Abstract
Sex differences in human survival have been extensively investigated in many studies that have in part uncovered the biological determinants that promote a longer life in females with respect to males. Moreover, researches performed in the past years have prompted increased awareness about the biological effects of environmental factors that can modulate the magnitude of the sex gap in survival. Besides the genetic background, epigenetic modifications like DNA methylation, that can modulate cell function, have been particularly studied in this framework. In this review, we aim to summarize the role of the genetic and epigenetic mechanisms in promoting female advantage from the early in life (“INNATE” features), and in influencing the magnitude of the gap in sex differences in survival and ageing (“VARIABLE” features). After briefly discussing the biological bases of sex determination in humans, we will provide much evidence showing that (i) “innate” mechanisms common to all males and to all females (both genetic and epigenetic) play a major role in sex differences in lifespan; (ii) “variable” genetic and epigenetic patterns, that vary according to context, populations and exposures to different environments, can affect the magnitude of the gap in sex differences in survival. Then we will describe recent findings in the use of epigenetic clocks to uncover sex differences in biological age and thus potentially in mortality. In conclusion, we will discuss how environmental factors cannot be kept apart from the biological factors providing evidence from the field of human ecology.
Similar content being viewed by others
Introduction
It is well known that females generally live longer than males in most mammals, including apes (Austad & Fischer, 2016; Bronikowski et al., 2022; Clutton-Brock & Isvaran, 2007; Lemaître et al., 2020). Females outlive males in almost all modern human populations, even under extreme events, such as famines, epidemics, and slavery (Colchero et al., 2016; Hägg & Jylhävä, 2021; Zarulli et al., 2018). However, the magnitude of this gap is variable and it is mainly shaped by complex interactions between biological and environmental factors that in humans also include sociocultural aspects (Lemaître et al., 2020). Data from German cloistered populations collected for the years 1890 to 1995 showed how peculiar sociocultural conditions such as enclosure and monastic life may influence the magnitude of this gap (Luy, 2003).
The classical viewpoint in the field tends to consider environmental factors as non-biological factors, with only genetic (innate) factors being considered biological. Recent discoveries have led to a blurring of the classic dichotomous view between biological (genetic) factors and non-biological factors (behavioural and environmental factors). In particular, the environment is increasingly becoming part of the concept of biology as demonstrated by recent discoveries in the field of epigenetics, a molecular mechanism at the interface between genetics and environment which is important in regulating gene expression.
Under these premises, in this review we will focus on the role of genetics and epigenetics in determining sex differences in survival. We will not discuss other biological aspects that certainly play an important role in this trait, like the effect of sex hormones that have been extensively described in other papers (Viña et al., 2005). First, we will briefly recall the process of sex determination in humans, then we will discuss current knowledge about (i) the genetic and epigenetic features that are characteristics of the female sex and of the male sex (that is, are common to all males and to all females) and that can promote female advantage in survival from the beginning of the life (we will refer to these characteristics as “INNATE”); (ii) the genetic and epigenetic patterns that vary according to context, populations and exposures to different environments, and that influence the magnitude of the gap in sex differences in survival and ageing (we will call them “VARIABLE”). We will also discuss how predictors of biological age that are based on epigenetic measures are differently associated to mortality in males and females. Finally, we will conclude the review with some considerations based on human ecology that support interdisciplinary reasoning and that contribute to explain sex differences in survival.
In order to assist the reader in dealing with some biological terms used across the text, a glossary is reported in Table 1.
Biological bases of sex determination in humans
In humans, like in the other mammals, sex is the result of two sequential processes known as primary sex determination and secondary sex determination. Primary sex determination refers to the development of gonads, i.e. the testis or the ovary, the organs responsible for the production of gametes (sperm and egg cells, respectively) (Table 1). Then, the hormones secreted by the gonads drive secondary sex determination: estrogens secreted from foetal ovaries induce the differentiation of uterus, oviducts, and cervix in females, while the testicular hormones (anti-Müllerian duct hormone, testosterone and dihydrotestosterone) drive the development of male phenotype (Table 1), including penis, seminal vesicles and prostate gland (Fig. 1). Finally, starting from embryogenesis, the production of sex hormones is finely regulated across the life of the individual and has a pervasive effect on body development and functioning.
Primary sex determination is strictly chromosomal, i.e. it depends on the set of chromosomes. In physiological conditions, 22 pairs of chromosomes (indicated with sequential numbers from 1 to 22) are present in both males and females and are called autosomes (Table 1). The remaining two chromosomes, indicated as X and Y chromosomes are termed sex chromosomes (Table 1): males have a single X and a single Y chromosome, i.e. a XY karyotype (Table 1), while females have two X chromosomes, i.e. a XX karyotype. Chromosome Y contains a gene, names SRY, which is pivotal in primary sex determination. Indeed, both testis and ovary develop from a common precursor, which forms at week 4 after fertilization and remains sexually indifferent until week 7. If SRY is present (XY karyotype) the gonad is committed towards testis, while if SRY is absent (XX karyotype) it will develop into ovary. Importantly, experiments in mouse models suggest that just the presence of SRY gene contributes to the shorter lifespan in males, as we will better discuss in Sect. "Genetic and epigenetic “variable” factors to explain the magnitude of sex differences in survival" (Davis et al., 2019).
Furthermore, the regulation of the expression of genes on the sex chromosomes depends on epigenetic modifications, including DNA methylation (DNAm) (Table 1). Indeed, females (XX karyotype) carry two copies of the about 1000 genes on X chromosome, while males (XY karyotype) have only one copy. As this different dosage in X-linked genes is potentially toxic, one of the X chromosomes in each cell of the developing female embryo undergoes a series of epigenetic modifications that prevent its expression, a process known as X chromosome inactivation (XCI) and that below is described as a potential biological mechanism involved in sex differences in survival. As a consequence of XCI, females will have only one functional copy of the X chromosome in each cell, like males. XCI normally occurs randomly in female cells during development, with some cells inactivating the X chromosome derived from the mother and other cells inactivating the X chromosome of paternal origin. As a consequence, females are a mosaic for the expression of genes on X chromosome.
Genetic and epigenetic “innate” factors to explain sex differences in survival
In this paragraph, we will discuss the genetic and epigenetic characteristics that are innate in females and males and that can contribute to sex differences in survival from conception. We will consider the role of sex chromosomes, the role of mitochondrial DNA (mtDNA) and the role of sex-specific epigenetic profiles.
The role of sex chromosomes
Sex chromosomes have a fundamental role in influencing the difference in survival between males and females. Already in 1985, Trivers (1985) hypothesized that in females (XX karyotype) each X chromosome protects the other from potentially damaging mutations, differently from what happens in male individuals (XY karyotype). Another interesting observation comes from the study of Davis et al. (2019). The authors made use of the “four core genotypes” mouse model, which consists in mice that have a sex chromosome complement (XX vs. XY) unrelated to their gonadal set-up. Through the relocation of the SRY gene (see Sect. “Biological bases of sex determination in humans”) from Y chromosome to an autosome, four different mouse strains were obtained: XX gonadal females (animals with an XX karyotype and ovaries), XX gonadal males (animals with and XX karyotype and testis), XY gonadal females (animals with and XY karyotype and ovaries) and XY gonadal males (animals with and XY karyotype and testes). The authors found that the risk of mortality for the XX karyotype was about 50% and 20% lower than the XY karyotype in animals, respectively, with ovaries and testis. Furthermore, they also demonstrated that the risk of mortality for mice with XX karyotype and ovaries was half of that observed for mice with XX karyotype and testis. Thus, the best combination that reduce the risk of mortality is XX karyotype and ovaries, the condition that physiologically characterized females. This experiment elegantly demonstrated that sex chromosomes have a main role in determining sex differences in survival.
