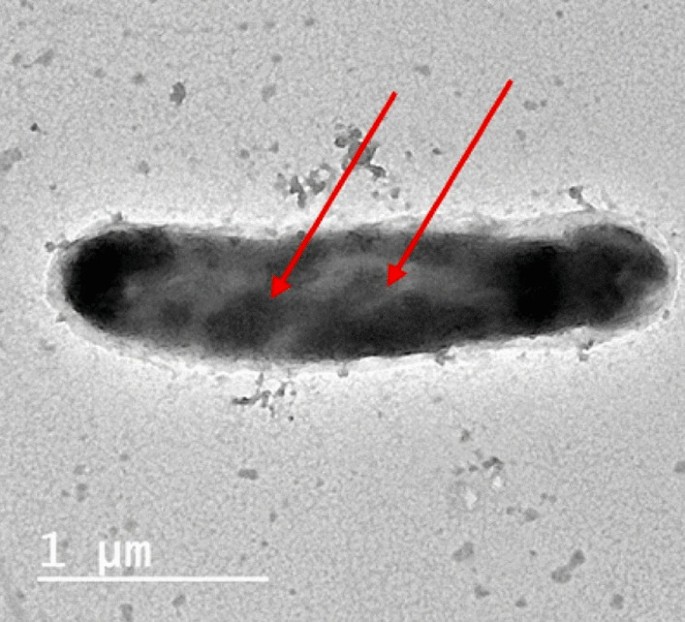
Intracellular sequestering (Bioaccumulation), is a process by which the complex form of cell-metal occurred inside the cytoplasm (Ramasamy and Banu 2007), as reported by (Abo-Alkasem et al. 2022a) and illustrated in Fig. 3, examination of Salipaludibacillus agaradhaerens strain NRC-R cells using the Transmission Electron Microscope (TEM) showed the accumulation of chromium inside the cell which also confirmed by EDX analysis, accumulation of metals conducted by attaching with the cell surface follows slow penetration to periplasm to the cell cytoplasm by a process that looks like nutrient uptake (Mishra and Malik 2013), it was reported that cysteine-rich protein plays an important role in sequestering Zn, Cd, and Cu in cadmium-tolerant Pseudomonas putida, also, glutathione helps in sequestering Cd by Rhizobium leguminosarum. Fungi also play a vital role in inorganic metal elimination using their rigid cell wall which works as a ligand in the decontamination process.
TEM image of the cells grown in the presence of Cr (VI) (Abo-Alkasem et al. 2022a)
Siderophore-mediated biosorption, also called a chelating agent, in aerobic soils some microorganisms produce siderophores that mediate the ability of the microorganisms to utilize low water-soluble metals using an energy-dependent process (John et al. 2001). Microbacterium flavescens was found to use siderophore to uptake their nutritional requirements of iron, also the organism uses the siderophore desferrioxamine-(DF) to bind with uranium, plutonium, and iron.
Biotransformation
Biotransformation relies on the cellular metabolic activity of the microorganism through the redox mechanism, reduction of metals by changing the oxidation number of the metal is common in nature, such as the reduction of chromium (Abo-Alkasem et al. 2022a, b), selenium (Lloyd et al. 2001), uranium (Chang et al. 2001) and mercury (Brim et al. 2000).
Oxidation and reduction mechanisms
The mechanism by which the microorganism works as an oxidizing agent by releasing electrons that react with the anions in the contaminated soil is the same mechanism utilized to decontaminate organic compounds under anaerobic conditions (Lovley and Phillips 1988). However, it was found that the presence of iron (III) stimulates the degradation process (Spormann and Widdel 2000). The reduction could be occurred directly using a bioreactor, (pump and treat) or after the excavation of soils, inoculated with the appropriate microbial consortium, or indirectly using sulfate-reducing bacteria which plays an important role in the ecological balance directly by sulfate reduction or indirectly by the formation of biofilms (Abo Elsoud and Abo-Alkasem 2022). The indirect mechanism is more favorable due to its cost-effectiveness and eco-friendly method (Asha and Sandeep 2013). Decontamination of uranium by Desulfosphorosinus spp. And Closteridium spp is an applicable example of utilizing sulfate-reducing bacteria (Prasad and Freitas 2003).
Methylation of metals (volatilization)
Volatilization of metal by microbial methylation plays a significant role in metal remediation, for instance, some Pseudomonas spp., Escherichia spp., Clostridium spp., and Bacillus spp. can convert Hg (II), Se, As, and Pb to a gaseous methylated form (Ramasamy and Banu 2007).
Bioleaching
Bioleaching is the secretion of low molecular weight compounds that aid the transformation of a toxic form of metals to a nontoxic form by dissolution or precipitation mechanisms, (Chanmugathas and Bollag 1988) reported that leaching of Cd is promoted by the secretion of organic acids by some microorganisms, also the production of inorganic phosphate by Citrobacter organism leads to precipitation of metal phosphate coat.
Plant-microbial remediation
Rhizoremediation is the association of microorganisms with plants to improve the potential of the bioremediation process and it now plays a crucial role in environmental bioremediation due to cost-effectiveness and outstanding efficiency (Nie et al. 2011; Marihal and Jagadeesh 2013; Prabha et al. 2017).
The capability of microorganisms to develop a symbiotic relationship enhances the biodegradability of different types of contaminants (Kumar et al. 2017). The predominant type of organisms associated with the plant-microorganism relationships is mycorrhizal fungi which can bio-sorb heavy metals (Bojorquez and Voltolina 2016). The potentiality of rhizoremediation was reported by Joner and Leyva (1997) who found that mycorrhizal plants when subjected to soil contaminated with Cd2+ 1, 10, and 100 mg/kg, Cd uptake of mycorrhizal was higher than non-mycorrhizal plants by 90%, 127%, and 131% respectively. The mechanisms utilized in rhizoremediation are mainly through the activation of metal phosphates, acidification, production of organic acids, chelating agents, and ion carriers.
