Abstract
Background
Leptin exerts both protective and deleterious effects on the heart; the first occurs under hypoxia- or ischemia-associated damage, the second is a pro-hypertrophic factor on cardiomyocytes. Therefore, leptin could represent a link between obesity and cardiovascular diseases. The study aimed to investigate the effect of leptin—the same concentration that is frequently measured in obesity and induces cardiac hypertrophy—on murine hearts following an episode of ischemia–reperfusion; moreover, we evaluated the heart's performance, hypertrophy, and activation of apoptosis. Rat hearts were perfused continuously with or without 3.1 nM leptin for one h before and 1 h after an episode of ischemia. Cardiac performance was evaluated, homogenates and mitochondria were prepared for western blot analysis of cardiac actin, leptin receptor, STAT3, pSTAT3, and apoptosis-related proteins Bax, Bcl-2, cytochrome c, and caspase 3.
Results
Leptin worsened heart recovery after ischemia (p < 0.05 Control vs IR + Lep of Heart Perform, Fig. 2). Although no hypertrophic response was observed, leptin induced the migration of Bax to the mitochondria and the release of cytochrome c into the cytosol (p < 0.05 Control vs IR + Lep, Fig. 5), essential events in the intrinsic/mitochondrial apoptosis.
Conclusions
Our results indicate that the presence of leptin for 1 h before and after the ischemic insult reduces heart recovery and amplifies apoptotic signaling through the mitochondrial pathway.
Similar content being viewed by others
Background
Obesity has become an impending global health issue because of its impact on the quality of people's lives and because it represents a risk factor for various health disorders, including cardiovascular diseases, diabetes, and metabolic syndrome (Yusuf et al. 2005; Poirier et al. 2006). A key factor explaining the association between obesity and cardiovascular diseases is adipokine leptin, an adipocyte-released hormone that regulates satiety in the central nervous system (Zhang et al. 2005). A growing body of evidence has highlighted the detrimental effect of leptin on the cardiovascular system; this includes a recent report from our group demonstrating the deleterious effect of a perfused high concentration of leptin on mitochondrial performance in isolated rat hearts (Berzabá-Evoli et al. 2018).
Whereas thin people have less than 10 ng/mL of circulating leptin, obese people can have as much as 200 ng/mL, (Maffei et al. 1995; Perego et al. 2005) which could have a systemic impact considering that all organs are exposed. The cardiovascular system is a primary target for elevated leptin, as it promotes fatty acid oxidation and the release of pro-inflammatory cytokines that activate hypertrophic signaling (Sweeney 2010; Agrawal et al. 2011; Rajapurohitam et al. 2003) and initiate mitochondrial dysfunction (Karmazyn and Rajapurohitam 2014; Martinez-Abundis et al. 2012).
Activation of leptin receptors (ObR) is associated with several intracellular signaling pathways, including Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3), Rho/Rho-associated protein kinase (ROCK), and P38 mitogen-activated protein kinase (Rajapurohitam et al. 2003). We previously described the presence of ObR in the mitochondrial inner membrane of cardiomyocytes, (Martinez-Abundis et al. 2015) which is particularly interesting given that STAT3 is localized in several cellular compartments, including the mitochondria, where it optimizes the function of respiratory complexes I and II (Wegrzyn et al. 2009). Recently, Karmazyn's group reported that leptin modulated mitochondrial dynamics in cardiomyocytes, which was associated with its pro-hypertrophic effect (Jong et al. 2019). Based on the above findings, it is possible that signaling through the leptin receptor pathway could influence mitochondrial function.
Despite several reports of leptin's negative effect, the latter also exerts a protective role, mainly in ischemia models (Erkasap et al. 2006; Smith et al. 2010; Yang et al. 2018); however, most experimental evidence has accrued from pathophysiological conditions that do not exist in obese people.
Here, we aimed to analyze the effect of a high concentration of leptin on cardiac performance and regulation of mitochondrial apoptosis in a pathologic environment. To this end, we used an ischemia–reperfusion insult and administered leptin throughout perfusion and reperfusion time.
