Abstract
Background
Yellow (stripe) rust, caused by Puccinia striiformis f. sp. tritici (Pst), is an economic disease of wheat. Growth-promoting fungi (GPF) such as Trichoderma asperellum and Penicillium simplicissimum have been investigated for their potential to control yellow rust and their involvement in gene expression of four PR proteins for all-stage resistance.
Results
Wheat plants (cv. Sids-12) treated individually with each of the biocontrol agents, P. simplicissimum and T. asperellum, at 24 and 48 hpi showed a resistance response (infection type = 2) to yellow rust, compared to the non-treated plants, which showed highly susceptible response (infection type = 9). Both biocontrol agents induced resistance against yellow rust on wheat plants, exhibiting a moderate resistance (10 MR) and reduced the colony size of Pst (0.6 mm2). Moreover, P. simplicissimum and T. asperellum increased (P ≤ 0.05) the grain yields of wheat plants infected with Pst. Scanning electron microscope (SEM) of yellow rust infected wheat leaves treated with P. simplicissimum and T. asperellum at 24 and 48 hpi showed hyperparasitism on Pst urediniospores and inhibition of the spore germination. Expressions of pathogenesis-related (PR) protein genes, PR1, PR2, PR3 and PR4 were higher in wheat plants treated with both biocontrol agents than the non-treated checks.
Conclusion
P. simplicissimum and T. asperellum exhibited biocontrol potential against yellow rust disease caused by P. striiformis f. sp. tritici (Pst) on wheat plants. It was found that wheat defence mechanism against Pst was activated by a high expression of PR protein genes induced by both biocontrol agents.
Similar content being viewed by others
Background
Yellow rust disease caused by P. striiformis f. sp. tritici (Pst) is one of the most devastating diseases to wheat worldwide (Wellings 2011). Yield losses of wheat due to this disease might exceed 90%, depending on the intensity of the outbreak and the degree of susceptibility of wheat varieties (Chen 2014). Fungicides and resistant cultivars may be useful to combat the disease. Cost-effective management may be achieved by the development of resistant cultivars. However, the fundamental disadvantage of this strategy is that certain virulent Pst races might quickly overcome cultivar resistance. Besides, the usage of fungicides is very harmful to the environment and human beings. Thus, the utilization of biocontrol agents to control yellow rust disease is now receiving the most research attention. Some fungal hyperparasites, such as Microdochium nivale, Lecanicillium lecanii, Cladosporium cladosporioides and Typhula idahoensis, were used to control Pst (Zhan et al. 2014). P. simplicissimum and T. asperellum were successfully utilized against the phytopathogen cucumber mosaic virus in Arabidopsis plants (Elsharkawy et al. 2013). Additionally, Trichoderma harzianum enhanced resistance against Plasmopara viticola (Kamble et al. 2021).
Wheat plants react to Pst infection by producing a variety of defensive proteins, which are mainly triggered by the pathogen itself. Plant responses could range from entirely resistant to completely susceptible. Wheat rust resistance may be grouped into durable and non-durable resistance (Chen et al. 2013). There is a more gradual increase of expression in the genes involved in adult plant heat resistance, compared to the rapid elevation of expression in the genes involved in all-stage resistance (Chen et al. 2013). Seedling-stage resistance may be identified during the seedling stage, but it manifests throughout the plant's life cycle. It is easy for virulent strains of the pathogen to overcome this form of resistance, hence it is not long-lasting against Pst infection. Plant resistance in wheat plants begins to express when plants enter the late jointing stage and the weather warms up, but often does not provide a complete protection when the disease begins early in the growing season, and weather is preferable for disease progression (Chen 2014). There are various defence-related genes that may be controlled by master genes, and both forms of resistance are controlled by the master gene known as the Yr gene, which is responsible for yellow rust resistance (Coram et al. 2008). In seedling-stage resistance, several genes have been implicated by transcriptomics research (Coram et al. 2008). However, the molecular systems that underline the various forms of resistance are still unknown. After adhering to the leaf surface under conditions of dew formation and temperatures suitable for germinating and penetrating, Pst urediniospores infect wheat resulting in a variety of histological and physiological responses. In response to these modifications, the plant will demonstrate either a resistant (incompatible) or a sensitive (compatible) response. The detection of the invading pathogen by the host plants is the first step in the initiation of these responses.