Moreover, some authors suggested that XCI, described in the previous paragraph, can be advantageous for longevity: females are protected from potentially deleterious variants, as these are silenced in about half of the cells (Libert et al., 2010). On the contrary, in males the unique copy of X chromosome is unguarded and therefore potentially more susceptible to disadvantageous variants (Marais et al., 2018).
If XCI in females is advantageous on one side, its deregulation can favour sex-specific disease. Skewing of XCI occurs when the inactivation of one X chromosome occurs more frequently than the inactivation of the other one. The degree of skewing increases during ageing (Sharp et al., 2000) and possibly contributes to the higher frequency of some age-related diseases in females, like Alzheimer’s disease (Bajic et al., 2015). Conversely, delayed skewing occurs in centenarians' offspring, a model of successful aging (Gentilini et al., 2012).
In humans, up to 30% of X-chromosome genes can escape XCI and displays sex-biased gene expression (Tukiainen et al., 2017). Some evidence suggest that escaper genes can contribute to the sexual dimorphism in aging. For example, the escaper gene miR-548am-5p confers to XX cells a higher resistance to cell death compared to XY cells, a characteristic that could favour the survival of the female organism (Matarrese et al., 2019).
It is worth noting that some studies demonstrated that cells from the elderly tend to lose sex chromosomes with higher frequencies compared to autosomal chromosomes (Forsberg, 2017; Marais et al., 2018). In females, age-related X-chromosome loss preferentially involves the inactivated chromosome (Machiela et al., 2016) and it has been suggested that this phenomenon contributes to brain aging and Alzheimer’s disease (Yurov et al., 2014). Loss of Y chromosome during aging has been associated with mortality in males (Forsberg et al., 2014) and to risk for cancer, autoimmune thyroiditis and Alzheimer’s disease (Dumanski et al., 2016; Forsberg, 2017; Persani et al., 2012).
The role of mtDNA: the mother’s curse hypothesis
Unlike the nuclear DNA (Table 1), which comes from both parents, mitochondrial DNA (mtDNA) (Table 1) is inherited only from the mother (Hutchison et al., 1974), and it can only give a direct and adaptive response across generations only through females. This means that mutations with a negative effect in males can accumulate in the population if they are neutral or beneficial for females (a phenomenon called “the Mother's Curse”, one of the asymmetric inheritance theories). Accordingly, a study on mutations causing Leber’s hereditary optical neuropathy, a disease with male-biased prevalence, suggests that deleterious mutations could accumulate in the mtDNA at higher cost for males in term of fecundity, health and lifespan (Milot et al., 2017).
A study on Drosophila melanogaster reported that SNPs (Table 1) in mtDNA sequence can contribute to the sex differences in survival, such that the females outlive the males (Camus et al., 2015). In particular, authors showed that a single SNP (Ala-278-Thr) located in CYTB gene has antagonistic pleiotropic effects (Table 1) and the males carrying this SNP are sterile but with greater longevity while the same variant in females is associated with fertility and shorter longevity. This constitutes an example of pleiotropic effect both within and between sexes (Camus et al., 2015). However, experimental evidence of this mechanism in humans is still an open field of research. Evidence of the sex-specific effect of mitochondrial variants on gene expression through ageing has been collected mainly on animal models while the data in humans are still scarce. A recent study performed on 955 individuals from a population-based Young Finns Study cohort considering the whole mtDNA sequence did not show any significant differences in gene expression between males and females (Laaksonen et al., 2019, 2021).
The role of sex-specific epigenetic profiles
Several studies reported that innate DNAm differences exist between males and females (Inoshita et al., 2015; Perzel Mandell et al., 2021; Singmann et al., 2015; Spiers et al., 2015; Yusipov et al., 2020). These innate epigenetic differences are not limited to sex chromosomes, but are widespread across the entire genome and possibly contribute to sex differences in the predisposition to some diseases, including neurological, psychiatric and metabolic pathologies (Hall et al., 2014; Maschietto et al., 2017; Migliore et al., 2021; Perzel Mandell et al., 2021). Epigenetic profiles are profoundly remodelled during ageing and can further change in age-related diseases. Several of the CpG sites with sex-associated DNAm undergo also age-associated DNAm changes, and in most of the cases the differences between males and females tend to be maintained during ageing (Pellegrini et al., 2021; Yusipov et al., 2020). However, some genomic regions display significant age-by-sex interactions, i.e. they change their DNAm during aging in a different way in males and in females (Masser et al., 2017; McCartney et al., 2019; Yusipov et al., 2020). It is tempting to speculate that these sex-specific DNAm trajectories during aging can contribute to sex differences in survival. For example, a recent paper showed that two CpG sites with significant age-by-sex interactions are differently modulated in human models of successful (centenarians) and unsuccessful (persons with Down syndrome) aging (Yusipov et al., 2020). In particular, centenarian males showed a DNAm pattern more similar to that characteristic of females, while females with Down syndrome tended to have a masculinization of their DNAm values. Kananen and Marttila evaluated how DNAm of CpG sites on X and Y chromosomes changes with ageing in whole blood considering a large cohort of males and females aged 14–92 years. They found that males and females shared a significant number of CpG sites on X chromosome showing age-associated DNAm changes and concluded that DNAm changes in the X chromosome are unlikely to be a major contributor of sex dimorphism in ageing (Kananen & Marttila, 2021). When investigating age-by-sex differences in blood-based DNAm, McCartney et al. identified almost 600 CpG sites located in X chromosome that had different age-dependent DNAm patterns in males and females (McCartney et al., 2020), although a subsequent work suggested that the identification of these sex-specific patterns could be influenced by XCI (Li et al., 2020a). Importantly, neither of these two studies discussed whether and how the observed age-dependent DNAm changes in X chromosome can influence sex differences in survival.
At least three studies (Li et al., 2022; Lund et al., 2020; Vidaki et al., 2021) evaluated whole blood DNAm from male participants with different ancestries and found a clear hypermethylation of Y-linked CpG sites during ageing. Furthermore, Li et al. reported the hypermethylation of one CpG site within Y chromosome was positively associated with all-cause mortality risk in a Chinese cohort (Li et al., 2022), while Lund et al. analysed four cohorts of European octogenarians and found that the rate of hypermethylation was higher at older ages and associated with reduced risk of all-cause mortality (Lund et al., 2020). These partially discordant results can be related to the specific characteristics of the cohorts evaluated in each study, but collectively demonstrate that the epigenetic regulation of Y chromosome during ageing is an important contributor of ageing trajectories in males.
Genetic and epigenetic “variable” factors to explain the magnitude of sex differences in survival
As described in the previous paragraphs, males and females have a different genetic and epigenetic asset, which possibly contributes to the longer life expectancy of females. However, as mentioned in the introduction, the extent of the sex gap in survival is variable. In the present paragraph we will discuss the possible contribution of genetic and epigenetic variability to this phenotypic variability. These genetic and epigenetic features are not shared by all the females and by all the males (for example, are present only in individuals with a certain genetic background or exposed to a certain environment), but contribute to the variability in the magnitude of the sex gap in survival.
