Abstract
Background
Patients may have signs of hypovolemia, but fluid administration is not always beneficial. We are in need of bedside devices and techniques, which can predict fluid responsiveness effectively and safely. This study is aiming to compare the effectiveness and reliability of the pleth variability index (PVI) and IVC distensibility index (dIVC) as predictors of fluid responsiveness by simultaneous recordings in all sedated mechanically ventilated patients in the surgical intensive care unit (ICU). We used the passive leg raising test (PLR) as a harmless reversible technique for fluid challenge, and patients were considered responders if the cardiac index (CI) measured by transthoracic echocardiography (TTE) increased ≥ 15% after passive leg raising test (PLR).
Results
This observational cross-sectional study was performed randomly on 88 intubated ventilated sedated patients. Compared with CI measured by transthoracic echocardiography, the dIVC provided 79.17% sensitivity and 80% specificity at a threshold value of > 19.42% for fluid responsiveness prediction and was statistically significant (P < .0001), with an area under the curve (AUC) of 0.886 (0.801–0.944), while PVI at a threshold value of > 14% provided 93.75% sensitivity and 87.5% specificity and was statistically significant (P < .0001), with an AUC of 0.969 (0.889–0.988).
Conclusion
PVI and dIVC are effective non-invasive bedside methods for the assessment of fluid responsiveness in ICU for intubated ventilated sedated patients with sinus rhythm, but PVI has the advantage of being continuous, operator-independent, and more reliable than dIVC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Perioperative prediction of fluid responsiveness has been a challenge for many years. It is known as the ability of the circulation to increase cardiac output (CO) in response to volume expansion. Accommodation of the large volume of venous return (VR) is done by stretching ventricles, which is known as cardiac preload. Since preload is related to CO, increased negativity of intrathoracic pressure (ITP) during inspiration subsequently increases VR and subsequently CO, and the reverse occurs during expiration (Chu et al., 2016).
Hemodynamic optimization by intravenous (IV) fluid administration is very important to correct any fluid deficits created by fasting, blood loss, and urinary excretion, or in septic and other critically ill patients to improve oxygen delivery and overall hemodynamic function. However, it may be ineffective or harmful to patients if no suitable monitoring methods are used. Interstitial fluid accumulation by more volume expansion may worsen oxygen diffusion to the tissues and decrease myocardial compliance. Therefore, it became essential to develop an approach for the evaluation of patients who are likely to get benefit from fluid administration (Cumpstey et al., 2016).
Methods for assessment of volume responsiveness depending on what and how surrogate is measuring can be classified into static and dynamic measures (Jalil & Cavallazzi, 2018).
Swan and Ganz developed in 1970 the pulmonary artery flow-directed catheter. It has the ability to measure the pulmonary artery occlusion pressure (PAOP), but due to its complications and the same limitations as central venous pressure (CVP) (Chatterjee, 2009; Marik & Cavallazzi, 2013), multiple studies found that it cannot be a good predictor of fluid responsiveness (Marik & Lemson, 2014), as these static techniques cannot predict the effect on ITP during inspiration and expiration (Wise et al., 2017).
Michard and others discovered that the interaction between the heart and lung during mechanical ventilation could be used for the prediction of fluid responsiveness (Michard et al., 2000). Based on this idea, dynamic measurements such as pulse pressure variation (PPV) and stroke volume variation (SVV) were used to predict fluid responsiveness in a more accurate way, especially in sedated mechanically ventilated patients, but due to being invasive and affected by multiple clinical factors (Marik & Lemson, 2014), non-invasive bedside and continuous techniques became more popular (Haas et al., 2012).
Pleth variability index (PVI) is a dynamic variable, which has recently gained a lot of interest. By using the amplitude of the pulse oximeter waveform, it measures the respiratory variation continuously and automatically. Therefore, it became an effective dynamic fluid response predictor in sedated mechanically ventilated patients with sinus rhythm by providing simple numeric value on the monitor screen (Chu et al., 2016).
Ultrasound calculation of inferior vena cava distensibility index (dIVC) in mechanically ventilated patients has been reported as a widely available and non-invasive measure for prediction of fluid responsiveness in multiple patient settings (De Backer & Fagnoul, 2014). It can also be used with an ultrasound of the lungs and heart to give a comprehensive sonographic picture especially for critically ill patients in ICU (Evans et al., 2014).