Microorganisms responsible for bioremediation
In nature, the presence of microorganisms guarantees the retrieval of ecological balance and the removal of contaminants that hinder biological life. The use of microorganisms for the removal of contaminants from the environment is described as "Bioremediation". The concept of environmental remediation using microorganisms was first registered as a patent in 1981 for the degradation of petroleum oil by Pseudomonas putida (Prescott et al. 2002; Glazer and Nikaido 2007). Bioremediation aims to stimulate microbial metabolic activity, with nutrients or other chemical agents, to be able to remove, destroy, or neutralize the effect of these contaminants. The microorganisms used for bioremediation should not only be able to tolerate a wide concentration range of the contaminant(s) but also be physiologically active. Once favorable conditions are obtained, the metabolic activity and growth rate of these microorganisms reach alarming levels as well as the bioremediation process. Many theories have been illustrated for the mechanism of microbial tolerance to heavy metals. These theories include the accumulation and formation of non-toxic complexes with the metal ions inside the cells, the efflux of toxic metals outside the cell, biotransformation of the toxic metal into a less toxic form, or methylation and/or de-methylation.
In nature, the type of micro-flora (microbial consortium) is a significant factor affecting the tolerance and rate of heavy metal bioremediation depending on the gene and metabolic diversity (Juwarkar et al. 2010). Two types of microorganisms are used for heavy metal bioremediation based on their sources: indigenous (microorganisms present in the site of contamination and have bioremediation capability) and extraneous (microorganisms introduced into the site of contamination and have bioremediation capability), Table 2 summarizes some of the organisms used in the bioremediation process and their target pollutants. The utilization of indigenous microorganisms excludes the need for continuous monitoring according to Asha and Sandeep (2013). After the bioremediation process, the soil and/or water retrieve their ability to be reused in various activities.
It was reported that many microorganisms including bacteria, Actinomycetes, fungi, yeast, and algae can remediate heavy metals from soil and water:
Bacteria
Endophytic bacteria and Plant Growth Promoting Rhizobacteria (PGPR) are the most common bacterial strains associated with heavy metal bioremediation.
The endophytic bacteria colonize the sub-epidermal layer of the plant tissues (Schulz and Boyle 2006). The presence of endophytic bacteria helps the protection of the plant cells from heavy metals stress conditions (Ryan et al. 2008). They diminish or remove the phytotoxicity of the heavy metals by altering their phyto-availability (Weyens et al. 2009; Ma et al. 2011) such as some species of Pseudomonas, Bacillus, and Rahnella that showed high resistance to Pb, Mn, and Cd (Luo et al. 2012; Yuan et al. 2014; Babu et al. 2015).
On the other hand, PGPR comprises a group of free-living, symbiotic, or endophytic bacteria (Glick 2012). For example, Bacillus, Enterobacter, Erwinia, Flavobacterium, Klebsiella, Gluconacetobacter, and Pseudomonas (Nadeem et al. 2010) can mitigate the toxicity of heavy metals, improve plant growth in heavy metal-contaminated soils (Seth 2012) and produce phytohormones and siderophores and help phosphate solubilization (Ullah et al. 2015).
Actinobacteria
In addition to their well-known ability to utilize complex organic matter as a carbon and energy source (Kieser et al. 2000), Actinobacteria, such as Amycolatopsis, Corynebacterium, Rhodococcus, and Streptomyces, can tolerate and remediate heavy metals, such as Hg(II), Co(II), Cd(II), Cr(VI), Zn(II) and Ni(II) (Oyetibo et al. 2010; Alvarez et al. 2017).
Fungi
Some fungal strains have been reported to possess metal chelating and sequestrating systems that increase their heavy metal tolerance and biotransformation into a less toxic form such as Allescheriella, Pleurotus, Phlebia, and Stachybotrys (D’Annibale et al. 2007). The hyphal and high biomass growth adds an advantage to this type of microorganism as it allows simple harvest along with the attached heavy metals (Aly et al. 2011). Aspergillus, Penicillium, Cephalosporium, and Rhizopus are the most studied fungal genera for their potential activity in the removal of heavy metals, such as Pb2+ and Zn2+, from aqueous solutions and soils (Volesky and Holan 1995; Huang and Huang 1996; Tunali et al. 2006; Akar et al. 2007).
Factors affecting the bioremediation process
To confine the biodegradation potential on selecting the most appropriate method, mechanism, and technique without paying attention to the factors that may affect the utilized application, limit the efficiency of the bioremediation process. A lot of factors could exhibit significant effects on the bioremediation process, for instance, metal ion concentration, valance state and chemical forms of the metal, the bioavailability of the metal, redox potential, availability of low molecular weight organic acids, and environmental factors such as temperature and pH (Bandowe et al. 2014).
Substrate concentration
To establish the process of bioremediation, bio-sorbent accumulation features should be quantified, two models could be used; the Langmuir model mainly defines adsorption by assuming an adsorbate behaves of the single-layer (Acar and Malkoc 2004), and the Freundlich model which mainly estimates the adsorption equilibrium (Febrianto et al. 2009). However, the main concept is that the adsorption efficiency increases with the increment of heavy metal concentration until a certain value.
Type of the substrate
The efficiency of the adsorption mechanism is affected by the type of soil, the type of heavy metal, and the type of soil additives. since the adsorption between the soil and heavy metals may lower the mobility of heavy metals and hence reduce microbial adsorption (Hu et al. 2010). Also, soil additives have a significant effect on heavy metals removal, Tyagi et al. (2014) found that increasing FeSO4.7H2O higher than 20 gm/l has an adverse effect on the leaching rate of Cu and Zn.
pH
The potential of hydrogen (pH) plays a vital role in both microbial activity and metal characteristics. Growing of microorganisms in unfavorable pH may affect the enzyme activity thereby lowering the rate of microbial metabolism, also, the charge of the microorganism surface will be changed that affects the binding capacity between the adsorbent and heavy metals (Bandowe et al. 2014; Galiulin and Galiulina 2008). Furthermore, changes in pH value may alter metal mobility and hydration as the metals tend to be free ionic at acidic pH (Bandowe et al. 2014; Dermont et al. 2008). According to (Rodríguez-Tirado et al. 2012; Wierzba 2015), the adsorption capacity of Pb2+ and Zn2+ increased by raising the pH value to 5.5, however, an observed decrease in the removal of metals was recorded upon increasing pH value over (5.5).