Plant-pathogen interactions are characterized by the presence of pathogen-related (PR) proteins, which are represented by a broad collection of 17 protein families (Van Loon et al. 2006). There are several different types of PR proteins, including PR1 (which has an unknown function), PR2 (1,3-glucanase), endochitinases (PR3, 4, 8, and 11), thaumatin-like proteins (PR5), proteinase inhibitors (PR6), proteinase (PR7), peroxidase (PR9), ribonuclease-like proteins (PR10), defensins (PR12), thionins (PR13), lipid transfer proteins (PR14), oxalate oxidase (PR15), oxalate oxidase-like proteins (PR16) and PR17 (unknown function). Most of these PR proteins have been shown to be involved in the plant defence against a variety of different environmental stresses, but their involvement in wheat-Pst interactions still needs to be further investigated (Van Loon et al. 2006).
There is a little knowledge regarding how various forms of yellow rust resistance affect the growth of the pathogen or the plant’s response to pathogen invasion in the presence of the biocontrol agents. Profiling the expression of distinct genes is one way to explore disease resistance mechanisms. Using the standard Affymetrix wheat microarray GeneChip or a custom GeneChip to compare the Yr5 resistance, a typical race-specific all-stage resistance, to a susceptible response showed the expression of just a few PR protein genes in contrast to the susceptible reaction (Coram et al. 2008). Additionally, the efforts to broaden gene expression and include some additional Yr genes for resistance were restricted by the limited number of genes available on the custom GeneChip that was employed in the previous research (Chen et al. 2013). In addition, it is unknown if any extra PR protein genes are involved in the various forms of resistance, and if they are, whether the expression of PR protein genes differs across the different types of resistance induced by biocontrol agents and their exact roles in resistance induced.
This study aimed to assess the potentiality of the two antagonistic fungi, P. simplicissimum and T. asperellum, as biocontrol agents against yellow rust of wheat caused by P. striiformis f. sp. tritici, and to specify the mechanisms of disease resistance by evaluating gene expressions of four PR proteins at seedling stage.
Methods
Efficacy of P. simplicissimum and T. asperellum against P. striiformis f. sp. tritici
The trials were performed under greenhouse conditions at the Wheat Disease Research Department, Sakha Agricultural Research Station, Plant Pathology Research Institute, Agricultural Research Centre, Egypt. Using a totally random design with three replications, the tests were carried out in plastic pots (10 cm diam) filled with clay soil. Wheat grains (cv. Sids-12) were sown in the pots at a density of 10 grains per pot. Eight days after germination, wheat seedlings were moistened and dusted with Pst urediniospores (6 × 105 spores/ml) according to the method described by Stubbs (1988). Pots were maintained in a dark, humid room at 10 °C for 24 h and then kept in a controlled greenhouse at 13 ± 2 °C and 100% RH under 102.6 μmol m−2 s−1 light intensity with a 16 h photoperiod.
Pure isolates of P. simplicissimum and T. asperellum were obtained from Prof. Mitsuro Hyakumachi, Gifu University, Japan. Each fungal antagonist inoculum was prepared according to the method reported by Biles and Hill (1988). After 14–18 days of culturing each isolate on PDA plates at 25 °C, the conidia were gently scraped with glass rods containing sterilized distilled water, and the spore suspension was filtered with a cheesecloth. Conidia were then counted with the aid of a hemocytometer. Wheat seedlings were inoculated with the fungal antagonists (1 × 106 spores/ml/seedling), 24 h before to pathogen inoculation. Non-treated plants were used as a control. Rust pustules were observed on plants for as long as possible. After 8–16 days of inoculation, the infection types (ITs) of yellow rust were scored on a 0–9 scale adopted by McNeal et al. (1971) where; ITs 0–6 refer to resistant response, while 7–9 refer to a susceptible response. Two parameters, i.e. the incubation period (IP) and latent period (LP), were evaluated according to Parlevliet (1975).