Genetic factors
Several studies in the last decades are addressing the role of single genetic variants in shaping differences between males and females in age-related onset of diseases and survival (Lagou et al., 2021). The vast amount of data does not provide a unidirectional association but showed that different regions of the genome may play a role in modulating the magnitude of sex difference in survival. We report some specific examples from genome-wide association studies (GWAS), which enable to analyse several hundreds of thousands of SNPs at the same time across the genome of many people to find genetic variation associated with complex traits and particular disease. GWAS are therefore one of the most powerful tools to address whether genetic variability contributes to sex differences in human longevity. A GWAS study was performed in the framework of the Genetics of Healthy Aging (GEHA) European project to identify SNPs associated with longer lives in a group of 1228 unrelated nonagenarian from 6 European nations (Finland, Denmark, UK, Netherlands, France, Italy) and 1907 geographically matched controls. Authors identified 3 alleles (Table 1) linked to longevity in a sex-specific manner, two beneficial for only females and one beneficial for only males (Beekman et al., 2013). Another GWAS (Zeng et al., 2018) compared 564 male and 1614 female participants older than 100 years with a control group of 773 male and 1526 female individuals aged 40 to 64 years. All were Chinese Longitudinal Healthy Longevity Study participants with Han Chinese ancestry who were recruited in 1998 and 2008 to 2014. Sex-specific longevity alleles, located on genes involved in inflammation and tryptophan metabolism, were found to be significant associated with longevity, respectively, in males and females. A recent GWAS (Liu et al., 2021) performed on the same Chinese population (N = 15,651 individuals) found 2 male-specific SNPs and 4 female-specific SNPs strongly associated with the longevity. Notably, although one of the four female-specific SNPs, named rs2075650, had been linked to longevity in multiple studies that performed sex-combined analysis, authors found that the effect of this SNP on longevity in female was about 1.5 times higher than in males. Furthermore, authors created a model to predict longevity and lifespan starting from a subset of 23,800 SNPs, which however represents part of the total genetic variability. They stated that this model could explain the 7% of the variance for lifespan in females but failed to provide a significant prediction for lifespan in males. The absence of a significant prediction of lifespan in men could be due to other factors, such as the lifestyle, the interaction of genetics with the sexual hormones, the different prevalence of disease in the two sexes, that could have a greater predictive capacity, “hiding” the effect of the genetic factors. Another interesting result emerged from this paper is about the genomic loci located in the well-known region of APOE/TOMM40, which presented a sex difference and a strong association with longevity in females (Liu et al., 2021). This last finding was partially confirmed by another study performed in the UK biobank dataset, which demonstrated that common variants near APOE have sex- and age-dependent effects (Joshi et al., 2016).
Furthermore, several studies in the last decades are addressing the role of single genetic variants in shaping differences between males and females in age-related onset of diseases and survival and demonstrated that some variants can have a beneficial effect in one sex but deleterious (or null) effects in the other and for that they are named “sexually antagonistic variants” (SAV) (Rice, 1984; Ruzicka & Connallon, 2020). In this perspective, it is likely that SAV can contribute to the magnitude of gap in the survival by predisposing males to age-related diseases more than females. A recent study, merging studies of evolutionary biology and biomedicine, described SNPs with sexually antagonistic effects on human diseases (Harper et al., 2021). The authors identified list of SNPs with sex-specific effect (meaning same direction but different magnitude) or the sex-limited ones (meaning that the SNP plays a role in one sex only) including several diseases (some of them age-related) (Harper et al., 2021). An example of study considered in this review is that of Sainz and colleagues (2012), who investigated the relationship between the colorectal cancer and the alleles that are known to affect the type 2 diabetes, for a deeper understanding of the relationship between these two diseases. They found that a mutation located on the LTA gene was underrepresented in female with colorectal cancer, but more present in control males with the type 2 diabetes, suggesting that this mutation has a protective role against colorectal cancer in female, but a detrimental role for the type 2 diabetes in male.
Another independent study investigated the effect of 174 variants on IL-6 serum levels, that are associated with diseases, disability and mortality in the elderly, in a total of 700 people from 60 to 110 years of age, including 323 centenarians, born and resident in Italy. Authors found that the same variants located in the IL6 gene are associated to higher level of the IL-6 serum levels in men than women (Bonafè et al., 2001).
Overall, these findings suggest the key role of the GWAS to identify genetic variants associated with sex differences in longevity and how SAVs can contribute to the onset of age-related diseases which in turn can affect the magnitude of the sex differences in survival. However, to date GWAS are mainly based on case–control studies with the final aim of identifying list of SNPs associated with survival and only recently with survival of males and females. In this scenario more studies that estimate the contribution of these SNPs to the magnitude of sex differences in survival between male and female are needed.
Furthermore, it is worth noting that GWAS generally consider only autosomes, as sex chromosomes are usually excluded from the analyses for methodological reasons and potential bias due to sex-specific demographic events. This scenario is going to change in next future, thanks to the growing consciousness on the role of sex chromosomes in several human traits, including aging, and to the generation of statistical pipelines specifically devoted to their analysis.
Epigenetic factors
Epigenetic variability, here described as the differences in DNAm profiles among individuals, is the result of the interaction between the genetic background of an individual and the environment to which he/she is exposed during the life course, starting from the in utero period (Cavalli & Heard, 2019; Gluckman, 2012; Tobi et al., 2018). Both genetics and environment can affect DNAm profiles in a sex-specific manner, and this can be relevant for development of diseases and for survival. For example, some studies demonstrated that some SNPs can have sex-specific effects on DNAm profiles, contributing to the different risks of psychiatric disorders and post-traumatic stress disorder in males and females (Vukojevic et al., 2014; Xia et al., 2021). A recent study on Faroe islanders showed that the exposure to different chemical compounds can have sex-specific effects on DNAm. Notably, epigenetic changes were remarkably more pronounced in males than in females and were enriched in X chromosome and occur in genes involved in several diseases (Leung et al., 2018). Future studies should systematically investigate whether the sex-specific effects of genetic or environmental factors can contribute to the magnitude of survival differences between males and females.
The use of epigenetic clocks to uncover sex differences in biological age
As mentioned in paragraph “The role of sex-specific epigenetic profiles”, ageing profoundly affects DNAm profiles. Some of this age-associated DNAm changes are so reproducible that they have been used to build mathematical models to predict the age of an individual. Although these models, termed epigenetic clocks, are highly predictive of chronological age, in some situations the estimated age can be different from chronological age. This discrepancy is generally considered informative of the biological age of an individual, as it has been associated with the presence of age-related diseases, morbidity and mortality (Horvath & Raj, 2018).
Epigenetic clocks have provided important insights into the biological basis of sex differences in survival. Horvath and colleagues (Horvath et al., 2016) analysed blood, saliva, and brain samples from seven different ethnic groups: Hispanics, Caucasians, African Americans, East Asians, Tsimane Amerindians, Pygmies hunter-gatherers and Bantus agrarians, both from Central Africa. According to epigenetic clocks computed from blood samples, they found that men were epigenetically older than woman in African Americans and Caucasians, even when controlling for education, diabetes, and hypertension. The other ethnic groups showed similar trends, although they did not reach statistical significance. Furthermore, blood cells count predicted from DNAm profiles suggested a more rapid immunosenescence in men than in woman for three ethnic groups: Caucasians, Tsimane and African Americans. Men resulted epigenetically older than woman also when measuring the epigenetic clock in saliva samples from Hispanic individuals and in brain samples from Caucasian individuals collected in six different studies. Kankaanpää and colleagues (2021) assessed the association between epigenetic age and sex in younger and older Finnish twin adults. They showed that men were epigenetically older than woman, and that the sex differences in biological ageing tended to increase with the chronological age at least until about 50 years old. In particular, men were about 1.2 years older than women in the younger twins (21–42 years of age) and about 4 years older in the older twins (50–76 years of age). The authors also found that the magnitude of this difference tended to remain constant from midlife onward. Another longitudinal study on the Swedish population supports this finding (Li et al., 2020b) and showed that average rates of epigenetic age increase are not significantly different in males and females after the age 50. A possible explanation for this phenomenon is that men experienced higher rates of epigenetic aging even before 50 years, whereas woman experienced higher rates of epigenetic aging only after menopause (Levine et al., 2018), and as a consequence offset this difference between the sexes. It cannot be excluded that male sexual hormones also could play a role in this phenomenon, since it is known that the decrease of testosterone production, that diminishes with chronological age, is associated with a deceleration of epigenetic aging (Sugrue et al., 2021).