Results
Leptin perfusion reduces contraction pressure and worsens the response to ischemia–reperfusion
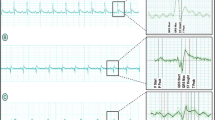
Rat hearts were subject to 45 min of ischemia and 1 h of reperfusion after 1 h of perfusion with/without 3.1 nM leptin (Fig. 1 describes the experimental design). Under these conditions, we noticed a decreased contraction pressure in leptin-perfused hearts during perfusion and after 45 min of global ischemia (Fig. 2A) compared with the control group, which was perfused continuously without leptin was not subjected to ischemia. The heart rate was not affected during the first hour of perfusion (Fig. 2B); however, it dropped significantly during reperfusion in those hearts perfused with leptin compared to those subjected to ischemia–reperfusion only and for which the decrease was much slighter. These results suggest an additive deleterious effect of leptin and ischemia, which can be translated to a decreased heart performance for the ischemia–reperfusion + leptin hearts compared with the ischemia–reperfusion only group (Fig. 2C).
Schematic representation of the experimental design. Hearts from the control group were continuously perfused with Krebs buffer; IR hearts were subjected to 45 min of ischemia before 60 min of perfusion and after 60 min of reperfusion; IR + Lep hearts were subjected to the same protocol as the IR group but the Krebs buffer contained 50 ng/mL leptin. IR ischemia–reperfusion; Lep leptin. For all experiments, n = 3
Effect of leptin on cardiac rate-pressure product (heart performance). A Plot of heart contraction pressure of leptin-perfused and control hearts (mmHg). B Plot of heart rate (bpm) of leptin-perfused and control hearts. C Heart rate-pressure product of leptin-perfused and control hearts; (n = 3). Ctrl control without ischemia; IR ischemia–reperfusion; Lep leptin. *p < 0.05 of AUC in reperfusion of Ctrl versus IR + Lep
Perfusion with leptin does not modify the expression of JAK/STAT-associated proteins
Hearts subjected to the different experimental protocols were homogenized. The expression of cACT, ObR, and total and phosphorylated STAT3 was determined by western blotting (Fig. 3A–D). Although some of the average values suggested an increased expression, mainly in ischemia–reperfusion hearts, statistical tests confirmed no differences between the experimental groups (Fig. 3). It is important to point out that these proteins are implicated in the pro-hypertrophic pathways described as activated by leptin.
Leptin does not change the expression of pro-hypertrophy-associated cACT, ObR, or STAT3 in heart homogenates. A cACT content; bars represent the median ± SEM of three independent preparations; B ObR content. C Total STAT3 and D phosphorylated STAT3 content; values indicate the mean ± SEM, n = 3. GAPDH was used as loading control. cACT cardiac actin (alpha-actin); ObR b isoform of the leptin receptor; STAT3 signal transducer and activator of transcription 3 total (t) and phosphorylated (p)
Effect of leptin on the apoptosis-related proteins
Ischemia–reperfusion is an insult that induces apoptosis in the heart. To determine the effect of leptin in this context, we quantified the expression of proteins associated with intrinsic (Bax and Bcl-2) or extrinsic (Caspase 3) pathways of apoptosis. Surprisingly, ischemia–reperfusion did not change the expression of Bcl-2 nor caspase 3; however, perfusion with leptin induced an increase in the median value of Bax. Additionally, ischemia–reperfusion groups down-regulated the expression of p53 (Fig. 5A), a ubiquitous regulator of apoptosis.
Leptin modifies the content of apoptosis-related proteins in mitochondria
Analysis of isolated mitochondria indicated that the pro-apoptotic protein Bax increased when leptin was present in ischemia–reperfusion hearts; this phenomenon did not occur in ischemia–reperfusion hearts (Fig. 5B). This result is exciting given that the movement of Bax from the cytosol to mitochondria is considered deleterious for the organelle's function and promotes cytochrome c releasing. Leptin also induced a higher release of cytochrome c from the mitochondria into the cytosol, which is usually associated with activating the intrinsic apoptotic pathway (Fig. 5C).
Discussion
Leptin has been described as having both protective and deleterious effects on the cardiovascular system (Rajapurohitam et al. 2003; Karmazyn and Rajapurohitam 2014; Erkasap et al. 2006; Smith et al. 2010; Yang et al. 2018), depending on the experimental conditions, the model employed (isolated, cultured cardiomyocytes, isolated hearts, in vivo), and the concentrations assayed. Most reports have adjudicated to leptin a cardioprotective effect against oxidative damage, associated with ischemia at concentrations of 160 or 3000 ng/mL (Erkasap et al. 2006; Smith et al. 2010; Yang et al. 2018); as opposed to basal circulating levels of up to 10 ng/mL in thin and 50–100 ng/mL in obese individuals (Maffei et al. 1995; Perego et al. 2005). In contrast, the deleterious pro-hypertrophic, mitochondrial dysfunction-inducing effect was described for obesity-associated leptin concentrations (50 ng/mL or 3.1 nM). Our study was designed to address this controversy by inducing cardiac ischemia–reperfusion in the presence of 50 ng/mL of leptin using the Langendorff system.