Temperature
Temperature is an important parameter in adjusting the optimum conditions for microbial growth, metabolism, and enzyme activity (Fang et al. 2011), increasing temperature affects the diffusion of metals across different layers and also, increases the bioavailability of metals. However, the optimum biodegradation temperature differs according to the type of metal, for instance, the biodegradation of Cd2+ by Bacillus jeotgali was the highest at 35 °C, however, it was 30 °C for Zn2+ biodegradation (Chanmugathas and Bollag 1988).
Role of biotechnology in the bioremediation process
Biotechnology is the discipline of using the engineering of scientific principles to improve the efficiency of organisms to serve humans and remediate the environmental toxic substance (McHughen 2016), by using genetic engineering, one of the biotechnology approaches, a single organism can be engineered to produce all the needed enzymes or to utilize all the degradative pathways for bioremediation process (Dangi et al. 2019). The purpose of utilizing genetic tools is to enhance efficiency and reduce the cost and time of the bioremediation process.
Degradation of Polychlorinated biphenyls (PCBs) is controlled by to group of genes that were found in the genetic material of two different organisms, thereby, using genetic engineering for achieving recombination between Pseudomonas pseudoalcaligenes KF707 and Burkholderia cepacia LB400 bph genes may enhance the degradation rate of PCBs and stimulate the remediation of toluene and benzene (Seeger et al. 2010), also the application of DNA probes helps in accelerating the process of the isolation and identification of a particular strain from a mixed population (Dua et al. 2002). Another example of using biotechnology is the fusion between metallothionein (MT) isolated from rats, IgA protease protein isolated from Neisseria gonorrhoeae, and the fusion vehicle lpp-ompA to provide the bacterial cell wall with metal ion-binding polypeptides (Bae et al. 2000 and Valls et al. 2000).
Another discipline of biotechnology involves the use of transgenic plants in the bioremediation process, this could be conducted by transferring a desirable gene from different sources (other plants, microorganisms, or even animals) to improve the ability of the plant to remove the toxic pollutant (Truu et al. 2015) this process of transmission increases the phytoremediation ability of the plant (Dixit et al. 2015).
Immobilized microorganism technology
Immobilization is one utilized technique in bioremediation, it possesses stability of the biological cell, also the immobilized cell did not compete with indigenous organisms, therefore it is considered eco-friendly and has high degradation efficiency (lone et al. 2008).
Advantage of bioremediation
-
A natural bioprocess is characterized by a safe effect on the environment which makes it globally accepted as a technique for treating wastes.
-
The consumed energy is lower than the technologies.
-
Cost-effectiveness is one of the most bioremediation features.
-
Several types of pollutants could be eliminated at the same time.
-
Minimize the risk of transferring the contamination from one site to another.
Disadvantages of bioremediation
-
Several factors could affect the efficiency of the bioremediation process.
-
Elimination of toxic metals to be achieved could take a lot of time.
-
Limited to those contaminates that can be biodegradable.
-
Biodegradation capacity and efficiency cannot be predicted because of dealing with a live organism (Zeyaullah et al. 2009).
Conclusions
Great efforts were spent during the last few decades to address the problem of heavy metal pollution by developing new strategies to fix this issue, however, the application of bioremediation techniques still represents the most favorable strategy due to the cost-effectiveness and safety impacts of bioremediation techniques on the environment and also due to the variability of bioremediation mechanisms which makes these techniques applicable and affordable, this article enumerates different types of bioremediation and the advantage and disadvantage of these types also the suitability of these types to different environments and conditions moreover, the article summarizes some of the mechanisms of action of different bioremediation techniques in addition to the microorganisms that play an important role and the factors that may affect the bioremediation process and how the newly developed technologies can improve the bioremediation techniques to be more efficient.
Availability of data and materials
Not applicable.
Abbreviations
- DNA:
-
Deoxyribonucleic acid
- DF:
-
Desferrioxamine
- EPS:
-
Extracellular polymeric substance
- PGPR:
-
Plant Growth Promoting Rhizobacteria
- PCBs:
-
Polychlorinated biphenyls
- ROS:
-
Reactive oxygen species
- USEPA:
-
The United States Environmental Protection Agency
- WHO:
-
World Health Organization
References
Abo Elsoud MM, Abo-Alkasem MI (2022) Environmental sulfate-reducing microorganisms. In: Ahamed MI, Prasad R (eds) Application of Microbes in environmental and microbial biotechnology. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-16-2225-0_23
Abo-Alkasem MI, Maany DA, El-Abd MA, Ibrahim AS (2022a) Bioreduction of hexavalent chromium by a novel haloalkaliphilic Salipaludibacillus agaradhaerens strain NRC-R isolated from hypersaline soda lakes. 3 Biotech 12(1):1–15