Biocontrol applications at the adult plant stage of wheat
P. simplicissimum and T. asperellum were used for treating wheat plants (cv. Sids-12) at the booting stage to control the yellow rust disease and its influences on the yield components were investigated. The experiment was performed over two growing seasons at the Wheat Disease Research Department's Experimental Farm, Sakha Agricultural Research Station, Agric. Res. Center, Egypt. Wheat seeds were sown in three rows of 1.5 m long and 30 cm apart for each treatment. The experiment was performed in a randomized complete block design (RCBD). A one-meter-wide spreader area was planted with the highly sensitive Morocco wheat variety and encircled each plot as a spreader of yellow rust. All commercial agricultural procedures, such as fertilization, irrigation, and other management, were used. The spreader was artificially inoculated using urediniospores (6 × 105 spores/ml). Conidial suspensions (1 × 106 spores/mL) of fungal antagonists mixed with Tween 20 (2 drops/100 ml) were sprayed on plants till runoff using a handheld sprayer after the appearance of rust pustules. Treatments were applied twice, 15-days apart. Yellow rust ratings were evaluated at the early dough stage when rust symptoms were severe on untreated control plants. The symptoms were used to assess disease severity, according to Roelfs et al. (1992). Rust severity was determined according to the proportion of leaves covered with rust pustules following Cobb’s scale (Peterson et al. 1948). A calibrated eyepiece micrometre was used to determine rust pustule colony sizes (mm2) (Saleem et al. 2019). The number of kernels per 10 spikes, weight of kernels/10 spikes and the weight of 1000 kernels were determined to compute yield components at the harvest stage.
Scanning electron microscope (SEM)
Using sterilized scissors, five specimens of wheat seedlings untreated or treated with P. simplicissimum and T. asperellum were harvested at 24-and 48-h post-inoculation (hpi) for scanning electron microscopy investigation. Sample preparation for SEM examination was carried out as described by Harley and Fergusen (1990). The central laboratory of the Faculty of Agriculture, Mansoura University, Egypt, used a Jeol scanning electron microscope (T.330 A) for inspection and photography. Ultra-structural changes on the pathogen were investigated in both treatments and control by scanning electron microscopy.
Analysis of gene expression of PR proteins for all-stage resistance
The transcription levels of defence genes were assessed to understand the mechanisms of disease resistance in wheat plants treated with the antagonistic fungi (P. simplicissimum and T. asperellum). Sterilized scissors were used to pick the leaves at 24- and 48-hpi. These time periods were chosen based on the most important stages of the infection process. Liquid nitrogen was used to freeze the leaves in an aluminium foil that had been washed with 95% ethanol. Using a sterile mortar and pestle, 200 mg of leaf tissue from each sample was crushed in liquid nitrogen to a fine powder. Each sample received a total of 1 ml of Trizol (Invitrogen, Carlsbad, CA, USA). The suspension was homogenized and incubated at 20–22 °C, followed by the addition of 0.2 ml chloroform. The samples were shaken for 15 s and incubated for an additional 2–3 min at 20–22 °C. Samples were centrifuged for 15 min at 13.000 rpm/min at 4 °C. To prepare the phenol: chloroform: isoamyl alcohol (25:24:1) mixture, a portion of the aqueous phase (550 µl) was transferred to an Eppendorf tube, and an equal amount of the mixture was added by vortexing and then centrifuged for 10 min at 13.000 rpm/min at 4 °C. An equivalent amount of chloroform was added to the aqueous phase in a fresh Eppendorf tube before centrifugation, then combined by inverting, held at 20–22 °C for 2–3 min, and centrifuged for 10 min at 13.000 rpm/min at 4 °C. The pellet was cleaned with 1 ml filter sterilized with 70% ethanol, and dried in a chemical hood after removing the supernatant. Sterilized water was added to dissolve the RNA pellet, and then the solution was maintained in the freezer until needed. A NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the RNA concentration in each sample. Four wheat PR protein genes were identified for their possible involvement in plant early defence in wheat. The primer sequences for PR1, PR2 and PR3 and PR4 are shown in Table 1 (Desmond et al. 2006). Polymerase chain reaction (PCR) procedures were carried out using the Life Science Research iTaq™ Universal One-Step RT-qPCR Kit (California, USA) (Desmond et al. 2006). Wheat leaf tissues from Pst-inoculated plants and treated with antagonistic fungi were analysed for the transcript levels of all four PR protein genes relative to their mean expression levels in mock-inoculated (control) plants at each time. To calibrate the expression levels of PR protein genes in each sample, the expression value of the β-tubulin gene, which is not influenced by pathogen infection, was used. Each time point was analysed using three separate samples. The comparative 2–ΔΔCT method was used to determine relative fold changes (Livak and Schmittgen 2001).