In obese people, hyperleptinemia is a chronic condition. In a previous study, whereby we assayed the long-term exposure of isolated hearts to leptin (Berzabá-Evoli et al. 2018) (over a maximum period of 4 h because of technical limitations inherent to the experimental model), leptin was seen to induce time-dependent mitochondrial dysfunction, manifested mainly as a decreased capability for calcium management. Here, we report that continuous perfusion with leptin 1 h before and 1 h after an ischemic insult worsened heart performance (Fig. 2C). These results were not associated with the pro-hypertrophic effect of leptin because we did not observe an increased expression of cACT or ObR and are in line with previous reports, whereby overexpression of hypertrophic markers was significant only after 6 h of leptin exposure (Martinez-Abundis et al. 2012), responding to the intracellular signaling pathways, including Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3), Rho/Rho-associated protein kinase (ROCK) (Karmazyn et al. 2013). In contrast, in our experimental design, hearts were exposed to leptin for less than 3 h. STAT3 activation was not detected because it is transient, with a peak in the first minutes of exposure (Smith et al. 2010) and a second and sustained increase after 6 h (Martinez-Abundis et al. 2012).
Apoptosis is part of the ischemia–reperfusion damage. Here, we could not confirm this mechanism's activation in ischemia–reperfusion hearts. Indeed, none of the commonly used markers for intrinsic or extrinsic pathways, such as increased expression of pro-apoptotic Bax with decreased anti-apoptotic Bcl-2, activation of caspase 3, or mitochondrial release of cytochrome c into the cytosol gave a clear-cut answer (Figs. 4 and 5). Although these results may be unexpected, we believe that the long perfusion period before ischemia (1 h) could exert a beneficial effect akin to that reported for cardiac preconditioning. Most ischemia–reperfusion protocols for the Langendorff system apply from 5 to 30 min of perfusion before ischemia, once apoptosis has been activated (Leistner et al. 2019; He et al. 2019; Stroethoff et al. 2019; Yasuda et al. 2019; Martínez-Abundis et al. 2009; Wang et al. 2017; Borutaite et al. 2003). Moreover, no apoptosis has been detected in some cases following ischemia–reperfusion (Klainguti et al. 2000). Our results support these findings and indicate that 1 h perfusion before ischemia–reperfusion decreases the levels of p53 (Fig. 5A), a phenomenon associated with inhibition of apoptosis and cardiac protection during hypoxic preconditioning (Xu et al. 2016).
Effect of leptin on the levels of apoptosis-associated proteins Bax, Bcl-2, and caspase 3 in heart homogenates. A Bax content; bars represent the median ± SEM of three independent preparations. B Bcl-2 content. C Caspase 3 and D activated caspase 3 content; values indicate the mean ± SEM, n = 3; *p < 0.05. GAPDH was used as loading control. Ctrl control without ischemia; IR ischemia–reperfusion; Lep leptin
Effect of leptin on the mitochondrial and cytosolic levels of apoptosis-associated proteins Bax, p53, and cytochrome c. A p53 content in heart homogenate; the image is representative. B Bax content in isolated mitochondria using VDAC as the loading control. C Cytochrome c content in heart mitochondrial (Mit) and cytosolic fractions of the same heart (Cyt). Values indicate the mean ± SEM, n = 3; *p < 0.05. Ctrl control without ischemia; IR ischemia–reperfusion; Lep leptin
Nevertheless, the results are very illustrative in terms of the effect of leptin. Recovery of heart performance after 45 min of ischemia was poorest when leptin was present in the perfusion buffer (Fig. 2C), in line with our previous report (Martínez-Abundis et al. 2015). Activation of apoptosis is one of the potential mechanisms explaining the deleterious effect of leptin on the heart (Martinez-Abundis et al. 2012); however, both the extrinsic and intrinsic apoptotic pathways may activate this mechanism. The latter involves the participation of mitochondria by releasing pro-apoptotic factors such as cytochrome c; whereas the first is associated with caspases activation. Our results indicate that recruitment of Bax from the cytosol to mitochondria was stimulated by leptin, and, as a result, cytochrome c was released from the mitochondria into the cytosol, which may trigger apoptosis (The intrinsic pathway of apoptosis, Fig. 5). In line with this result, Karmazyn's group recently reported that leptin induced the migration of Drp1, a mitochondrial fission activator, from the cytosol to the mitochondria (Jong et al. 2019). This event is associated, as well as Bax recruitment, with mitochondrial apoptosis.