In an increasing number of studies, epigenetic clocks are used to evaluate the biological readout of social, economic and physical environments (Fiorito et al., 2017; Oblak et al., 2021). The effects of these factors on the magnitude of sex-specific differences in survival have only been partially investigated. Fiorito and colleagues (2017) investigated the association of low socio-economic status (SES) with epigenetic age acceleration in three independent cohorts from Italy, Ireland and Australia. For each individual the SES was computed as the combination of their childhood SES (defined as the father’s occupational position) and their adult SAS (defined as his highest occupational position), both categorized as “low” and “high”. Authors found that lower SES was associated with higher epigenetic age acceleration, with an increase in epigenetic age of about 1 years in individuals with low SES when compared with those with high SES. Although also this study reported that males are epigenetically older than females, no significant evidence of SES–sex interaction was detected. Zhao and colleagues (2019), explored the association between education (defined by the highest level of degree and the years of education) or lifestyle risk factors for age-related diseases (such as smoking, alcohol consumption, physical activity) and the epigenetic age in the African American participants of the Genetic Epidemiology Network of Arteriopathy. The authors found that effect of education and smoking on epigenetic age varied according to sex. In fact, although men were epigenetically older than woman overall, the magnitude of the sex-specific difference in the epigenetic clocks tend to decrease with the level of education. In particular, the gap in the epigenetic clocks between women and men without high school licence was about 5 years, whereas this gap reduced to about 3.5 years between women and men with a college degree. Furthermore, they also found that the magnitude of sex-specific difference in the epigenetic clocks was higher in current smokers than in never smokers.
Also, the effect of interventions aimed at promoting healthy ageing can be sex-specific. For example, a recent study showed that the administration of Mediterranean diet for one year promoted a rejuvenation in epigenetic age and that this effect was more pronounced in females, although the magnitude of sex-specific gap in the epigenetic clocks was not reported (Gensous et al., 2020).
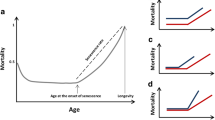
In this perspective, more interdisciplinary studies are needed to explore how socio-economic factors and physical environment contribute to the magnitude of sex-specific difference by modulating biological ageing and its epigenetic readout (Fig. 2).
Ecological insights
Survival and reproduction are the two most basic components of fitness, and they drive the evolution of a life cycle. A trade-off between survival and reproduction was first theorized by Kirkwood in the framework of the “Disposable Soma theory” (Kirkwood, 1977). According to this theory, longevity in women requires investments in somatic maintenance that reduce the resources available for the reproduction. In other words, the “Disposable Soma theory” assumes that an organism divides its energy between maintenance and reproduction according to the evolutionary niche in which it has developed. In particular, it has been observed that when survival increases, fertility decreases, and vice versa (Jasienska et al., 2017; Kirkwood & Rose, 1991; Penn & Smith, 2007; Salinari et al., 2022; Westendorp & Kirkwood, 1998), although this relationship could be confounded by unobserved demographic factors (Gavrilov & Gavrilova, 1999) and by the frailty and the mortality selection during childbearing age (Doblhammer & Oeppen, 2003).
The trade-off between survival and reproduction is also supported by biological measurements (Edward & Chapman, 2011; Ghalambor & Martin, 2001; Partridge et al., 2005). A study on 397 young (20–22 year-old) Filipino women investigated the relationship between the number of pregnancies and epigenetic age (Ryan et al., 2018). The authors found that each additional pregnancy was associated with 0.44-year increase in the epigenetic age acceleration, supporting the hypothesis that a higher number of pregnancies was associated with a worst biological aging. This observation is in line with a study performed in 2356 non-Hispanic white women, aged 35–74, enrolled in the Sister Study cohort and living in US (Kresovich et al., 2019). Two major results emerged from this study: (i) an increase in the epigenetic age acceleration estimates were seen with more births; (ii) the age at the first birth was negatively associated with epigenetic age acceleration, suggesting that the negative effect of reproduction on survival is much higher in the early reproductive ages.
The trade-off between survival and fertility is also influenced by the variability of ecological niches, thus contributing to creating a high level of population variability in many life-history traits. For example, in environment with high extrinsic mortality, defined as the age-specific risk of death due to external forces that is equally shared by all members of a population (Quinlan, 2007), metabolic investment in reproduction is prioritized, thus reducing energy allocated to somatic maintenance (or growth). This leads an organisms to physiological and molecular changes towards a “fast life-history”, characterized by peculiar development characteristics, such as younger age at first birth, accelerated pubertal timing, suboptimal tissue defence, but also earlier age at menopause, increased risk of cardiovascular disease and to accelerate the biological process of aging (Fraser et al., 2020; Hidaka & Boddy, 2016; Nettle, 2010; Stearns et al., 2000).
These trade-offs can be interpreted in the light of the principle of energy allocation (Baudisch & Vaupel, 2012) that define sex-specific developmental, behavioural and metabolic strategies that optimize energy allocation in males and females across life stages. When resources are allocated to reproduction (female) or growth (male)—rather than to somatic maintenance—molecular errors should accumulate more rapidly in somatic cells, leading to a stronger senescence. Lemaitre et al. reviewed many biological studies that provide evidence that the ageing process is embedded in the evolution of life-history strategies and covaries with other biological processes like growth and reproduction in a sex-specific way and in relation to environmental conditions (Lemaitre et al., 2015). One example is the nutrient-sensing and growth-promoting TOR signalling pathway that is considered a universal molecular link between growth and ageing from yeast to humans (Blagosklonny & Hall, 2009). It has been suggested that sex differences in survival can be related to accelerated aging in males relative to females as a by-product of physical robustness to prevent death from extrinsic causes, that at molecular level is visible through a hyper-activation of mTOR that contributes to physical robustness/growth of young males. This hyperactive mTOR is beneficial earlier in life at the cost of accelerated ageing (Blagosklonny, 2010).
Conclusions
Genetics and epigenetics are gaining increasing attention in the field for their emerging role in determining sex differences in survival, but this research field is still in its infancy and only in the last decades the differences between sexes started to be considered and included in experimental design. Despite these limitations here we provided evidence showing that “innate” mechanisms (both genetic and epigenetic) common to all males and to all females play a major role in sex differences in lifespan, but solely on their own are not sufficient to explain the magnitude of this gap between sexes. Genetic and epigenetic patterns that vary according to context, populations and exposures to different environments, influence the magnitude of the gap in sex differences in survival, whose ultimate causes can be analysed through the principle of energy allocation. Many external factors such as parental investment, sexual selection, nutritional factors and ecological factors—whose role is central in this trait (Austad & Fischer, 2016)—could become embodied through epigenetic mechanisms thus increasing the complexity of this trait. In conclusion, the use of epigenetic clocks to uncover sex differences in biological age seems the most promising biomarkers for interdisciplinary research at the interface between biology and demography.
Availability of data and materials
Not applicable.