We aim to compare the accuracy and reliability of the PVI and dIVC as predictors of fluid responsiveness by simultaneous recordings in mechanically ventilated sedated patients in our surgical ICU.
Methods
After approval of the ethical committee in the Faculty of Medicine, number FMASU M D 89/2018, this observational prospective study was conducted over 88 patients for 1 year from March 2018 to March 2019. Written informed consent was obtained from the patients’ legal guardians after explaining the procedure and its potential complications.
Inclusion criteria
The inclusion criteria are age ≥ 18 years, admission to the ICU preoperative (in need of ventilation) or postoperative, and sedated on controlled mechanical ventilation with arterial and central venous catheters. The included patients were in need of IV fluid challenge for resuscitation based on the clinical characteristics (systolic arterial blood pressure (BP) < 90 mmHg, or mean arterial pressure (MAP) < 65 mmHg, or a fall of > 20 mmHg from the baseline of MAP, or with signs of hypoperfusion as oliguria less than 0.5 ml/kg/h and arterial lactate > 2.5 mmol/L).
Exclusion criteria
The exclusion criteria are spontaneous breathing, poor cardiac echogenicity, cardiac arrhythmia, severe valvular heart disease or intracardiac shunt, impaired left ventricular function (ejection fraction < 40%), ascites, pregnancy, and any contraindication to fluid resuscitation, such as congestive heart failure, evidence of fluid overload, and renal dysfunction.
All patients were fully assessed with general physical examination, and their baseline characteristics, including age, gender, height, weight, body mass index, body surface area, and Acute Physiology and Chronic Health Evaluation score (APACHE) II score (Knaus et al., 1985), were recorded.
Patients were sedated using IV titration of fentanyl starting with 0.5 mcg/kg and midazolam starting with 0.5–1 mg to achieve a Ramsay sedation score of 4–6 (Ramsay et al., 1974), before muscle relaxation with 0.6 mg/kg rocuronium IV. Patients were mechanically ventilated (Newport™ e360 Ventilator; Newport Medical Instrument, CA) in volume control mode with tidal volume = 8 ml/kg of predicted body weight, inspiratory to expiratory ratio = 1:2, and positive end-expiratory pressure = 5 cm/H2O, and end-tidal carbon dioxide was monitored and maintained between 30 and 35 mmHg by adjusting the respiratory rate.
Recorded measurements were heart rate (HR), MAP, CVP with a zero referenced to the middle axillary line, PVI, dIVC, and cardiac index (CI). At first, every patient was positioned semi-recumbent with an angle of 45° between the trunk and the bed plane to record the initial measurements. Then, in the passive leg raising (PLR) position, other measurements were recorded after 1 min from leg elevation to 45° with the horizontal plane of the trunk. Positioning was done by an automatic bed mechanism controlled by a remote controller.
CO was measured by the same operator using transthoracic echocardiography (TTE) (ACUSON X300™ Ultrasound System, Premium Edition, Siemens Healthcare, Mountain View), as the product of stroke volume (SV) and heart rate (HR). SV is equal to the product of the aortic cross-sectional area, and the distance of a column of blood travels through it with each stroke, which is known as the velocity time integral (VTI) (Desai & Garry, 2018).
The cross-sectional area of the aortic annulus was estimated from the diameter (D) of the annulus using the parasternal long-axis view of the left ventricle. From this view, D is the maximal distance between the hinge points of the anterior and posterior aortic cusps in early systole, and the cross-sectional area is equal to the product of square D by 3.14 divided by 4 (Desai & Garry, 2018).
VTI can be estimated by obtaining the apical four-chamber (A4C) view, then tilting the ultrasound beams anteriorly to get the apical five-chamber (A5C) view, where the proximal ascending aorta, aortic valve, and left ventricular outflow tract (LVOT) come in the view (Matta et al., 2019). The pulse-wave (PW) Doppler is positioned within 15° to the LVOT, just in proximity to the aortic valve for the best flow estimation (Miller & Mandeville, 2016).