Interestingly, in this report, the intracellular movement of Drp1 was dependent on its de-phosphorylation by the phosphatase calcineurin. The same behavior was described for Bax, (Gardai et al. 2004) which is retained in the cytosol in a phosphorylated form but is de-phosphorylated by apoptotic stimuli. Our experiments showed that mitochondrial Bax increased following exposure to leptin, even though total Bax did not augment, indicating an elevated relocation of the pro-apoptotic protein. The similarity between the regulation of Drp1 and Bax opens up the possibility that the mechanism by which leptin induces mitochondrial recruitment of Bax is also the same.
Some limitations of the present study should also be noted. First, we did not demonstrate activation of apoptosis following 45 min of ischemia. Second, although p53 down-regulation could provide a possible explanation for the observed lack of apoptotic markers' expression, it does not preclude other options. Under our experimental model, hearts were exposed to leptin for a limited time. In obese subjects, a chronic exposition may induce a different profile of effects; however, the results obtained from experimental models always bring important information to the understanding of a specific problem.
Conclusions
In conclusion, our results strongly support the hypothesis of a deleterious effect of a high concentration of leptin on cardiac and mitochondrial function under pathologic conditions such as those elicited by ischemia–reperfusion and the consequent activation of apoptosis. These results may explain, at least in part, the prognosis of recovery after a heart attack in obese people. The exact mechanism by which leptin elicits activation of the mitochondrial apoptotic pathway remains determined.
Methods
Reagents
Primary and secondary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and Bio-Rad (Hercules, CA, USA). Unless otherwise stated, all remaining chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Ischemia–reperfusion model
Eighteen male Wistar rats weighing 300–350 g were purchased from the Production, Care and Animal Experimentation Unit (UPCEA), Juarez Autonomous University of Tabasco and keep in acrylic cages with 24 h dark/light cycles and 25 °C for acclimatization. A Langendorff System (Radnoti LTD, Dublin, Ireland) was used to expose isolated hearts to 50 ng/mL (3.1 nM) leptin. Rats were deeply anesthetized by intraperitoneal injection with sodium pentobarbital plus heparin (anticoagulant); then, the heart was extracted by thoracotomy and quickly cannulated through the aorta for retrograde perfusion at 12 mL/min with physiologic Krebs–Henseleit buffer, pH 7.39 without leptin (Control group). The buffer was oxygenated continuously (O2 95%/CO2 5%) and maintained at 37 °C along with the experiment (1 h perfusion, 45 min ischemia, 1 h reperfusion, IR group and IR + LEP group) as explained in Fig. 1. Cardiac performance was measured at a left ventricular end-diastolic pressure of 10 mmHg using a latex balloon inserted into the left ventricle and connected to a pressure transducer. The measured heart rate (beats per minute, bpm) and contraction force (pressure, P) were used to calculate the rate-pressure product (heart performance). For the control group without ischemia–reperfusion, hearts were perfused continuously for 165 min. (See Fig. 1 for a better reference of the experimental design). After hearts were used, animal bodies were collected by a Biohazard Waste Management company for a final disposition.
Isolation of mitochondrial and cytosolic fractions
After ischemia–reperfusion experiments, the hearts were washed with cold STE isolation buffer (250 mM sucrose, 10 mM Tris–HCl, and 1 mM EDTA), cut into small pieces, and incubated for 10 min with nagarse (1 mg/heart in cold STE buffer). Nagarse was removed by centrifugation at 2500×g, and the tissue was homogenized in a Potter–Elvehjem homogenizer before the second centrifugation at 2500×g. A supernatant sample was preserved as total homogenate, whereas further centrifugation at 10,000×g yielded the mitochondrial fraction (pellet) and a crude cytosolic fraction (supernatant). Pellet was incubated for 10 min with 0.1% bovine serum albumin (BSA) in cold STE buffer. Finally, the total protein concentration for each fraction was calculated with the Bradford reagent using a standard curve with BSA. Cytosolic proteins were concentrated by precipitation with trichloroacetic acid (TCA) 5%.