Abo-Alkasem M, El-Abd M, Maany D, Ibrahim A (2022b) Hexavalent Chromium Reduction by a Potent Novel Haloalkaliphilic Nesterenkonia sp strain NRC-Y Isolated from Hypersaline Soda Lakes. Egypt J Chem. https://doi.org/10.21608/ejchem.2022.136986.6039
Acar FN, Malkoc E (2004) The removal of chromium (VI) from aqueous solutions by Fagus orientalis L. Biores Technol 94(1):13–15
Achal V, Kumari D, Pan X (2011) Bioremediation of chromium-contaminated soil by a brown-rot fungus. Gloeophyllum Sepiarium Res J Microbiol 6(2):166
Ahemad M (2019) Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: paradigms and prospects. Arab J Chem 12(7):1365–1377
Ahuti S (2015) Industrial growth and environmental degradation. Int Educ Res J 1(5):5–7
Akar T, Tunali S, Çabuk A (2007) Study on the characterization of lead (II) biosorption by fungus Aspergillus parasiticus. Appl Biochem Biotechnol 136(3):389–405
Al-Garni SM, Ghanem KM, Ibrahim AS (2010) Biosorption of mercury by capsulated and slime layerforming Gram-ve bacilli from an aqueous solution. Afr J Biotech 9(38):6413–6421
Alvarez A, Saez JM, Costa JSD, Colin VL, Fuentes MS, Cuozzo SA, Amoroso MJ (2017) Actinobacteria: current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 166:41–62
Aly AH, Debbab A, Proksch P (2011) Fungal endophytes: unique plant inhabitants with great promises. Appl Microbiol Biotechnol 90(6):1829–1845
Arfala Y, Douch J, Assabbane A, Kaaouachi K, Tian H, Hamdani M (2018) Assessment of heavy metals released into the air from the cement kilns co-burning waste: case of Oujda cement manufacturing (Northeast Morocco). Sustain Environ Res 28(6):363–373
Asha LP, Sandeep RS (2013) Review on bioremediation-potential tool for removing environmental pollution. Int J Basic Appl Chem Sci 3(3):21–33
Ashokkumar P, Loashini VM, Bhavya V (2017) Effect of pH, Temperature and biomass on biosorption of heavy metals by Sphaerotilus natans. Int J Microbiol Mycol 6(1):32–38
Atlas RM, Philp J (2005) Bioremediation. Applied microbial solutions for real-world environmental cleanup. ASM press
Babák L, Šupinova P, Zichova M, Burdychova R, Vítová E (2013) Biosorption of Cu, Zn and Pb by thermophilic bacteria–effect of biomass concentration on biosorption capacity. Acta Universitatis Agriculturae Et Silviculturae Mendelianae Brunensis 60(5):9–18
Babu AG, Shea PJ, Sudhakar D, Jung IB, Oh BT (2015) Potential use of Pseudomonas koreensis AGB-1 in association with Miscanthus sinensis to remediate heavy metal (loid)-contaminated mining site soil. J Environ Manage 151:160–166
Bae W, Chen W, Mulchandani A, Mehra RK (2000) Enhanced bioaccumulation of heavy metals by bacterial cells displaying synthetic phytochelatins. Biotechnol Bioeng 70(5):518–524
Bae W, Mehra RK, Mulchandani A, Chen W (2001) Genetic engineering of Escherichia coli for enhanced uptake and bioaccumulation of mercury. Appl Environ Microbiol 67(11):5335–5338
Bae W, Mulchandani A, Chen W (2002) Cell surface display of synthetic phytochelatins using ice nucleation protein for enhanced heavy metal bioaccumulation. J Inorg Biochem 88(2):223–227
Bae W, Wu CH, Kostal J, Mulchandani A, Chen W (2003) Enhanced mercury biosorption by bacterial cells with surface-displayed MerR. Appl Environ Microbiol 69(6):3176–3180
Bandowe BAM, Bigalke M, Boamah L, Nyarko E, Saalia FK, Wilcke W (2014) Polycyclic aromatic compounds (PAHs and oxygenated PAHs) and trace metals in fish species from Ghana (West Africa): bioaccumulation and health risk assessment. Environ Int 65:135–146
Benazir JF, Suganthi R, Rajvel D, Pooja MP, Mathithumilan B (2010) Bioremediation of chromium in tannery effluent by microbial consortia. Afr J Biotech 9(21):3140–3143
Bhattacharya A, Gupta A (2013) Evaluation of Acinetobacter sp. B9 for Cr (VI) resistance and detoxification with potential application in bioremediation of heavy-metals-rich industrial wastewater. Environ Sci Pollut Res 20(9):6628–6637
Bhattacharya A, Gupta A, Kaur A, Malik D (2015) Simultaneous bioremediation of phenol and Cr (VI) from tannery wastewater using bacterial consortium. Int J Appl Sci Biotechnol 3(1):50–55
Bissen M, Frimmel FH (2003) Arsenic—a review: Part I: occurrence, toxicity, speciation, mobility. Acta Hydrochim Hydrobiol 31(1):9–18
Bojórquez C, Espericueta MGF, Voltolina D (2016) Removal of cadmium and lead by adapted strains of Pseudomonas aeruginosa and Enterobacter cloacae. Revista Internacional De Contaminación Ambiental 32(4):407–412
Bondarenko O, Rõlova T, Kahru A, Ivask A (2008) Bioavailability of Cd, Zn and Hg in soil to nine recombinant luminescent metal sensor bacteria. Sensors 8(11):6899–6923
Brar SK, Verma M, Surampalli RY, Misra K, Tyagi RD, Meunier N, Blais JF (2006) Bioremediation of hazardous wastes—a review. Pract Period Hazard Toxic Radioact Waste Manag 10(2):59–72
Brim H, McFarlan SC, Fredrickson JK, Minton KW, Zhai M, Wackett LP, Daly MJ (2000) Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat Biotechnol 18(1):85–90
Chang YJ, Peacock AD, Long PE, Stephen JR, McKinley JP, Macnaughton SJ, White DC (2001) Diversity and characterization of sulfate-reducing bacteria in groundwater at a uranium mill tailings site. Appl Environ Microbiol 67(7):3149–3160
Chanmugathas P, Bollag JM (1988) A column study of the biological mobilization and speciation of cadmium in soil. Arch Environ Contam Toxicol 17(2):229–237
Chaturvedi MK (2011) Studies on chromate removal by chromium-resistant Bacillus sp. isolated from tannery effluent. J Environ Prot 2(01):76
Cuevas J, Ruiz AI, de Soto IS, Sevilla T, Procopio JR, Da Silva P, Leguey S (2012) The performance of natural clay as a barrier to the diffusion of municipal solid waste landfill leachates. J Environ Manag 95:S175–S181