We calculated CI using CO, BW, and height, and we defined patients as “volume responders” if they had a ≥ 15% increase in the CI after the PLR test, and “non-responders” if they had no change or a change of < 15%.
The PVI was automatically calculated and recorded using an adhesive oximeter probe (LNCS Adtx, Masimo, Irvine, CA) placed on the finger and wrapped with a black protector for minimizing light interference and then connected to a Masimo Radical-7 monitor (Masimo Corp., Irvine, CA).
The dIVC is the percentage of variation in the IVC diameter during inspiration vs expiration. Which we measured by another single operator simultaneously, using the convex probe of ultrasound (Mindray M5, UMT-200/China) by the subcostal view approach using M-mode during the same respiratory cycle, then we calculated dIVC percentage by the following formula:
Statistical analysis
Data were collected, revised, coded, and entered to the MedCalc software (ver. 19.1.0; MedCalc Software, Ostend, Belgium), and the Statistical Package for Social Sciences, version 25.0 (SPSS Inc., Chicago, IL, USA). The quantitative data were presented as mean, standard deviations, and ranges. Also, qualitative variables were presented as number and percentages.
The paired Student t test was used to compare between hemodynamic data before and after PLR. Independent sample t test was used to compare between responders and non-responders for normally distributed parametric variables, while for the non-parametric data, we used the Mann-Whitney test.
The receiver operating characteristic (ROC) curves and area under the curve (AUC; with 95% confidence intervals [CIs]) of PVI, dIVC, and CVP were calculated and compared for the assessment of the ability of these methods to predict fluid responsiveness.
The confidence interval was set to 95%, and the margin of error accepted was set to 5%. So, a P value < 0.05 was considered significant, a P value < 0.001 was considered as highly significant, and a P value > 0.05 was considered insignificant.
Sample size
The PASS program is used, setting alpha error at 5% and power at 80%. The result from the previous study by Pişkin and Öz (Pişkin & Öz, 2017) showed that the AUC for diagnostic accuracy for PVI was 0.939, while for the diagnostic accuracy for IVC, the AUC was 0.79 in the study done by Long et al. (Long et al., 2017) Based on this, the needed sample is 40 responders and 40 non-responders.
Results
After the exclusion of 24 patients due to exclusion criteria, mainly poor echogenicity (18 patients), the study was conducted on 88 patients; we divided them into two groups (48 responders and 40 non-responders). There were no significant differences in patient demographics between the groups as shown in Table 1.
Hemodynamic data presented in Table 2 show a significant difference after PLR in the responders group (P < 0.5), while only HR showed a significant difference after PLR in the non-responders group. The baseline MAP, CVP, and CI of the responders were significantly lower than the non-responders (P < .05); the baseline PVI and dIVC were significantly higher than responders (P < 0.5), while HR readings showed no significant difference (P > .05) between the baselines of the two groups.
The difference between CVP, PVI, and dIVC regarding performance is presented in Table 3. CVP at a threshold value ≤ 5 mmHg had 70.83% sensitivity and 47.50% specificity, with AUC = 0.612 (0.502–0.714), and was not significant (P = 0.0648). dIVC had 79.17% sensitivity and 80% specificity at a threshold value of > 19.42% with AUC = 0.886 (0.801–0.944) and was highly significant (P < 0.0001).
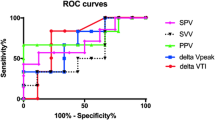
PVI had a 93.75 sensitivity and an 87.50 specificity at a threshold value of > 14% with AUC = 0.955 (0.889–0.988) and was highly significant (P < 0.0001). Figure 1 shows the ROC curves of the three methods comparing their effectiveness in the prediction of fluid responsiveness.
Discussion
This observational cross-sectional study was done for 1 year in the surgical ICU. Collected data from 88 patients were analyzed for reaching the final results. We compared between PVI and dIVC regarding their reliability and effectiveness as simple non-invasive dynamic techniques in mechanically ventilated sedated patients with sinus rhythm.
The best way to test the sensitivity of the heart to preload changes is to detect the change in CO after a fluid bolus (Pierrakos et al., 2012). The usual fluid challenge has two concerns. First, one is that we should not use a procedure, which may entangle in itself harm and volume overload due to irreversibility (Muller et al., 2011). Therefore, we used PLR as a dependable test for the recognition of preload responsiveness (Monnet et al., 2016).