References
Argentieri, M. A., Nagarajan, S., Seddighzadeh, B., Baccarelli, A. A., & Shields, A. E. (2017). Epigenetic pathways in human disease: The impact of DNA methylation on stress-related pathogenesis and current challenges in biomarker development. eBioMedicine, 18, 327–350. https://doi.org/10.1016/j.ebiom.2017.03.044
Austad, S. N., & Fischer, K. E. (2016). Sex differences in lifespan. Cell Metabolism, 23(6), 1022–1033. https://doi.org/10.1016/j.cmet.2016.05.019
Bajic, V., Mandusic, V., Stefanova, E., Bozovic, A., Davidovic, R., Zivkovic, L., Cabarkapa, A., & Spremo-Potparevic, B. (2015). Skewed X-chromosome inactivation in women affected by Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD, 43(4), Article 4. https://doi.org/10.3233/JAD-141674
Baudisch, A., & Vaupel, J. W. (2012). Getting to the root of aging. Science, 338(6107), 618–619. https://doi.org/10.1126/science.1226467
Beekman, M., Blanché, H., Perola, M., Hervonen, A., Bezrukov, V., Sikora, E., Flachsbart, F., Christiansen, L., De Craen, A. J. M., Kirkwood, T. B. L., Rea, I. M., Poulain, M., Robine, J.-M., Valensin, S., Stazi, M. A., Passarino, G., Deiana, L., Gonos, E. S., Paternoster, L., et al., & GEHA consortium. (2013). Genome-wide linkage analysis for human longevity: Genetics of Healthy Aging Study. Aging Cell, 12(2), 184–193. https://doi.org/10.1111/acel.12039
Blagosklonny, M. V. (2010). Why the disposable soma theory cannot explain why women live longer and why we age. Aging, 2(12), 884–887. https://doi.org/10.18632/aging.100253
Blagosklonny, M. V., & Hall, M. N. (2009). Growth and aging: A common molecular mechanism. Aging, 1(4), 357–362. https://doi.org/10.18632/aging.100040
Bonafè, M., Olivieri, F., Cavallone, L., Giovagnetti, S., Mayegiani, F., Cardelli, M., Pieri, C., Marra, M., Antonicelli, R., Lisa, R., Rizzo, M. R., Paolisso, G., Monti, D., & Franceschi, C. (2001). A gender-dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. European Journal of Immunology, 31(8), 2357–2361. https://doi.org/10.1002/1521-4141(200108)31:8%3c2357::aid-immu2357%3e3.0.co;2-x
Bronikowski, A. M., Meisel, R. P., Biga, P. R., Walters, J. R., Mank, J. E., Larschan, E., Wilkinson, G. S., Valenzuela, N., Conard, A. M., Magalhães, J. P., Duan, J. E., Elias, A. E., Gamble, T., Graze, R. M., Gribble, K. E., Kreiling, J. A., & Riddle, N. C. (2022). Sex-specific aging in animals: Perspective and future directions. Aging Cell. https://doi.org/10.1111/acel.13542
Camus, M. F., Wolf, J. B. W., Morrow, E. H., & Dowling, D. K. (2015). Single nucleotides in the mtDNA sequence modify mitochondrial molecular function and are associated with sex-specific effects on fertility and aging. Current Biology, 25(20), 2717–2722. https://doi.org/10.1016/j.cub.2015.09.012
Cavalli, G., & Heard, E. (2019). Advances in epigenetics link genetics to the environment and disease. Nature, 571(7766), 489–499. https://doi.org/10.1038/s41586-019-1411-0
Clutton-Brock, T. H., & Isvaran, K. (2007). Sex differences in ageing in natural populations of vertebrates. Proceedings of the Royal Society b: Biological Sciences, 274(1629), 3097–3104. https://doi.org/10.1098/rspb.2007.1138
Colchero, F., Rau, R., Jones, O. R., Barthold, J. A., Conde, D. A., Lenart, A., Nemeth, L., Scheuerlein, A., Schoeley, J., Torres, C., Zarulli, V., Altmann, J., Brockman, D. K., Bronikowski, A. M., Fedigan, L. M., Pusey, A. E., Stoinski, T. S., Strier, K. B., Baudisch, A., et al. (2016). The emergence of longevous populations. Proceedings of the National Academy of Sciences. https://doi.org/10.1073/pnas.1612191113
Davis, E. J., Lobach, I., & Dubal, D. B. (2019). Female XX sex chromosomes increase survival and extend lifespan in aging mice. Aging Cell, 18(1), Article 1. https://doi.org/10.1111/acel.12871
Doblhammer, G., & Oeppen, J. (2003). Reproduction and longevity among the British peerage: The effect of frailty and health selection. Proceedings of the Royal Society of London Series b: Biological Sciences, 270(1524), 1541–1547. https://doi.org/10.1098/rspb.2003.2400
Dor, Y., & Cedar, H. (2018). Principles of DNA methylation and their implications for biology and medicine. The Lancet, 392(10149), 777–786. https://doi.org/10.1016/S0140-6736(18)31268-6
Dumanski, J. P., Lambert, J.-C., Rasi, C., Giedraitis, V., Davies, H., Grenier-Boley, B., Lindgren, C. M., Campion, D., Dufouil, C., European Alzheimer’s Disease Initiative Investigators, Pasquier, F., Amouyel, P., Lannfelt, L., Ingelsson, M., Kilander, L., Lind, L., & Forsberg, L. A. (2016). Mosaic loss of chromosome Y in blood is associated with Alzheimer disease. American Journal of Human Genetics, 98(6), Article 6. https://doi.org/10.1016/j.ajhg.2016.05.014
Edward, D. A., & Chapman, T. (2011). Mechanisms underlying reproductive trade-offs: Costs of reproduction. In T. Flatt & A. Heyland (Eds.), Mechanisms of life history evolution (pp. 137–152). Oxford University Press. https://doi.org/10.1093/acprof:oso/9780199568765.003.0011
Fiorito, G., Polidoro, S., Dugué, P.-A., Kivimaki, M., Ponzi, E., Matullo, G., Guarrera, S., Assumma, M. B., Georgiadis, P., Kyrtopoulos, S. A., Krogh, V., Palli, D., Panico, S., Sacerdote, C., Tumino, R., Chadeau-Hyam, M., Stringhini, S., Severi, G., Hodge, A. M., et al. (2017). Social adversity and epigenetic aging: A multi-cohort study on socioeconomic differences in peripheral blood DNA methylation. Scientific Reports, 7(1), Article 1. https://doi.org/10.1038/s41598-017-16391-5
Forsberg, L. A. (2017). Loss of chromosome Y (LOY) in blood cells is associated with increased risk for disease and mortality in aging men. Human Genetics, 136(5), Article 5. https://doi.org/10.1007/s00439-017-1799-2
Forsberg, L. A., Rasi, C., Malmqvist, N., Davies, H., Pasupulati, S., Pakalapati, G., Sandgren, J., Diaz de Ståhl, T., Zaghlool, A., Giedraitis, V., Lannfelt, L., Score, J., Cross, N. C. P., Absher, D., Janson, E. T., Lindgren, C. M., Morris, A. P., Ingelsson, E., Lind, L., & Dumanski, J. P. (2014). Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nature Genetics, 46(6), Article 6. https://doi.org/10.1038/ng.2966
Fraser, A., Johnman, C., Whitley, E., & Alvergne, A. (2020). The evolutionary ecology of age at natural menopause: Implications for public health. Evolutionary Human Sciences, 2, e57. https://doi.org/10.1017/ehs.2020.59
Gavrilov, L. A., & Gavrilova, N. S. (1999). Is there a reproductive cost for human longevity? Journal of Anti-Aging Medicine, 2(2), 121–123. https://doi.org/10.1089/rej.1.1999.2.121