D’Annibale A, Leonardi V, Federici E, Baldi F, Zecchini F, Petruccioli M (2007) Leaching and microbial treatment of a soil contaminated by sulphide ore ashes and aromatic hydrocarbons. Appl Microbiol Biotechnol 74(5):1135–1144
Dangi AK, Sharma B, Hill RT, Shukla P (2019) Bioremediation through microbes: systems biology and metabolic engineering approach. Crit Rev Biotechnol 39(1):79–98
De J, Ramaiah N, Vardanyan L (2008) Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar Biotechnol 10(4):471–477
Deng X, Yi XE, Liu G (2007) Cadmium removal from aqueous solution by gene-modified Escherichia coli JM109. J Hazard Mater 139(2):340–344
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152(1):1–31
Dixit R, Malaviya D, Pandiyan K, Singh UB, Sahu A, Shukla R, Paul D (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7(2):2189–2212
Dua M, Singh A, Sethunathan N, Johri A (2002) Biotechnology and bioremediation: successes and limitations. Appl Microbiol Biotechnol 59(2–3):143–152
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2(5):112–118
EEA (2019) National emissions reported to the Convention on Long-range Transboundary Air Pollution (LRTAP Convention) provided by European Environment Agency (EEA). https://www.eea.europa.eu/data-andmaps/indicators/eea32-heavy-metal-hm-emissions-1/assessment-10
EPA (2003) EPA protocol for the review of existing national primary drinking water regulations, united states environmental protection agency office of water office of ground water and drinking water standards and risk management division targeting and analysis branch1200 pennsylvania avenue, NW (4607M) Washington, DC 20460
Fang L, Zhou C, Cai P, Chen W, Rong X, Dai K, Huang Q (2011) Binding characteristics of copper and cadmium by cyanobacterium Spirulina platensis. J Hazard Mater 190(1–3):810–815
Fashola MO, Ngole-Jeme VM, Babalola OO (2016) Heavy metal pollution from gold mines: environmental effects and bacterial strategies for resistance. Int J Environ Res Public Health 13(11):1047
Favero N, Costa P, Massimino ML (1991) In vitro uptake of cadmium by basidiomycetes Pleurotus ostreatus. Biotech Lett 13(10):701–704
Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162(2–3):616–645
Gabriel J, Kofroňová O, Rychlovský P, Krenželok M (1996) Accumulation and effect of cadmium in the wood-rotting basidiomycete Daedalea quercina. Bull Environ Contam Toxicol 57(3):383–390
Gabriel J, Mokrejš M, Bílý J, Rychlovský P (1994) Accumulation of heavy metals by some wood-rotting fungi. Folia Microbiol 39(2):115–118
Galiulin RV, Galiulina RA (2008) Removing heavy metals from soil with plants. Her Russ Acad Sci 78(2):141–143
Gautam RK, Soni S, Chattopadhyaya MC (2015) Functionalized magnetic nanoparticles for environmental remediation. In: Handbook of research on diverse applications of nanotechnology in biomedicine, chemistry, and engineering. IGI Global, pp 518–551
Gauthier PT, Norwood WP, Prepas EE, Pyle GG (2014) Metal–PAH mixtures in the aquatic environment: a review of co-toxic mechanisms leading to more-than-additive outcomes. Aquat Toxicol 154:253–269
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17(11):3782
Glazer AN, Nikaido H (2007) Microbial biotechnology: fundamentals of applied microbiology. Cambridge University Press
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012.
Glombitza F, Reichel S (2013) Metal-containing residues from industry and in the environment: Geobiotechnological urban mining. In: Geobiotechnology I. Springer, Berlin, Heidelberg, pp 49–107
Goher ME, Abdel-Satar AM, Ali MH, Hussian AE, Napiórkowska-Krzebietke A (2016) Biosorption of some toxic metals from aqueous solution using non-living algal cells of Chlorella vulgaris. J Elementol 21(3)
Gomaa ABM, Al-Hazmi RH, Al-Fassi FA, Al-Garhi TF (2016) Bio-remediation of Cd-contaminated soil cultivated with Faba bean via application of Alcaligenes faecalis Rhizobacterium. Rev Res J 5(6):1–7
Gomaa AM, Al-Fassi FA, Al-Kenawy Z, Al-Gharbawi HT (2012) Role of Azospirillum and Rhizobium in bio-remediating Cd and Zn polluted soil cultivated with wheat plant. Aust J Basic Appl Sci 6(10):550–556
Gomez F, Sartaj M (2014) Optimization of field scale biopiles for bioremediation of petroleum hydrocarbon contaminated soil at low temperature conditions by response surface methodology (RSM). Int Biodeterior Biodegrad 89:103–109
Gorby YA, Caccavo F, Bolton H (1998) Microbial reduction of cobaltIIIEDTA-in the presence and absence of manganese (IV) oxide. Environ Sci Technol 32(2):244–250
Guiné V, Spadini L, Sarret G, Muris M, Delolme C, Gaudet JP, Martins JMF (2006) Zinc sorption to three gram-negative bacteria: combined titration, modeling, and EXAFS study. Environ Sci Technol 40(6):1806–1813
He Y, Gong Y, Su Y, Zhang Y, Zhou X (2019) Bioremediation of Cr (VI) contaminated groundwater by Geobacter sulfurreducens: environmental factors and electron transfer flow studies. Chemosphere 221:793–801
Held T, Don H (2000) In situ remediation. Biotechnology 11b:350–370
Hildebrandt U, Regvar M, Bothe H (2007) Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 68(1):139–146
Hobson AM, Frederickson J, Dise NB (2005) CH4 and N2O from mechanically turned windrow and vermicomposting systems following in-vessel pre-treatment. Waste Manage 25(4):345–352
Hogan CM (2010) Heavy metal. Encyclopedia of Earth. National Council for Science and the Environment. Eds E. Monosson & C. Cleveland, Washington DC.