The second concern is that we need to measure CO directly, so we used TTE as it is considered the gold standard dynamic technique in ICU (Desai & Garry, 2018), Moreover, a meta-analysis in 2016 revealed that it could predict fluid responsiveness accurately by PLR in ICU (Xiang et al., 2016). Patients in our study were defined as responders when their CO increased by 15% or more after PLR. The responders’ fraction for PLR in our work was 54.55%.
The deleterious effect of over fluid transfusion on different organs made a continuous need for new dynamic indicators for fluid management which are more preferable than unreliable static ones (Sakr et al., 2017). That was obvious in our study as CVP was a weak predictor for fluid responsiveness with best threshold value ≥ 5 mmHg and AUC of 0.612 (0.502 to 0.714).
Dynamic methods in fluid responsiveness identification are more accurate (Theerawit et al., 2016; Guérin et al., 2013), but some of them require invasive procedures like arterial line insertion, and also, not all of them can provide continuous readings. Minimally or non-invasive cardiac output monitors have the least prerequisites and can be used in a variety of critically ill patients for estimating CO rapidly (Jalil & Cavallazzi, 2018) to minimize the risk (Scheer et al., 2002) to already ill patients.
PVI differs from other invasive dynamic techniques in providing the physicians with a numerical value continuously, automatically, and non-invasively (Sandroni et al., 2012). In a study done by Forget and others, they found that PVI-based goal-directed fluid therapy reduced the volume of infused fluids intraoperatively, so reduced lactate levels intraoperative and postoperative (Forget et al., 2010). It is suitable only for patients on mechanical ventilation (Keller et al., 2008), and it is not suitable for patients with open chest, irregular heart rhythm, RV failure, or high intra-abdominal pressure (Cannesson et al., 2011).
Different other factors can affect the accuracy of PVI as the anatomical region where we made our readings. The fingertips may be affected more than the cephalic region (Desgranges et al., 2011) (forehead and earlobe); also, vasoactive drugs such as norepinephrine, perfusion problems, or hypothermia may affect the vasomotor tone and decrease peripheral perfusion (Broch et al., 2011; Yamaura et al., 2007; Monnet et al., 2013).
Our observational study included only patients on mechanical ventilation with the exclusion of any patient with factors affecting the heart-lung interaction or with vasoactive drug infusion, so our study results are not affected by these factors. On the other hand, we used fingertip measurements as an easier way to calculate PVI for fluid responsiveness identification.
The ROC analysis in our study revealed that the best PVI threshold value was more than 14%, with 93.75% sensitivity, 87.50% specificity, and AUC (95% CI) for the ROC curve of 0.955 (0.889–0.988).
In a recent meta-analysis done by Liu et al. which was including 25 studies, they concluded that PVI has a good bedside reliability in ICU especially with 79% sensitivity, 88% specificity, and AUC of 0.89 (0.86–0.92) also its limited ability in the prediction of fluid responsiveness in general (Liu et al, 2019). However, Chu et al.’s meta-analysis showed higher sensitivity of fluid responsiveness in operating theaters than ICU but with the same specificity and nearly equal AUC of 0.89 (0.85–0.92) in operating theater (OR) and AUC of 0.90 (0.82–0.94) in ICU (Chu et al., 2016). This is in line with our results; also, our study was in a surgical ICU using PLR with the exclusion of any patient on vasoactive drugs, which was not met with the two included meta-analysis studies.
Lately, ultrasound became one of the most important devices in ICU; it can be used as a bedside non-invasive method for the assessment of fluid responsiveness in daily ICU practice (Theerawit et al., 2016; Orso et al., 2016) by dIVC using variation in the diameter of the IVC (Barbier et al., 2004).
In a systematic review and meta-analysis done by Long et al. (Long et al., 2017), they found that pooled results of dIVC in mechanically ventilated patients had AUC of 0.79 with a sensitivity of 67% and a specificity of 68%, which is not in line with our results.