Western blotting
Heart homogenates, mitochondria, and concentrated cytosolic fractions were mixed with loading buffer (30% glycerol, 10% SDS, 0.5 M Tris, 0.01% bromophenol blue, and β-mercaptoethanol) and boiled at 95 °C for 10 min. Proteins were resolved on 12% acrylamide/bis-acrylamide gels and transferred to nitrocellulose membranes. The membranes were then blocked for 2 h with 5% fat-free milk in washing buffer and incubated overnight at 4 °C with the following primary antibodies diluted 1:1000 with 3% BSA in washing buffer: mouse anti-cardiac alpha-actin (cACT), chicken anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH), rabbit anti-STAT3, mouse anti-pSTAT3, mouse anti-Bax, mouse anti-caspase 3, rabbit anti-voltage-dependent anion channel (VDAC), rabbit anti-Bcl-2, and mouse anti-cytochrome c. The next day, membranes were washed three times and incubated with horseradish peroxidase-conjugated secondary antibodies diluted 1:10,000 and then detected using the Immobilon Western AP chemiluminescent substrate (Millipore, Bedford, MA, USA).
Statistics
Values were assessed by one-way ANOVA before Dunnett's post hoc or t test for comparison between groups. Results are given as the mean ± standard error of the mean (SEM). A p value < 0 05 was considered the threshold for statistical significance between the compared groups. The area under the curve in Fig. 2 was assessed by Mann–Whitney U test. The GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis and ImageJ (NIH, Bethesda, MD, USA) for densitometry analysis of western blots.
Availability of data and materials
The results of western blot used and analyzed during the current study are included as supplementary material. The data set are available from the corresponding author on reasonable request (Additional file 1).
Abbreviations
- BSA:
-
Bovine serum albumin
- cACT:
-
Cardiac actin or alpha-actin
- min.:
-
Minutes
- EDTA:
-
Ethylenediamine tetraacetic acid
- Fig:
-
Figure
- JAK:
-
Janus Kinase
- JAK2:
-
Janus kinase 2
- ObR:
-
Leptin receptors
- ROCK:
-
Rho/Rho-associated protein kinase
- SDS:
-
Sodium dodecyl sulfate
- SEM:
-
Standard error of the mean
- STAT3:
-
Signal transducer and activator of transcription 3
- STE:
-
Sucrose/Tris/EDTA buffer
- VDAC:
-
Voltage dependent anion channel
References
Agrawal S, Gollapudi S, Su H, Gupta S (2011) Leptin activates human B cells to secrete TNF-alpha, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin Immunol 31:472–478
Berzabá-Evoli E, Zazueta C, Cruz Hernández JH, Gómez-Crisóstomo NP, Juárez-Rojop IE, De la Cruz-Hernández EN et al (2018) Leptin modifies the rat heart performance associated with mitochondrial dysfunction independently of its prohypertrophic effects. Int J Endocrinol 2018:6081415
Borutaite V, Jekabsone A, Morkuniene R, Brown GC (2003) Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. J Mol Cell Cardiol 35:357–366
Erkasap N, Ikizler M, Shneyvays V, Zinman T, Mamedova LK, Uyar R et al (2006) Leptin protects the cardiac myocyte cultures from hypoxic damage. Life Sci 78:1098–1102
Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N et al (2004) Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem 279:21085–21095
He L, Hao S, Wang Y, Yang W, Liu L, Chen H, Qian J (2019) Dexmedetomidine preconditioning attenuates ischemia/reperfusion injury in isolated rat hearts with endothelial dysfunction. Biomed Pharmacother 114:108837
Jong CJ, Yeung J, Tseung E, Karmazyn M (2019) Leptin-induced cardiomyocytes hypertrophy is associated with enhanced mitochondrial fission. Mol Cell Biochem 454:33–44
Karmazyn M, Rajapurohitam V (2014) Leptin as a cardiac pro-hypertrophic factor and its potential role in the development of heart failure. Curr Pharm Des 20:646–651
Karmazyn M, Gan XT, Rajapurohitam V (2013) The potential contribution of circulating and locally produced leptin to cardiac hypertrophy and failure. Can J Physiol Pharmacol 91(11):883–888
Klainguti M, Aigner S, Kilo J, Eppenberger HM, Mandinova A, Aebi U et al (2000) Lack of nuclear apoptosis in cardiomyocytes and increased endothelin-1 levels in a rat heart model of myocardial stunning. Basic Res Cardiol 95:308–315
Leistner M, Sommer S, Kanofsky P, Leyh R, Sommer SP (2019) Ischemia time impacts on respiratory chain functions and Ca(2+)-handling of cardiac subsarcolemmal mitochondria subjected to ischemia reperfusion injury. J Cardiothorac Surg 14:92
Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y et al (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161