Hrynkiewicz K, Baum C (2014) Application of microorganisms in bioremediation of environment from heavy metals. In: Environmental deterioration and human health. Springer, Dordrecht, pp 215–227
Hu N, Luo Y, Song J, Wu L, Zhang H (2010) Influences of soil organic matter, pH and temperature on Pb sorption by four soils in Yangtze River Delta. Acta Pedol Sin 47(2):246–252
Huang C, Huang CP (1996) Application of Aspergillus oryze and Rhizopus oryzae for Cu (II) removal. Water Res 30(9):1985–1990
Huët MAL, Puchooa D (2017) Bioremediation of heavy metals from aquatic environment through microbial processes: a potential role for probiotics. J Appl Biol Biotechnol 5(6):14–23
Hyma M, Dupont RR (2001) Groundwater and soil remediation: process design and cost estimating of proven technologies. American Society of Civil Engineers, Chicago
Iqbal M, Edyvean RGJ (2004) Biosorption of lead, copper and zinc ions on loofa sponge immobilized biomass of Phanerochaete chrysosporium. Miner Eng 17(2):217–223
Iranzo M, Sainz-Padro I, Boluda R, Sanchez J, Mormeneo S (2001) The use of microorganisms in environmental engineering. Ann Microbiol 51:135–143
Ivask A, Dubourguier HC, Põllumaa L, Kahru A (2011) Bioavailability of Cd in 110 polluted top soils to recombinant bioluminescent sensor bacteria: effect of soil particulate matter. J Soils Sedim 11(2):231–237
Jafari SA, Cheraghi S, Mirbakhsh M, Mirza R, Maryamabadi A (2015) Employing response surface methodology for optimization of mercury bioremediation by Vibrio parahaemolyticus PG02 in coastal sediments of Bushehr, Iran. CLEAN-Soil Air Water 43(1):118–126
Jain AN, Udayashankara TH, Lokesh KS (2012) Review on bioremediation of heavy metals with microbial isolates and amendments on soil residue. Int J Sci Res 6:2319–7064
Jaishankar M, Mathew BB, Shah MS, Murthy TPK, Gowda KRS (2014) Biosorption of few heavy metal ions using agricultural wastes. J Environ Pollut Hum Health 2(1):1–6
Javaid AMNA, Bajwa R, Manzoor T (2011) Biosorption of heavy metals by pretreated biomass of Aspergillus niger. Pak J Bot 43(1):419–425
John SG, Ruggiero CE, Hersman LE, Tung CS, Neu MP (2001) Siderophore mediated plutonium accumulation by Microbacterium flavescens (JG-9). Environ Sci Technol 35(14):2942–2948
Joner EJ, Leyval C (1997) Uptake of 109Cd by roots and hyphae of a Glomus mosseae/Trifolium subterraneum mycorrhiza from soil amended with high and low concentrations of cadmium. New Phytol 135(2):353–360
Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Rev Environ Sci Bio/technol 9(3):215–288
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical streptomyces genetics, vol 291. John Innes Foundation, Norwich
Kim IH, Choi JH, Joo JO, Kim YK, Choi JW, Oh BK (2015) Development of a microbe-zeolite carrier for the effective elimination of heavy metals from seawater. J Microbiol Biotechnol 25(9):1542–1546
Koning M, Hupe K, Stegmann R (2001) Thermal processes, scrubbing/extraction, bioremediation and disposal. Biotechnology Set 304–317
Kulshreshtha A, Agrawal R, Barar M, Saxena S (2014) A review on bioremediation of heavy metals in contaminated water. IOSR J Environ Sci Toxicol Food Technol 8(7):44–50
Kumar A, Bisht BS, Joshi VD, Dhewa T (2011a) Review on bioremediation of polluted environment: a management tool. Int J Environ Sci 1(6):1079–1093
Kumar A, Chanderman A, Makolomakwa M, Perumal K, Singh S (2016) Microbial production of phytases for combating environmental phosphate pollution and other diverse applications. Crit Rev Environ Sci Technol 46(6):556–591
Kumar R, Bhatia D, Singh R, Rani S, Bishnoi NR (2011b) Sorption of heavy metals from electroplating effluent using immobilized biomass Trichoderma viride in a continuous packed-bed column. Int Biodeterior Biodegrad 65(8):1133–1139
Kumar SS, Kadier A, Malyan SK, Ahmad A, Bishnoi NR (2017) Phytoremediation and rhizoremediation: uptake, mobilization and sequestration of heavy metals by plants. In: Plant-microbe interactions in agro-ecological perspectives. Springer, Singapore, pp 367–394
Kumaran NS, Sundaramanicam A, Bragadeeswaran S (2011) Adsorption studies on heavy metals by isolated cyanobacterial strain (nostoc sp.) from uppanar estuarine water, southeast coast of India. J Appl Sci Res 7(11):1609–1615
Lloyd JR, Mabbett AN, Williams DR, Macaskie LE (2001) Metal reduction by sulphate-reducing bacteria: physiological diversity and metal specificity. Hydrometallurgy 59(2–3):327–337
Lloyd JR, Sole VA, Van Praagh CVG, Lovley DR (2000) Direct and Fe (II)-mediated reduction of technetium by Fe (III)-reducing bacteria. Appl Environ Microbiol 66(9):3743–3749
Lone MI, He ZL, Stoffella PJ, Yang XE (2008) Phytoremediation of heavy metal polluted soils and water: progresses and perspectives. J Zhejiang Univ Sci B 9(3):210–220
Lovley DR, Phillips EJ (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54(6):1472–1480
Luo S, Xu T, Chen L, Chen J, Rao C, Xiao X, Liu Y (2012) Endophyte-assisted promotion of biomass production and metal-uptake of energy crop sweet sorghum by plant-growth-promoting endophyte Bacillus sp. SLS18. Appl Microbiol Biotechnol 93(4):1745–1753
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29(2):248–258
Ma Y, Oliveira RS, Freitas H, Zhang C (2016) Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front Plant Sci 7:918
Mane PC, Bhosle AB (2012) Bioremoval of Some Metals by Living Algae Spirogyra sp. and Spirullina sp. from aqueous solution.