In another recent systematic review and meta-analysis done by Huang et al. (Huang et al., 2018), dIVC has better performance in mechanically ventilated shocked patients with a pooled AUC of 0.82 (95% CI 0.79–0.85) with a specificity of 80% and a sensitivity of 69%, which is near to our study results with 79.17% sensitivity and 80% specificity at a threshold value of > 19.42% with AUC = 0.886 (0.801−0.944).
However, several studies showed poor performance of dIVC. A study included 44 medical and surgical septic mechanically ventilated patients who had an AUC of 0.43 and 95% CI 0.25–0.61) with 38% sensitivity and 61% specificity (Charbonneau et al., 2014).
Many factors can affect dIVC measurements and cause this difference between studies as respiratory compliance (Lakhal et al., 2011), difference in adjusted tidal volume, amount and type of infused fluids (Barbier et al., 2004), and factors affecting intra-abdominal pressure (Bendjelid & Romand, 2012; Santa-Teresa et al., 2012).
The results of our study showed that assessment of PVI and dIVC non-invasively were good predictors for fluid management and responsiveness prediction using PLR technique in the surgical ICU mechanically ventilated patients.
Limitations
Our observational study has many limitations. The first one was in the accuracy of measuring CO by VTI using TTE. It necessitates good echogenicity of the patients, so we excluded 18 patients not fulfilling these criteria before data analysis.
The second limitation was in the comparison between PVI and dIVC; it is difficult to equalize blood volume added to circulation to all patients after PLR. However, the reversible PLR technique was proven trustworthy for preload responder identification (Monnet et al., 2016) with less harm to the candidate patient than irreversible transfusing of the same volume of crystalloid or colloid to all patients including the non-responders with no benefit.
The third limitation was intra-abdominal pressure, as we did not measure it in our patients. However, we excluded all patients with any expected cause of increased intra-abdominal pressure like ascites, pregnancy, abdominal malignancy, distension, and acute abdomen.
Conclusion
PVI and dIVC can be used in the assessment of fluid responsiveness of the intubated ventilated sedated patients with sinus rhythm in ICU, and both methods are non-invasive and can be performed at the bedside, but PVI has the advantage of being continuous, operator-independent, and more reliable than dIVC.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CI:
-
Cardiac index
- CO:
-
Cardiac output
- CVP:
-
Central venous pressure
- D :
-
Diameter
- dIVC:
-
Inferior vena cava distensibility index
- HR:
-
Heart rate
- ICU:
-
Intensive care unit
- ITP:
-
Intrathoracic pressure
- IV:
-
Intravenous
- IVC:
-
Inferior vena cava
- LVOT:
-
Left ventricular outflow tract
- MAP:
-
Mean arterial pressure
- OR:
-
Operating theater
- PAOP:
-
Pulmonary artery occlusion pressure
- PLR:
-
Passive leg raising
- PPV:
-
Pulse pressure variation
- PVI:
-
Pleth variability index
- PW:
-
Pulse-wave
- SV:
-
Stroke volume
- SSV:
-
Stroke volume variation
- TTE:
-
Transthoracic echocardiography
- VR:
-
Venous return
- VTI:
-
Velocity time integral
References
Barbier C, Loubières Y, Schmit C, Hayon J, Ricôme JL, Jardin F, Vieillard-Baron A (2004) Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Medicine 30(9):1740–1746
Bendjelid K, Romand JA (2012) Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care, In Applied Physiology in Intensive Care Medicine 2 (pp. 3-11). Springer, Berlin, Heidelberg
Broch O, Bein B, Gruenewald M, Höcker J, Schöttler J, Meybohm P, Renner J (2011) Accuracy of the pleth variability index to predict fluid responsiveness depends on the perfusion index. Acta Anaesthesiologica Scandinavica 55(6):686–693
Cannesson M, De Backer D, Hofer CK (2011) Using arterial pressure waveform analysis for the assessment of fluid responsiveness. Expert Review of Medical Devices 8(5):635–646
Charbonneau H, Riu B, Faron M, Mari A, Kurrek MM, Ruiz J et al (2014) Predicting preload responsiveness using simultaneous recordings of inferior and superior vena cavae diameters. Critical Care 18(5):473