Statistical analysis
Data were subjected to the analysis of variance (ANOVA), and means were separated using Tukey's studentized range test at P ≤ 0.05 (SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Antagonistic effect of biocontrol agents against Pst
P. simplicissimum and T. asperellum significantly reduced yellow rust (YR) in terms of infection type, incubation period and latent period (Fig. 1). Treatment with each antagonistic isolate resulted in infection type 2, which refers to resistance response to yellow rust, compared to the susceptible response expressed on untreated control (IT 9). Both isolates exhibited the longest incubation period (IP) and latent period (LP), recording 12 d each for IP and 14 and 15 d for LP, respectively (Fig. 1).
Effect of P. simplicissimum and T. asperellum on the infection of wheat plants
Data in Table 2 showed that the application of P. simplicissimum and T. asperellum on wheat plants reduced the symptoms of yellow rust on wheat plants cv. Sids-12 at adult stage, relative to the non-treated control, which reached 90% susceptibility (90 S). P. simplicissimum and T. asperellum treatments reduced the size of Pst pustules (0.6 mm2), relative to the non-treated control (8.0 mm2).
Data illustrated in Table 3 showed that P. simplicissimum and T. asperellum treatments increased grain yield components in terms of the number of kernels/10 spikes, the weight of kernels/10 spikes and 1000-kernel weight in both seasons. P. simplicissimum recorded 522.3 and 41.6, while T. asperellum recorded 497.7 and 40.8 for the number of kernels/10 spikes and 1000-kernel weight, respectively, in the first season.
Effect of P. simplicissimum and T. asperellum treatments on spore germination
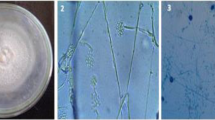
Scanning electron microscope (SEM) examinations of wheat leaves infected with Pst, the causal agent of yellow rust disease, and treated with P. simplicissimum and T. asperellum revealed fungal hyperparasitism on the surface of Pst urediniospores and the germination of urediniospores was reduced (Figs. 2 and 3). Hyphae of P. simplicissimum were seen growing from or between Pst urediniospores and the shrivelled urediniospores at 24 hpi (Fig. 2). At 48 hpi, ruptured or malformed urediniospores were seen invaded by the hyphae of P. simplicissimum, suggesting that they lost their ability to germinate. Abundance of P. simplicissimum hyperparasite inhibited the germination of urediospores (Fig. 2). Hyphae of T. asperellum were also seen growing extensively on the surface of Pst urediniospores (Fig. 3). At 24 hpi, the hyphae grew over and strangling urediniospore and shape of non-germinated urediniospores changed from round to oval. At 48 hpi, swelling and shrivelled urediniospore were observed. Abundance of T. asperellum hyperparasite inhibited the germination of Pst urediniospores. Normal shape and germination of urediniospores were observed on control leaves (Fig. 3).