Marihal AK, Jagadeesh KS (2013) Plant–microbe interaction: a potential tool for enhanced bioremediation. In: Plant microbe symbiosis: fundamentals and advances. Springer, New Delhi, pp 395–410
Marzan LW, Hossain M, Mina SA, Akter Y, Chowdhury AMA (2017) Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong city, Bangladesh: Bioremediation viewpoint. Egypt J Aquatic Res 43(1):65–74
McDonald J, Gaston L, Elbana T, Andres K, Cranfield E (2013) Dimoxystrobin sorption and degradation in sandy loam soil: impact of different landscape positions. Soil Sci 178(12):662–670
McHughen A (2016) A critical assessment of regulatory triggers for products of biotechnology: Product vs process. GM Crops Food 7(3–4):125–158
Mihopoulos PG, Sayles GD, Suidan MT, Shah J, Bishop DF (2000) Vapor phase treatment of PCE in a soil column by lab-scale anaerobic bioventing. Water Res 34(12):3231–3237
Mihopoulos PG, Suidan MT, Sayles GD, Kaskassian S (2002) Numerical modeling of oxygen exclusion experiments of anaerobic bioventing. J Contam Hydrol 58(3–4):209–220
Mishra A, Malik A (2013) Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol 43(11):1162–1222
Muneer B, Iqbal MJ, Shakoori FR, Shakoori AR (2013) Tolerance and biosorption of mercury by microbial consortia: potential use in bioremediation of wastewater. Pak J Zool 45(1):247–254
Nadeem SM, Zahir ZA, Naveed M, Asghar HN, Arshad M (2010) Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci Soc Am J 74(2):533–542
Nayak AK, Panda SS, Basu A, Dhal NK (2018) Enhancement of toxic Cr (VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and Vetiveria zizanioides L. Int J Phytorem 20(7):682–691
Naz N, Young HK, Ahmed N, Gadd GM (2005) Cadmium accumulation and DNA homology with metal resistance genes in sulfate-reducing bacteria. Appl Environ Microbiol 71(8):4610–4618
Nie M, Wang Y, Yu J, Xiao M, Jiang L, Yang J, Li B (2011) Understanding plant-microbe interactions for phytoremediation of petroleum-polluted soil. PLoS ONE 6(3):e17961
Nissim WG, Palm E, Mancuso S, Azzarello E (2018) Trace element phytoextraction from contaminated soil: a case study under Mediterranean climate. Environ Sci Pollut Res 25(9):9114–9131
Oyetibo GO, Ilori MO, Adebusoye SA, Obayori OS, Amund OO (2010) Bacteria with dual resistance to elevated concentrations of heavy metals and antibiotics in Nigerian contaminated systems. Environ Monit Assess 168(1–4):305–314
Parvathi K, Nagendran R, Nareshkumar R (2007) Effect of pH on chromium biosorption by chemically treated Saccharomyces
Paschoalini AL, Bazzoli N (2021) Heavy metals affecting Neotropical freshwater fish: a review of the last 10 years of research. Aquat Toxicol 237:105906
Pepper IL, Gerba CP, Gentry TJ, Maier RM (eds) (2011) Environmental microbiology. Academic press
Philip L, Iyengar L, Venkobachar C (2000) Site of interaction of copper on Bacillus polymyxa. Water Air Soil Pollut 119(1–4):11–21
Prabha R, Singh DP, Verma MK (2017) Microbial interactions and perspectives for bioremediation of pesticides in the soils. In: Plant-microbe interactions in agro-ecological perspectives. Springer, Singapore, pp 649–671
Prescott LM, Harley JP, Klein DA (2002) Microbiology. 5th International Edition
Qasem NA, Mohammed RH, Lawal DU (2021) Removal of heavy metal ions from wastewater: a comprehensive and critical review. Npj Clean Water 4(1):36
Raghunandan K, Kumar A, Kumar S, Permaul K, Singh S (2018) Production of gellan gum, an exopolysaccharide, from biodiesel-derived waste glycerol by Sphingomonas spp. 3 Biotech 8(1):71
Raghunandan K, Mchunu S, Kumar A, Kumar KS, Govender A, Permaul K, Singh S (2014) Biodegradation of glycerol using bacterial isolates from soil under aerobic conditions. J Environ Sci Health Part A 49(1):85–92
Rajendran P, Muthukrishnan J, Gunasekaran P (2003) Microbes in heavy metal remediation.
Ramasamy K, Banu SP (2007) Bioremediation of metals: microbial processes and techniques. In: Environmental bioremediation technologies. Springer, Berlin, Heidelberg, pp 173–187
Ray SA, Ray MK (2009) Bioremediation of heavy metal toxicity-with special reference to chromium. Al Ameen J Med Sci 2(2):57–63
Riseh RS, Vazvani MG, Hajabdollahi N, Thakur VK (2022) Bioremediation of heavy metals by Rhizobacteria. Appl Biochem Biotechnol 1–23
Rodríguez-Tirado V, Green-Ruiz C, Gómez-Gil B (2012) Cu and Pb biosorption on Bacillus thioparans strain U3 in aqueous solution: Kinetic and equilibrium studies. Chem Eng J 181:352–359
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278(1):1–9
Salehizadeh H, Shojaosadati SA (2003) Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res 37(17):4231–4235
Sar P, D’Souza SF (2001) Biosorptive uranium uptake by a Pseudomonas strain: characterization and equilibrium studies. J Chem Technol Biotechnol 76(12):1286–1294
Saranya K, Sundaramanickam A, Shekhar S, Swaminathan S, Balasubramanian T (2017) Bioremediation of mercury by Vibrio fluvialis screened from industrial effluents. BioMed Res Int 2017
Schulz B, Boyle C (2006) What are endophytes?. In: Microbial root endophytes. Springer, Berlin, Heidelberg, pp 1–13
Seeger M, Hernández M, Méndez V, Ponce B, Córdova M, González M (2010) Bacterial degradation and bioremediation of chlorinated herbicides and biphenyls. J Soil Sci Plant Nutr 10(3):320–332
Seema S, Alok A (2012) Hexavalent chromium reduction in tannery effluent by bacterial species isolated from tannery effluent contaminated soil. J Environ Sci Technol 5(3):142–154
Seth CS (2012) A review on mechanisms of plant tolerance and role of transgenic plants in environmental clean-up. Bot Rev 78(1):32–62
Shah JK, Sayles GD, Suidan MT, Mihopoulos P, Kaskassian S (2001) Anaerobic bioventing of unsaturated zone contaminated with DDT and DNT. Water Sci Technol 43(2):35–42