Chatterjee K (2009) The Swan-Ganz catheters: past, present, and future: a viewpoint. Circulation 119(1):147–152
Chu H, Wang Y, Sun Y, Wang G (2016) Accuracy of pleth variability index to predict fluid responsiveness in mechanically ventilated patients: a systematic review and meta-analysis. Journal of Clinical Monitoring and Computing 30(3):265–274
Cumpstey AF, Grocott MP, Mythen MMG (2016) Fluid management and its role in enhanced recovery, In Perioperative Fluid Management (pp. 299-321). Springer, Cham
De Backer D, Fagnoul D (2014) Intensive care ultrasound: VI. Fluid responsiveness and shock assessment. Annals of the American Thoracic Society 11(1):129–136
Desai N, Garry D (2018) Assessing dynamic fluid-responsiveness using transthoracic echocardiography in intensive care. BJA Education 18(7):218–226
Desgranges FP, Desebbe O, Ghazouani A, Gilbert K, Keller G, Chiari P, Cannesson M (2011) Influence of the site of measurement on the ability of plethysmographic variability index to predict fluid responsiveness. British Journal of Anaesthesia 107(3):329–335
Evans D, Ferraioli G, Snellings J, Levitov A (2014) Volume responsiveness in critically ill patients: use of sonography to guide management. Journal of Ultrasound in Medicine 33(1):3–7
Forget P, Lois F, De Kock M (2010) Goal-directed fluid management based on the pulse oximeter–derived pleth variability index reduces lactate levels and improves fluid management. Anesthesia & Analgesia 111(4):910–914
Guérin L, Monnet X, Teboul JL (2013) Monitoring volume and fluid responsiveness: from static to dynamic indicators. Best Practice & Research Clinical Anaesthesiology 27(2):177–185
Haas S, Trepte C, Hinteregger M, Fahje R, Sill B, Herich L, Reuter DA (2012) Prediction of volume responsiveness using pleth variability index in patients undergoing cardiac surgery after cardiopulmonary bypass. Journal of Anesthesia 26(5):696–701
Huang H, Shen Q, Liu Y, Xu H, Fang Y (2018) Value of variation index of inferior vena cava diameter in predicting fluid responsiveness in patients with circulatory shock receiving mechanical ventilation: a systematic review and meta-analysis. Critical Care 22(1):1–7
Jalil BA, Cavallazzi R (2018) Predicting fluid responsiveness: a review of literature and a guide for the clinician. The American Journal of Emergency Medicine 36(11):2093–2102
Keller G, Cassar E, Desebbe O, Lehot JJ, Cannesson M (2008) Ability of pleth variability index to detect hemodynamic changes induced by passive leg raising in spontaneously breathing volunteers. Critical Care 12(2):R37
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Critical Care Medicine 13(10):818–829
Lakhal K, Ehrmann S, Benzekri-Lefèvre D, Runge I, Legras A, Dequin PF et al (2011) Respiratory pulse pressure variation fails to predict fluid responsiveness in acute respiratory distress syndrome. Critical Care 15(2):R85
Liu T, Xu C, Wang M, Niu Z, Qi D (2019) Reliability of pleth variability index in predicting preload responsiveness of mechanically ventilated patients under various conditions: a systematic review and meta-analysis. BMC Anesthesiology 19(1):1–7
Long E, Oakley E, Duke T, Babl FE, Paediatric Research in Emergency Departments International Collaborative (PREDICT) (2017) Does respiratory variation in inferior vena cava diameter predict fluid responsiveness: a systematic review and meta-analysis. Shock (Augusta, Ga.) 47(5):550–559
Marik PE, Cavallazzi R (2013) Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Critical Care Medicine 41(7):1774–1781
Marik PE, Lemson J (2014) Fluid responsiveness: an evolution of our understanding. British Journal of Anaesthesia 112(4):617–620
Matta JL, Kraemer CE, Tuinman PR, van Westerloo DJ (2019) POCUS series: the use of velocity time integral in assessing cardiac output and fluid responsiveness. Netherlands Journal of Critical Care 27(5):196–198
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y et al (2000) Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. American Journal of Respiratory and Critical Care Medicine 162(1):134–138
Miller A, Mandeville J (2016) Predicting and measuring fluid responsiveness with echocardiography. Echo Research and Practice 3(2):G1–G12
Monnet X, Guérin L, Jozwiak M, Bataille A, Julien F, Richard C, Teboul JL (2013) Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. British Journal of Anaesthesia 110(2):207–213