Hyperparasitism of Penicillium simplicissimum on urediniospores of Puccinia striiformis f. sp. tritici (Pst). At 24 hpi: A normal shape and germination of urediniospores of Pst, B hyphae of P. simplicissimum growing from or between Pst urediniospores and C numerous hyphae of P. simplicissimum growing on Pst urediniospores. Note the shrivelled urediniospores. At 48 hpi: D hypha of P. simplicissimum invading a Pst urediniospore, E ruptured or malformed Pst urediniospore invaded by P. simplicissimum hyphae and F abundance of P. simplicissimum hyperparasite inhibiting the germination of urediospores
Hyperparasitism of Trichoderma asperellum on urediniospores of Puccinia striiformis f. sp. tritici (Pst). At 24 hpi: A normal shape of Pst urediniospores, B hyphae of T. asperellum growing over and strangle Pst urediniospores and C change in shape of non-germinated Pst urediospores from round to oval. At 48 hpi: D swelling of Pst urediniospore, E shrivelled Pst urediniospore and F abundance of T. asperellum hyperparasite inhibiting the germination of Pst urediospores
Gene expression of PR proteins for all-stage resistance
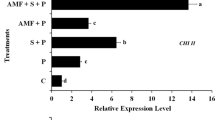
The mean relative expression values of the PR protein genes at 24 and 48 hpi in Pst-inoculated wheat leaves treated with P. simplicissimum and T. asperellum are presented in figs. 4 and 5. PR protein genes, PR1 and PR2, were enhanced in wheat plants treated with both P. simplicissimum and T. asperellum at 24 hpi, compared to the non-treated control (Fig. 4). However, gene expression values of PR1 and PR2 at 48 hpi were higher than those at 24 hpi. P. simplicissimum substantially raised the transcription of PR protein genes PR3 and PR4 at both 24 and 48 hpi, whereas T. asperellum did not significantly stimulate PR3 and PR4 expressions than the non-treated control (Figs. 4 and 5). At 48 hpi, the expression levels of PR genes were increased (Fig. 5). The expression levels of PR2 induced by both biological treatments, P. simplicissimum and T. asperellum were almost equal. Expression values of PR1 recorded 7.3 and 2.4 folds, while expression values of PR2 recorded 6.1 and 6.2 folds for P. simplicissimum and T. asperellum, respectively. However, the expressions of PR3 (6.9) and PR4 (5.1) induced by P. simplicissimum were significantly higher than their expression (1.4 and 1.2) induced by T. asperellum, which was non-significantly changed over control.
Discussion
In-plant defence against pathogens, pathogenesis-related protein genes play a critical role and also elicit resistance. In many plant pathosystems, the increase of PR protein gene transcripts is a well-characterized plant defensive response. However, PR proteins induced by fungal antagonists against Pst had not been characterized enough. Results of this study revealed the involvement of P. simplicissimum and T. asperellum in gene expression of four PR protein genes for all-stage resistance to yellow rust disease of wheat. Both biocontrol agents showed promising results in controlling yellow rust on wheat plants. At the adult plant stage, both antagonists induced resistance against yellow rust, exhibiting moderate resistance. They also reduced the colony size of Pst pustules. These results suggest that ruptured urediniospores, germinating spores with germ tubes smaller than the spore radius and non-germinating spores were all affected by the presence of P. simplicissimum and T. asperellum resulting in reduced disease rating. Pst urediniospores and the fungal hyphae competed for the site of the entrance. Released metabolites by biological agents slowed the growth of the germ tubes. Zheng et al. (2017) reported that A. alternata could parasitize Pst, which could be useful in the biological control of wheat yellow rust. Bacillus megaterium 6A and Paenibacillus xylanexedens 7A were also shown to greatly suppress the disease severity of wheat yellow rust (Kiani et al. 2021). Both antagonistic fungi, tested in this study, improved grain yield components in terms of the number of kernels/spike and 1000-kernel weight. Similarly, P. simplicissimum and T. asperellum improved the growth of Arabidopsis and cucumber plants (Elsharkawy et al. 2013).