Gensous, N., Garagnani, P., Santoro, A., Giuliani, C., Ostan, R., Fabbri, C., Milazzo, M., Gentilini, D., di Blasio, A. M., Pietruszka, B., Madej, D., Bialecka-Debek, A., Brzozowska, A., Franceschi, C., & Bacalini, M. G. (2020). One-year Mediterranean diet promotes epigenetic rejuvenation with country- and sex-specific effects: A pilot study from the NU-AGE project. GeroScience. https://doi.org/10.1007/s11357-019-00149-0
Gentilini, D., Castaldi, D., Mari, D., Monti, D., Franceschi, C., Di Blasio, A. M., & Vitale, G. (2012). Age-dependent skewing of X chromosome inactivation appears delayed in centenarians’ offspring. Is there a role for allelic imbalance in healthy aging and longevity? Aging Cell, 11(2), Article 2. https://doi.org/10.1111/j.1474-9726.2012.00790.x
Ghalambor, C. K., & Martin, T. E. (2001). Fecundity-survival trade-offs and parental risk-taking in birds. Science, 292(5516), 494–497. https://doi.org/10.1126/science.1059379
Gluckman, P. D. (2012). Epigenetics, the life-course and metabolic disease. Nature Reviews Endocrinology, 8(2), 74–76. https://doi.org/10.1038/nrendo.2011.226
Hägg, S., & Jylhävä, J. (2021). Sex differences in biological aging with a focus on human studies. eLife, 10, e63425. https://doi.org/10.7554/eLife.63425
Hall, E., Volkov, P., Dayeh, T., Esguerra, J. L. S., Salö, S., Eliasson, L., Rönn, T., Bacos, K., & Ling, C. (2014). Sex differences in the genome-wide DNA methylation pattern and impact on gene expression, microRNA levels and insulin secretion in human pancreatic islets. Genome Biology, 15(12), 522. https://doi.org/10.1186/s13059-014-0522-z
Harper, J. A., Janicke, T., & Morrow, E. H. (2021). Systematic review reveals multiple sexually antagonistic polymorphisms affecting human disease and complex traits. Evolution, 75(12), 3087–3097. https://doi.org/10.1111/evo.14394
Hidaka, B. H., & Boddy, A. M. (2016). Is estrogen receptor negative breast cancer risk associated with a fast life history strategy? Evolution, Medicine, and Public Health, 2016(1), 17–20. https://doi.org/10.1093/emph/eov034
Horvath, S., Gurven, M., Levine, M. E., Trumble, B. C., Kaplan, H., Allayee, H., Ritz, B. R., Chen, B., Lu, A. T., Rickabaugh, T. M., Jamieson, B. D., Sun, D., Li, S., Chen, W., Quintana-Murci, L., Fagny, M., Kobor, M. S., Tsao, P. S., Reiner, A. P., et al. (2016). An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biology, 17(1), 171. https://doi.org/10.1186/s13059-016-1030-0
Horvath, S., & Raj, K. (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics, 19(6), 371–384. https://doi.org/10.1038/s41576-018-0004-3
Hutchison, C. A., Newbold, J. E., Potter, S. S., & Edgell, M. H. (1974). Maternal inheritance of mammalian mitochondrial DNA. Nature, 251(5475), 536–538. https://doi.org/10.1038/251536a0
Inoshita, M., Numata, S., Tajima, A., Kinoshita, M., Umehara, H., Yamamori, H., Hashimoto, R., Imoto, I., & Ohmori, T. (2015). Sex differences of leukocytes DNA methylation adjusted for estimated cellular proportions. Biology of Sex Differences, 6(1), 11. https://doi.org/10.1186/s13293-015-0029-7
Jasienska, G., Bribiescas, R. G., Furberg, A.-S., Helle, S., & Núñez-de la Mora, A. (2017). Human reproduction and health: An evolutionary perspective. The Lancet, 390(10093), 510–520. https://doi.org/10.1016/S0140-6736(17)30573-1
Joshi, P. K., Fischer, K., Schraut, K. E., Campbell, H., Esko, T., & Wilson, J. F. (2016). Variants near CHRNA3/5 and APOE have age- and sex-related effects on human lifespan. Nature Communications, 7, 11174. https://doi.org/10.1038/ncomms11174
Kananen, L., & Marttila, S. (2021). Ageing-associated changes in DNA methylation in X and Y chromosomes. Epigenetics & Chromatin, 14(1), 33. https://doi.org/10.1186/s13072-021-00407-6
Kankaanpää, A., Tolvanen, A., Saikkonen, P., Heikkinen, A., Laakkonen, E. K., Kaprio, J., Ollikainen, M., & Sillanpää, E. (2021). Do epigenetic clocks provide explanations for sex differences in lifespan? A cross-sectional twin study. The Journals of Gerontology. Series a, Biological Sciences and Medical Sciences. https://doi.org/10.1093/gerona/glab337
Kirkwood, T. B. L. (1977). Evolution of ageing. Nature, 270(5635), 301–304. https://doi.org/10.1038/270301a0
Kirkwood, T. B., & Rose, M. R. (1991). Evolution of senescence: Late survival sacrificed for reproduction. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 332(1262), 15–24. https://doi.org/10.1098/rstb.1991.0028
Kresovich, J. K., Harmon, Q. E., Xu, Z., Nichols, H. B., Sandler, D. P., & Taylor, J. A. (2019). Reproduction, DNA methylation and biological age. Human Reproduction, 34(10), 1965–1973. https://doi.org/10.1093/humrep/dez149
Laaksonen, J., Mishra, P. P., Seppälä, I., Lyytikäinen, L.-P., Raitoharju, E., Mononen, N., Lepistö, M., Almusa, H., Ellonen, P., Hutri-Kähönen, N., Juonala, M., Raitakari, O., Kähönen, M., Salonen, J. T., & Lehtimäki, T. (2021). Examining the effect of mitochondrial DNA variants on blood pressure in two Finnish cohorts. Scientific Reports, 11(1), 611. https://doi.org/10.1038/s41598-020-79931-6
Laaksonen, J., Seppälä, I., Raitoharju, E., Mononen, N., Lyytikäinen, L.-P., Waldenberger, M., Illig, T., Lepistö, M., Almusa, H., Ellonen, P., Hutri-Kähönen, N., Juonala, M., Kähönen, M., Raitakari, O., Salonen, J. T., & Lehtimäki, T. (2019). Discovery of mitochondrial DNA variants associated with genome-wide blood cell gene expression: A population-based mtDNA sequencing study. Human Molecular Genetics, 28(8), 1381–1391. https://doi.org/10.1093/hmg/ddz011
Lagou, V., Mägi, R., Hottenga, J.-J., Grallert, H., Perry, J. R. B., Bouatia-Naji, N., Marullo, L., Rybin, D., Jansen, R., Min, J. L., Dimas, A. S., Ulrich, A., Zudina, L., Gådin, J. R., Jiang, L., Faggian, A., Bonnefond, A., Fadista, J., Stathopoulou, M. G., et al. & Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC). (2021). Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nature Communications, 12(1), 24. https://doi.org/10.1038/s41467-020-19366-9
Lemaitre, J.-F., Berger, V., Bonenfant, C., Douhard, M., Gamelon, M., Plard, F., & Gaillard, J.-M. (2015). Early-late life trade-offs and the evolution of ageing in the wild. Proceedings of the Royal Society B: Biological Sciences, 282(1806), 20150209–20150209. https://doi.org/10.1098/rspb.2015.0209
Lemaître, J.-F., Ronget, V., Tidière, M., Allainé, D., Berger, V., Cohas, A., Colchero, F., Conde, D. A., Garratt, M., Liker, A., Marais, G. A. B., Scheuerlein, A., Székely, T., & Gaillard, J.-M. (2020). Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proceedings of the National Academy of Sciences, 117(15), 8546–8553. https://doi.org/10.1073/pnas.1911999117