Martínez-Abundis E, Correa F, Pavón N, Zazueta C (2009) Bax distribution into mitochondrial detergent-resistant microdomains is related to ceramide and cholesterol content in postischemic hearts. FEBS J 276:5579–5588
Martinez-Abundis E, Rajapurohitam V, Haist JV, Gan XT, Karmazyn M (2012) The obesity-related peptide leptin sensitizes cardiac mitochondria to calcium-induced permeability transition pore opening and apoptosis. PLoS ONE 7:e41612
Martinez-Abundis E, Rajapurohitam V, Gertler A, Karmazyn M (2015) Identification of functional leptin receptors expressed in ventricular mitochondria. Mol Cell Biochem 408:155–162
Perego L, Pizzocri P, Corradi D, Maisano F, Paganelli M, Fiorina P et al (2005) Circulating leptin correlates with left ventricular mass in morbid (grade III) obesity before and after weight loss induced by bariatric surgery: a potential role for leptin in mediating human left ventricular hypertrophy. J Clin Endocrinol Metab 90:4087–4093
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX et al (2006) Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol 26:968–976
Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M (2003) The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res 93:277–279
Smith CC, Dixon RA, Wynne AM, Theodorou L, Ong SG, Subrayan S et al (2010) Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol 299:H1265–H1270
Stroethoff M, Behmenburg F, Meierkord S, Bunte S, Mayer F, Mathes A et al (2019) Cardioprotective properties of omecamtiv mecarbil against ischemia and reperfusion injury. J Clin Med 8:E375
Sweeney G (2010) Cardiovascular effects of leptin. Nat Rev Cardiol 7:22–29
Wang X, Yang L, Kang L, Li J, Yang L, Zhang J et al (2017) Metformin attenuates myocardial ischemia-reperfusion injury via up-regulation of antioxidant enzymes. PLoS ONE 12:e0182777
Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T et al (2009) Function of mitochondrial Stat3 in cellular respiration. Science 323:793–797
Xu R, Sun Y, Chen Z, Yao Y, Ma G (2016) Hypoxic preconditioning inhibits hypoxia-induced apoptosis of cardiac progenitor cells via the PI3K/Akt-DNMT1-p53 pathway. Sci Rep 6:30922
Yang F, Wu R, Jiang Z, Chen J, Nan J, Su S et al (2018) Leptin increases mitochondrial OPA1 via GSK3-mediated OMA1 ubiquitination to enhance therapeutic effects of mesenchymal stem cell transplantation. Cell Death Dis 9:556
Yasuda J, Okada M, Yamawaki H (2019) Protective effect of T3 peptide, an active fragment of tumstatin, against ischemia/reperfusion injury in rat heart. J Pharmacol Sci 139:193–200
Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P et al (2005) Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 366:1640–1649
Zhang F, Chen Y, Heiman M, Dimarchi R (2005) Leptin: structure, function and biology. Vitam Horm 71:345–372
Acknowledgements
The authors wish to dedicate this work to the memory of our colleague and friend Teresa Ramón Frias, who passed away in 2020 and thank Fernanda Hernández Landero for her technical assistance.
Funding
Partially supported by the National Council of Science and Technology, CONACYT, Mexico; under Grant Nos. 2013-222290-M to EM-A and 2015-1/257849 to NPG-C.
Author information
Authors and Affiliations
Contributions
NPG-C, ENDC-H and EM-A contributed to the conception and design of the work; WNRL, JHCH, NPG-C, CFA-G and EM-A participated in performing the experiments, the acquisition, analysis, and interpretation of data; NPG-C, ENDC_H and EM-A were major contributors in writing the manuscript, drafted the work and revised it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Protocol was registered at the Juarez Autonomous University of Tabasco before to begin. All protocols were following politics of the "Institutional Committee for Ethics in Research" (protocol number 0272); with the Mexican regulations for the use of animals in research (Norma Oficial Mexicana NOM-062-ZOO-1999, technical specifications for production, use, and care of experimental animals) and following the "Three Rs": replacement, reduction, and refinement in research with animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
WB raw images.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rosado Loman, W.N., Cruz Hernández, J.H., Gómez-Crisóstomo, N.P. et al. A murine model of ischemia–reperfusion: the perfusion with leptin promotes the apoptosis-related relocation of mitochondrial proteins Bax and cytochrome c. Bull Natl Res Cent 46, 213 (2022). https://doi.org/10.1186/s42269-022-00899-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00899-6