Treatments with biological agents demonstrated a form of response alteration related to the development of systemic resistance. A correlation exists between this effect and the success of colonization. SEM examination revealed the infection of Pst urediniospores with P. simplicissimum and T. asperellum. Although treatments with these two fungal antagonists resulted in different reaction types, the mechanism of resistance response in inoculated plants may be generated by the activation of PR protein genes. Pst-infected wheat leaves treated with P. simplicissimum and T. asperellum at 24 and 48 hpi showed the greatest expression of PR protein genes PR1-PR4, which was substantially different from the non-treated control, except for PR3 and PR4 treated with T. asperellum. Pathogen-induced systemic acquired resistance in plants has been utilized as a marker for PR1 protein gene, which has been shown to boost defence against pathogens (Elsharkawy et al. 2013). This gene is employed as a marker for the salicylic acid (SA) pathway because of its responsiveness to SA (Elsharkawy et al. 2013). Resistance to Puccinia triticina leaf rust in adult wheat plants is mediated by Lr35, which was associated with the PR1 protein gene (Li et al. 2016). Wheat lines resistant to Mycosphaerella graminicola and Fusarium graminearum accumulate a lot of PR1 transcripts (Ray et al. 2003). P. simplicissimum and T. asperellum induced the greatest PR1 expression in wheat leaves at 24 hpi in the present research, indicating that PR1 is important in the modulation of all-stage resistance in wheat controlled by different Yr genes.
PR2 is a β-1, 3-glucanase, a group of enzymes that play a crucial role in plant defence and general stress responses by regulating the deposition of callose in the plant (Levy et al. 2007). Additionally, β-1, 3-glucanases are able to hydrolyze β-1, 3-glucans present in the fungal cell wall. Active host defence is elicited as a result of the deposition of cell wall fragments (Yoshikawa et al. 1993). Gene transcripts coding for β-1, 3-glucanases were observed on tobacco leaves within 24 to 48 h and enhanced up to 21 times by Pseudomonas syringae, an antibiotic-resistant bacterium (Alonso et al. 1995). Wheat adult-plant resistance to P. triticina was shown to have a high level of PR2 expression (Casassola et al. 2015) and in resistant wheat plants infected with F. graminearum (Li et al. 2001). Wheat leaves treated with P. simplicissimum and T. asperellum had the greatest expression of PR2 for all-stage resistance at 24 and 48 hpi in this study. Gene expression of β-1,3-glucanase genes was greatest at 24 and 48 hpi in all-stage resistance mediated by Yr5, which was consistent with other studies (Coram et al. 2008) and other genes (Chen et al. 2013). The results of this research clearly demonstrate that the PR2 protein correlates with race-specific all-stage resistance to yellow rust generated by the biocontrol agents; P. simplicissimum and T. asperellum. In fungi, the cell wall is composed mainly of chitin, and the genes PR3 and PR4 are endochitinases that break bonds between the C1 and C4 of the two successive N-acetylglucosamines of chitin (Collinge et al. 1993). It has been revealed that in vitro test incorporating wheat PR4 protein suppresses the formation of both spores and hyphae (Caruso et al. 2001). Wheat resistance to yellow rust may also be mediated by chitinase (Chen et al. 2013). The maximum expression of PR3 and PR4 was seen in all-stage resistance generated by biocontrol agents at 24 and 48 hpi in the present study, which was significant in P. simplicissimum but not in T. asperellum. In the same sample at various periods, the plant stage, in combination with the infection process by yellow rust of wheat, provided even larger activation of PR genes and improved induction of defensive activities (Esmail et al. 2020).
Conclusion
The fungal hyperparasitism of P. simplicissimum and T. asperellum against yellow rust disease of wheat showed the potential of inhibiting Pst urediniospores germination, which is involved in gene expression of PR1-PR4 proteins for all stage resistance. The reduction in yellow rust disease rating could be attributed to the expression of pathogenesis-related protein genes (PR1-PR4). The two fungal hyperparasites P. simplicissimum and T. asperellum used in this study showed the most promising results with great potential and can be used to induce resistance against the disease in susceptible cultivars.