Monnet X, Marik PE, Teboul JL (2016) Prediction of fluid responsiveness: an update. Annals of Intensive Care 6(1):111
Muller L, Toumi M, Bousquet PJ, Riu-Poulenc B, Louart G, Candela D et al (2011) An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: the mini-fluid challenge study. Anesthesiology: The Journal of the American Society of Anesthesiologists 115(3):541–547
Orso D, Guglielmo N, Federici N, Cugini F, Ban A, Mearelli F, Copetti R (2016) Accuracy of the caval index and the expiratory diameter of the inferior vena cava for the diagnosis of dehydration in elderly. Journal of Ultrasound 19(3):203–209
Pierrakos C, Velissaris D, Scolletta S, Heenen S, De Backer D, Vincent JL (2012) Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Medicine 38(3):422–428
Pişkin Ö, Öz İİ (2017) Accuracy of pleth variability index compared with inferior vena cava diameter to predict fluid responsiveness in mechanically ventilated patients. Medicine 96(47):e8889
Ramsay MAE, Savege TM, Simpson BRJ, Goodwin R (1974) Controlled sedation with alphaxalone-alphadolone. Br med J 2(5920):656–659
Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S et al (2017) Higher fluid balance increases the risk of death from sepsis: results from a large international audit. Critical Care Medicine 45(3):386–394
Sandroni C, Cavallaro F, Marano C, Falcone C, De Santis P, Antonelli M (2012) Accuracy of plethysmographic indices as predictors of fluid responsiveness in mechanically ventilated adults: a systematic review and meta-analysis. Intensive Care Medicine 38(9):1429–1437
Santa-Teresa P, Muñoz J, Montero I, Zurita M, Tomey M, Álvarez-Sala L, García P (2012) Incidence and prognosis of intra-abdominal hypertension in critically ill medical patients: a prospective epidemiological study. Annals of Intensive Care 2(S1):S3
Scheer BV, Perel A, Pfeiffer UJ (2002) Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Critical Care 6(3):199
Theerawit P, Morasert T, Sutherasan Y (2016) Inferior vena cava diameter variation compared with pulse pressure variation as predictors of fluid responsiveness in patients with sepsis. Journal of Critical Care 36:246–251
Wise R, Faurie M, Malbrain ML, Hodgson E (2017) Strategies for intravenous fluid resuscitation in trauma patients. World Journal of Surgery 41(5):1170–1183
Xiang SI, Cao D, Wu J, Chen J, Liu Z, Chen M et al (2016) Diagnostic accuracy of transthoracic echocardiography to predict fluid responsiveness by passive leg raising in the critically ill: a meta-analysis. Open Journal of Emergency Medicine 4(04):83
Yamaura K, Irita K, Kandabashi T, Tohyama K, Takahashi S (2007) Evaluation of finger and forehead pulse oximeters during mild hypothermic cardiopulmonary bypass. Journal of Clinical Monitoring and Computing 21(4):249–252
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
DB designed the study, revised the literature, performed the analysis, followed up the patients, measured the inferior vena cava distensibility index, and wrote the manuscript. MI designed the study, performed the analysis, and wrote and critically revised the manuscript. YA revised the literature, performed the analysis, and critically reviewed the manuscript. IM revised the literature, followed up the patients, measured and calculated the cardiac index, collected the data, performed the analysis, and critically reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
After approval of the ethical committee in the Faculty of Medicine, Ain Shams University, number FMASU M D 89/2018, this observational prospective study was conducted over 88 patients for 1 year from March 2018 to March 2019. Written informed consent was obtained from patients’ legal guardians after explaining the procedure and its potential complications.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aboelnile, D.B.M.K., Elseidy, M.I.A., Kenawey, Y.A.E.M. et al. Prediction of fluid responsiveness in mechanically ventilated patients in surgical intensive care unit by pleth variability index and inferior vena cava diameter. Ain-Shams J Anesthesiol 12, 48 (2020). https://doi.org/10.1186/s42077-020-00097-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-020-00097-4