Leung, Y.-K., Ouyang, B., Niu, L., Xie, C., Ying, J., Medvedovic, M., Chen, A., Weihe, P., Valvi, D., Grandjean, P., & Ho, S.-M. (2018). Identification of sex-specific DNA methylation changes driven by specific chemicals in cord blood in a Faroese birth cohort. Epigenetics, 13(3), Article 3. https://doi.org/10.1080/15592294.2018.1445901
Levine, M. E., Lu, A. T., Quach, A., Chen, B. H., Assimes, T. L., Bandinelli, S., Hou, L., Baccarelli, A. A., Stewart, J. D., Li, Y., Whitsel, E. A., Wilson, J. G., Reiner, A. P., Aviv, A., Lohman, K., Liu, Y., Ferrucci, L., & Horvath, S. (2018). An epigenetic biomarker of aging for lifespan and healthspan. Aging, 10(4), 573–591. https://doi.org/10.18632/aging.101414
Li, S., Lund, J. B., Christensen, K., Baumbach, J., Mengel-From, J., Kruse, T., Li, W., Mohammadnejad, A., Pattie, A., Marioni, R. E., Deary, I. J., & Tan, Q. (2020a). Exploratory analysis of age and sex dependent DNA methylation patterns on the X-chromosome in whole blood samples. Genome Medicine, 12(1), 39. https://doi.org/10.1186/s13073-020-00736-3
Li, X., Ploner, A., Wang, Y., Magnusson, P. K., Reynolds, C., Finkel, D., Pedersen, N. L., Jylhävä, J., & Hägg, S. (2020b). Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife, 9, e51507. https://doi.org/10.7554/eLife.51507
Li, G., Wang, C., Guan, X., Bai, Y., Feng, Y., Wei, W., Meng, H., Fu, M., He, M., Zhang, X., Lu, Y., Lin, Y., & Guo, H. (2022). Age-related DNA methylation on Y chromosome and their associations with total mortality among Chinese males. Aging Cell. https://doi.org/10.1111/acel.13563
Libert, C., Dejager, L., & Pinheiro, I. (2010). The X chromosome in immune functions: When a chromosome makes the difference. Nature Reviews. Immunology, 10(8), Article 8. https://doi.org/10.1038/nri2815
Liu, X., Song, Z., Li, Y., Yao, Y., Fang, M., Bai, C., An, P., Chen, H., Chen, Z., Tang, B., Shen, J., Gao, X., Zhang, M., Chen, P., Zhang, T., Jia, H., Liu, X., Hou, Y., Yang, H., et al. (2021). Integrated genetic analyses revealed novel human longevity loci and reduced risks of multiple diseases in a cohort study of 15,651 Chinese individuals. Aging Cell. https://doi.org/10.1111/acel.13323
Lund, J. B., Li, S., Christensen, K., Mengel-From, J., Soerensen, M., Marioni, R. E., Starr, J., Pattie, A., Deary, I. J., Baumbach, J., & Tan, Q. (2020). Age-dependent DNA methylation patterns on the Y chromosome in elderly males. Aging Cell. https://doi.org/10.1111/acel.12907
Luy, M. (2003). Causes of male excess mortality: insights from cloistered populations. Population and Development Review, 29(4), 647–676. https://doi.org/10.1111/j.1728-4457.2003.00647.x
Machiela, M. J., Zhou, W., Karlins, E., Sampson, J. N., Freedman, N. D., Yang, Q., Hicks, B., Dagnall, C., Hautman, C., Jacobs, K. B., Abnet, C. C., Aldrich, M. C., Amos, C., Amundadottir, L. T., Arslan, A. A., Beane-Freeman, L. E., Berndt, S. I., Black, A., Blot, W. J., et al. (2016). Female chromosome X mosaicism is age-related and preferentially affects the inactivated X chromosome. Nature Communications, 7, 11843. https://doi.org/10.1038/ncomms11843
Marais, G. A. B., Gaillard, J.-M., Vieira, C., Plotton, I., Sanlaville, D., Gueyffier, F., & Lemaitre, J.-F. (2018). Sex gap in aging and longevity: Can sex chromosomes play a role? Biology of Sex Differences. https://doi.org/10.1186/s13293-018-0181-y
Martin, E. M., & Fry, R. C. (2018). Environmental influences on the epigenome: Exposure-associated DNA methylation in human populations. Annual Review of Public Health, 39(1), 309–333. https://doi.org/10.1146/annurev-publhealth-040617-014629
Maschietto, M., Bastos, L. C., Tahira, A. C., Bastos, E. P., Euclydes, V. L. V., Brentani, A., Fink, G., de Baumont, A., Felipe-Silva, A., Francisco, R. P. V., Gouveia, G., Grisi, S. J. F. E., Escobar, A. M. U., Moreira-Filho, C. A., Polanczyk, G. V., Miguel, E. C., & Brentani, H. (2017). Sex differences in DNA methylation of the cord blood are related to sex-bias psychiatric diseases. Scientific Reports, 7, 44547. https://doi.org/10.1038/srep44547
Masser, D. R., Hadad, N., Porter, H. L., Mangold, C. A., Unnikrishnan, A., Ford, M. M., Giles, C. B., Georgescu, C., Dozmorov, M. G., Wren, J. D., Richardson, A., Stanford, D. R., & Freeman, W. M. (2017). Sexually divergent DNA methylation patterns with hippocampal aging. Aging Cell, 16(6), Article 6. https://doi.org/10.1111/acel.12681
Matarrese, P., Tieri, P., Anticoli, S., Ascione, B., Conte, M., Franceschi, C., Malorni, W., Salvioli, S., & Ruggieri, A. (2019). X-chromosome-linked miR548am-5p is a key regulator of sex disparity in the susceptibility to mitochondria-mediated apoptosis. Cell Death & Disease, 10(9), Article 9. https://doi.org/10.1038/s41419-019-1888-3
McCartney, D. L., Zhang, F., Hillary, R. F., Zhang, Q., Stevenson, A. J., Walker, R. M., Bermingham, M. L., Boutin, T., Morris, S. W., Campbell, A., Murray, A. D., Whalley, H. C., Porteous, D. J., Hayward, C., Evans, K. L., Chandra, T., Deary, I. J., McIntosh, A. M., Yang, J., et al. (2019). An epigenome-wide association study of sex-specific chronological ageing. Genome Medicine, 12(1), Article 1. https://doi.org/10.1186/s13073-019-0693-z
McCartney, D. L., Zhang, F., Hillary, R. F., Zhang, Q., Stevenson, A. J., Walker, R. M., Bermingham, M. L., Boutin, T., Morris, S. W., Campbell, A., Murray, A. D., Whalley, H. C., Porteous, D. J., Hayward, C., Evans, K. L., Chandra, T., Deary, I. J., McIntosh, A. M., Yang, J., et al. (2020). An epigenome-wide association study of sex-specific chronological ageing. Genome Medicine, 12(1), 1. https://doi.org/10.1186/s13073-019-0693-z
Migliore, L., Nicolì, V., & Stoccoro, A. (2021). Gender specific differences in disease susceptibility: The role of epigenetics. Biomedicines, 9(6), 652. https://doi.org/10.3390/biomedicines9060652
Milot, E., Moreau, C., Gagnon, A., Cohen, A. A., Brais, B., & Labuda, D. (2017). Mother’s curse neutralizes natural selection against a human genetic disease over three centuries. Nature Ecology & Evolution, 1(9), 1400–1406. https://doi.org/10.1038/s41559-017-0276-6
Nettle, D. (2010). Dying young and living fast: Variation in life history across English neighborhoods. Behavioral Ecology, 21(2), 387–395. https://doi.org/10.1093/beheco/arp202
Oblak, L., van der Zaag, J., Higgins-Chen, A. T., Levine, M. E., & Boks, M. P. (2021). A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Research Reviews, 69, 101348. https://doi.org/10.1016/j.arr.2021.101348