Availability of data and materials
All data and materials are available.
Abbreviations
- Pst:
-
Puccinia striiformis f. sp. tritici
- GPF:
-
Growth-promoting fungi
- PR proteins:
-
Pathogen-related proteins
- Hpi:
-
Hours post-inoculation
- SEM:
-
Scanning electron microscope
- SAS:
-
Statistical analysis system
- ANOVA:
-
Analysis of variance
- YR:
-
Yellow rust
- IP:
-
Incubation period
- SAR:
-
Systemic acquired resistance
References
Alonso E, De Carvalho NF, Obregon P, Gheysen G, Inze D, Van Montagu M, Castresana C (1995) Differential in vitro DNA-binding activity to a promoter element of the gn1 β-1,3-glucanase gene in hyper sensitively reacting tobacco plants. Plant J 7:309–320
Biles CL, Hill JP (1988) Effect of Trichoderma harzianum on sporulation of Cochliobolus sativus on excised wheat seedling leaves. Phytopathology 78:656–659
Caruso C, Nobile M, Leonardi L, Bertini L, Buonocore V, Caporale C (2001) Isolation and amino acid sequence of two new PR-4 proteins from wheat. J Protein Chem 20:327–335
Casassola A, Brammer SP, Chaves MS, Martinelli JA, Stefanato F, Boyd LA (2015) Changes in gene expression profiles as they relate to the adult plant leaf rust resistance in the wheat cv. Toropi Physiol Mol Plant Pathol 89:49–54
Chen XM (2014) Integration of cultivar resistance and fungicide application for control of wheat stripe rust. Can J Plant Path 36:311–326
Chen XM, Coram TE, Huang XL, Wang MN, Dolezal A (2013) Understanding molecular mechanisms of durable and non-durable resistance to stripe rust in wheat using a transcriptomics approach. Curr Genom 14:111–126
Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad1 K (1993) Plant chitinases. Plant J 3(1):31–40
Coram TE, Wang MN, Chen XM (2008) Transcriptome analysis of the wheat-Puccinia striiformis f. sp. tritici interaction. Mol Plant Pathol 9:157–169
Desmond OJ, Edgar CI, Manners JM, Maclean DJ, Schenk PM, Kazan K (2006) Methyl jasmonate induced gene expression in wheat delays symptom development by the crown rot pathogen Fusarium pseudograminearum. Physiol Mol Plant Pathol 67:171–179
Elsharkawy MM, Shimizu M, Takahashi H, Ozaki K, Hyakumachi M (2013) Induction of Systemic Resistance against Cucumber mosaic virus in Arabidopsis thaliana by Trichoderma asperellum SKT-1. Plant Pathol J 29(2):193–200. https://doi.org/10.5423/PPJ.SI.07.2012.01
Esmail SM, Aboulila AA, Abd El-Moneim D (2020) Variation in several pathogenesis-related (PR) protein genes in wheat (Triticum aestivum) involved in defence against Puccinia striiformis f. sp. tritici. Physiol Mol Plant Pathol 112:101545
Harley MM, Fergusen LK (1990) Thee of SEM in pollen morphology and plant systemic. In: Clangher D (ed) Scanning electron microscopy studies in taxonomy and functional morphology, vol 41. Clarendon press, Oxford, pp 45–68
Kamble MV, Joshi SM, Hadimani S, Jogaiah S (2021) Biopriming with rhizosphere Trichoderma harzianum elicit protection against grapevine downy mildew disease by triggering histopathological and biochemical defence responses. Rhizosphere 19:100398. https://doi.org/10.1016/j.rhisph.2021.100398
Kiani T, Mehboob F, Hyder MZ, Zainy Z, Xu L, Huang L, Farrakh S (2021) Control of stripe rust of wheat using indigenous endophytic bacteria at seedling and adult plant stage. Sci Rep 11:14473. https://doi.org/10.1038/s41598-021-93939-6
Levy A, Guenoune-Gelbart D, Epel BL (2007) β-1,3- Glucanases: plasmodesmal gate keepers for intercellular communication. Plant Signal Behav 2:404–407
Li WL, Faris JD, Muthukrishnan S, Liu DJ, Chen PD, Gill BS (2001) Isolation and characterization of novel cDNA clones of acidic chitinases and β-1,3-glucanases from wheat spikes infected by Fusarium graminearum. Theor Appl Genet 102:353–362