Shannon MJ, Unterman R (1993) Evaluating bioremediation: distinguishing fact from fiction. Annu Rev Microbiol 47(1):715–736
Sharma PK, Balkwill DL, Frenkel A, Vairavamurthy MA (2000) A new Klebsiella planticola strain (Cd-1) grows anaerobically at high cadmium concentrations and precipitates cadmium sulfide. Appl Environ Microbiol 66(7):3083–3087
Shipra J, Dikshit SN, Pandey G (2011) Comparative study of agitation rate and stationary phase for the removal of Cu2+ by A. Lentulus. Int J Pharma Bio Sci 2(3)
Singh N, Verma T, Gaur R (2013) Detoxification of hexavalent chromium by an indigenous facultative anaerobic Bacillus cereus strain isolated from tannery effluent. Afr J Biotechnol 12(10)
Spormann AM, Widdel F (2000) Metabolism of alkylbenzenes, alkanes, and other hydrocarbons in anaerobic bacteria. Biodegradation 11(2–3):85–105
Sun X, Meng J, Huo S, Zhu J, Zheng S (2020) Remediation of heavy metal pollution in soil by microbial immobilization with carbon microspheres. Int J Environ Sci Dev 11(1)
Tamele IJ, Vázquez Loureiro P (2020) Lead, mercury and cadmium in fish and shellfish from the Indian Ocean and Red Sea (African Countries): public health challenges. J Mar Sci Eng 8(5):344
Tan H, Champion JT, Artiola JF, Brusseau ML, Miller RM (1994) Complexation of cadmium by a rhamnolipid biosurfactant. Environ Sci Technol 28(13):2402–2406
Tang X, Huang Y, Li Y, Wang L, Pei X, Zhou D, Hughes SS (2021) Study on detoxification and removal mechanisms of hexavalent chromium by microorganisms. Ecotoxicol Environ Saf 208:111699
Taştan BE, Ertuğrul S, Dönmez G (2010) Effective bioremoval of reactive dye and heavy metals by Aspergillus versicolor. Biores Technol 101(3):870–876
Tebo BM, Obraztsova AY (1998) Sulfate-reducing bacterium grows with Cr (VI), U (VI), Mn (IV), and Fe (III) as electron acceptors. FEMS Microbiol Lett 162(1):193–199
Tigini V, Prigione V, Giansanti P, Mangiavillano A, Pannocchia A, Varese GC (2010) Fungal biosorption, an innovative treatment for the decolourisation and detoxification of textile effluents. Water 2(3):550–565
Truu J, Truu M, Espenberg M, Nõlvak H, Juhanson J (2015) Phytoremediation and plant-assisted bioremediation in soil and treatment wetlands: a review. Open Biotechnol J 9(1)
Tunali S, Çabuk A, Akar T (2006) Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem Eng J 115(3):203–211
Tyagi S, Kumar V, Singh J, Teotia P, Bisht S, Sharma S (2014) Bioremediation of pulp and paper mill effluent by dominant aboriginal microbes and their consortium. Int J Environ Res 8(3):561–568
Ullah A, Mushtaq H, Ali H, Munis MFH, Javed MT, Chaudhary HJ (2015) Diazotrophs-assisted phytoremediation of heavy metals: a novel approach. Environ Sci Pollut Res 22(4):2505–2514
Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K (2016) Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 90(1):1–37
Valls M, Atrian S, de Lorenzo V, Fernández LA (2000) Engineering a mouse metallothionein on the cell surface of Ralstonia eutropha CH34 for immobilization of heavy metals in soil. Nat Biotechnol 18(6):661–665
Vara Prasad MN, de Oliveira Freitas HM (2003) Metal hyperaccumulation in plants: biodiversity prospecting for phytoremediation technology. Electron J Biotechnol 6(3):285–321
Verma A (2022) Bioremediation techniques for soil pollution: an introduction. Biodegrad Technol Organ Inorgan Pollut. https://doi.org/10.5772/intechopen.99028
Vinopal S, Ruml T, Kotrba P (2007) Biosorption of Cd2+ and Zn2+ by cell surface-engineered Saccharomyces cerevisiae. Int Biodeterior Biodegrad 60(2):96–102
Volesky B (1990) Removal and recovery of heavy metals by biosorption. Biosorption of Heavy Metals 7–43
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11(3):235–250
Wang D, Li H, Wei Z, Wang X, Hu F (2006) Effect of earthworms on the phytoremediation of zinc-polluted soil by ryegrass and Indian mustard. Biol Fertil Soils 43(1):120–123
Weyens N, van der Lelie D, Taghavi S, Newman L, Vangronsveld J (2009) Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol 27(10):591–598
Whelan MJ, Coulon F, Hince G, Rayner J, McWatters R, Spedding T, Snape I (2015) Fate and transport of petroleum hydrocarbons in engineered biopiles in polar regions. Chemosphere 131:232–240
Wierzba S (2015) Biosorption of lead (II), zinc (II) and nickel (II) from industrial wastewater by Stenotrophomonas maltophilia and Bacillus subtilis. Pol J Chem Technol 17(1):79–87
Wu MC, Hou CY, Jiang CM, Wang YT, Wang CY, Chen HH, Chang HM (2007) A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem 101(4):1753–1758
Yan G, Viraraghavan T (2001) Heavy metal removal in a biosorption column by immobilized M. rouxii biomass. Bioresour Technol 78(3):243–249
Yuan M, He H, Xiao L, Zhong T, Liu H, Li S, Jing Y (2014) Enhancement of Cd phytoextraction by two Amaranthus species with endophytic Rahnella sp. JN27. Chemosphere 103:99–104
Zakaria ZA, Zakaria Z, Surif S, Ahmad WA (2007) Hexavalent chromium reduction by Acinetobacter haemolyticus isolated from heavy-metal contaminated wastewater. J Hazard Mater 146(1–2):30–38
Zeyaullah MD, Atif M, Islam B, Abdelkafe AS, Sultan P, ElSaady MA, Ali A (2009) Bioremediation: a tool for environmental cleaning. Afr J Microbiol Res 3(6):310–314
Acknowledgements
The authors would like to acknowledge the support of all members of the Department of Chemistry of Natural and Microbial Products and the Department of Microbial Biotechnology at the National Research Centre.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
All the authors participate in writing, editing, and revising the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abo-Alkasem, M.I., Hassan, N.H. & Abo Elsoud, M.M. Microbial bioremediation as a tool for the removal of heavy metals. Bull Natl Res Cent 47, 31 (2023). https://doi.org/10.1186/s42269-023-01006-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-023-01006-z