Li X, Zhang Y, Zhang W, Zhang J, Wang H, Liu D (2016) Expression profiles of pathogenesis-related gene, TaLr35PR1 as it relate to Lr35-mediated adult plant leaf rust resistance. Plant Mol Biol Report 34:1127–1135
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25:402–408
McNeal FH, Konzak EP, Smith WS, Tate H, Russell TS (1971) A uniform system for recording and processing cereal research data. United States Department of Agriculture (USDA), Oxford, p 142
Parlevliet JE (1975) Partial resistance of barley to leaf rust, Puccinia hordei. I. effect of cultivars and development stage on latent period. Euphytica 24:21–27
Peterson RF, Compbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity on leaves and stems of cereal. Can J Res 26:496–500
Ray S, Anderson JM, Urmeev FI, Goodwin SB (2003) Rapid induction of a protein disulfide isomerase and defence- related genes in wheat in response to the hemibiotrophic fungal pathogen Mycosphaerella graminicola. Plant Mol Biol 53:701–714
Roelfs AP, Singh RP, Saari EE (1992) Rust diseases of wheat: concepts and methods of disease management. CIMMYT, Mexico, p 81
Saleem K, Sørensen CK, Labouriau R, Hovmøller MS (2019) Spatiotemporal changes in fungal growth and host responses of six yellow rust resistant near-isogenic lines of wheat. Plant Pathol 68:1320–1330. https://doi.org/10.1111/ppa.13052
Stubbs RW (1988) Pathogenicity analysis of yellow (stripe) rust of wheat and its significance in a global context. In: Simmonds NW, Rajaram S (eds) Breeding Strategies for Resistance to the Rusts of Wheat. CIMMYT, Mexico, pp 96–127
Van Loon LC, Rep M, Pieterse CMJ (2006) Significance of inducible defence-related proteins in infected plants. Annu Rev Phytopathol 44:135–162
Wellings CR (2011) Global status of stripe rust: a review of historical and current threats. Euphytica 179:129–141
Yoshikawa M, Yamaoka N, Rakeuchi Y (1993) Elicitors: their significance and primary modes of action in the induction of plant defence reactions. Plant Cell Physiol 34:1163–1173
Zhan G, Tian Y, Wang F, Chen X, Guo J, Jiao M, Kang Z (2014) A novel fungal hyperparasite of Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust. PLoS One 9(11):e111484
Zheng L, Zhao J, Liang X, Zhan G, Jiang S, Kang Z (2017) Identification of a novel Alternaria alternata strain able to hyperparasitize Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust. Front Microbiol 8:71
Acknowledgements
The authors greatly acknowledge the staff of Department of Agricultural Botany, Faculty of Agriculture, Kafrelsheikh University, Egypt, and Wheat Diseases Research Department, Plant Pathology Research Institute, Agricultural Research Center, Egypt.
Funding
Funding is by the authors.
Author information
Authors and Affiliations
Contributions
SME and MME were involved in the conceptualization and methodology; SME and MME contributed to the software and validation; SME and MME were involved in the investigation and resources; SME and MME helped in the data curation and software; SME and MME contributed to the resources and formal analysis; SME, ESD and MME were involved in writing—original draft preparation; SME, ESD, MHS, SM and MME were involved in the reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esmail, S.M., Draz, I.S., Saleem, M.H. et al. Penicillium simplicissimum and Trichoderma asperellum counteract the challenge of Puccinia striiformis f. sp. tritici in wheat plants. Egypt J Biol Pest Control 32, 116 (2022). https://doi.org/10.1186/s41938-022-00614-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-022-00614-7