Partridge, L., Gems, D., & Withers, D. J. (2005). Sex and death: What is the connection? Cell, 120(4), 461–472. https://doi.org/10.1016/j.cell.2005.01.026
Pellegrini, C., Pirazzini, C., Sala, C., Sambati, L., Yusipov, I., Kalyakulina, A., Ravaioli, F., Kwiatkowska, K. M., Durso, D. F., Ivanchenko, M., Monti, D., Lodi, R., Franceschi, C., Cortelli, P., Garagnani, P., & Bacalini, M. G. (2021). A meta-analysis of brain DNA methylation across sex, age, and Alzheimer’s disease points for accelerated epigenetic aging in neurodegeneration. Frontiers in Aging Neuroscience, 13, 639428. https://doi.org/10.3389/fnagi.2021.639428
Penn, D. J., & Smith, K. R. (2007). Differential fitness costs of reproduction between the sexes. Proceedings of the National Academy of Sciences, 104(2), 553–558. https://doi.org/10.1073/pnas.0609301103
Persani, L., Bonomi, M., Lleo, A., Pasini, S., Civardi, F., Bianchi, I., Campi, I., Finelli, P., Miozzo, M., Castronovo, C., Sirchia, S., Gershwin, M. E., & Invernizzi, P. (2012). Increased loss of the Y chromosome in peripheral blood cells in male patients with autoimmune thyroiditis. Journal of Autoimmunity, 38(2–3), Article 2-3. https://doi.org/10.1016/j.jaut.2011.11.011
Perzel Mandell, K. A., Price, A. J., Wilton, R., Collado-Torres, L., Tao, R., Eagles, N. J., Szalay, A. S., Hyde, T. M., Weinberger, D. R., Kleinman, J. E., & Jaffe, A. E. (2021). Characterizing the dynamic and functional DNA methylation landscape in the developing human cortex. Epigenetics, 16(1), 1–13. https://doi.org/10.1080/15592294.2020.1786304
Quinlan, R. J. (2007). Human parental effort and environmental risk. Proceedings of the Royal Society b: Biological Sciences, 274(1606), 121–125. https://doi.org/10.1098/rspb.2006.3690
Rice, W. R. (1984). Sex chromosomes and the evolution of sexual dimorphism. Evolution, 38(4), 735. https://doi.org/10.2307/2408385
Ruzicka, F., & Connallon, T. (2020). Is the X chromosome a hot spot for sexually antagonistic polymorphisms? Biases in current empirical tests of classical theory. Proceedings of the Royal Society b: Biological Sciences, 287(1937), 20201869. https://doi.org/10.1098/rspb.2020.1869
Ryan, C. P., Hayes, M. G., Lee, N. R., McDade, T. W., Jones, M. J., Kobor, M. S., Kuzawa, C. W., & Eisenberg, D. T. A. (2018). Reproduction predicts shorter telomeres and epigenetic age acceleration among young adult women. Scientific Reports, 8(1), 11100. https://doi.org/10.1038/s41598-018-29486-4
Ryan, J., Wrigglesworth, J., Loong, J., Fransquet, P. D., & Woods, R. L. (2020). A systematic review and meta-analysis of environmental, lifestyle, and health factors associated with DNA methylation age. The Journals of Gerontology: Series A, 75(3), 481–494. https://doi.org/10.1093/gerona/glz099
Sainz, J., Rudolph, A., Hoffmeister, M., Frank, B., Brenner, H., Chang-Claude, J., Hemminki, K., & Försti, A. (2012). Effect of type 2 diabetes predisposing genetic variants on colorectal cancer risk. The Journal of Clinical Endocrinology & Metabolism, 97(5), E845–E851. https://doi.org/10.1210/jc.2011-2565
Salinari, G., De Santis, G., Zarulli, V., Giuliani, C., Franceschi, C., & Breschi, M. (2022). Fertility decline and the emergence of excess female survival in post-reproductive ages in Italy. Genus, 78(1), 19. https://doi.org/10.1186/s41118-022-00166-6
Sharp, A., Robinson, D., & Jacobs, P. (2000). Age- and tissue-specific variation of X chromosome inactivation ratios in normal women. Human Genetics, 107(4), Article 4. https://doi.org/10.1007/s004390000382
Singmann, P., Shem-Tov, D., Wahl, S., Grallert, H., Fiorito, G., Shin, S.-Y., Schramm, K., Wolf, P., Kunze, S., Baran, Y., Guarrera, S., Vineis, P., Krogh, V., Panico, S., Tumino, R., Kretschmer, A., Gieger, C., Peters, A., Prokisch, H., et al. (2015). Characterization of whole-genome autosomal differences of DNA methylation between men and women. Epigenetics & Chromatin, 8(1), 43. https://doi.org/10.1186/s13072-015-0035-3
Spiers, H., Hannon, E., Schalkwyk, L. C., Smith, R., Wong, C. C. Y., O’Donovan, M. C., Bray, N. J., & Mill, J. (2015). Methylomic trajectories across human fetal brain development. Genome Research, 25(3), Article 3. https://doi.org/10.1101/gr.180273.114
Stearns, S. C., Ackermann, M., Doebeli, M., & Kaiser, M. (2000). Experimental evolution of aging, growth, and reproduction in fruitflies. Proceedings of the National Academy of Sciences, 97(7), 3309–3313. https://doi.org/10.1073/pnas.97.7.3309
Sugrue, V. J., Zoller, J. A., Narayan, P., Lu, A. T., Ortega-Recalde, O. J., Grant, M. J., Bawden, C. S., Rudiger, S. R., Haghani, A., Bond, D. M., Hore, R. R., Garratt, M., Sears, K. E., Wang, N., Yang, X. W., Snell, R. G., Hore, T. A., & Horvath, S. (2021). Castration delays epigenetic aging and feminizes DNA methylation at androgen-regulated loci. eLife, 10, e64932. https://doi.org/10.7554/eLife.64932
Tobi, E. W., van den Heuvel, J., Zwaan, B. J., Lumey, L. H., Heijmans, B. T., & Uller, T. (2018). Selective survival of embryos can explain DNA methylation signatures of adverse prenatal environments. Cell Reports, 25(10), 2660-2667.e4. https://doi.org/10.1016/j.celrep.2018.11.023
Trivers, R. (1985). Social evolution. Benjamin/Cummings Pub, Co.
Tukiainen, T., Villani, A.-C., Yen, A., Rivas, M. A., Marshall, J. L., Satija, R., Aguirre, M., Gauthier, L., Fleharty, M., Kirby, A., Cummings, B. B., Castel, S. E., Karczewski, K. J., Aguet, F., Byrnes, A., GTEx Consortium, Laboratory, Data Analysis &Coordinating Center (LDACC)—Analysis Working Group, Statistical Methods groups—Analysis Working Group, Enhancing GTEx (eGTEx) groups, … MacArthur, D. G. (2017). Landscape of X chromosome inactivation across human tissues. Nature, 550(7675), Article 7675. https://doi.org/10.1038/nature24265
Vidaki, A., González, D. M., Jiménez, B. P., & Kayser, M. (2021). Male-specific age estimation based on Y-chromosomal DNA methylation. Aging, 13(5), 6442–6458. https://doi.org/10.18632/aging.202775
Viña, J., Borrás, C., Gambini, J., Sastre, J., & Pallardó, F. V. (2005). Why females live longer than males: Control of longevity by sex hormones. Science of Aging Knowledge Environment. https://doi.org/10.1126/sageke.2005.23.pe17
Vukojevic, V., Kolassa, I.-T., Fastenrath, M., Gschwind, L., Spalek, K., Milnik, A., Heck, A., Vogler, C., Wilker, S., Demougin, P., Peter, F., Atucha, E., Stetak, A., Roozendaal, B., Elbert, T., Papassotiropoulos, A., & de Quervain, D.J.-F. (2014). Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and post-traumatic stress disorder risk in genocide survivors. Journal of Neuroscience, 34(31), 10274–10284. https://doi.org/10.1523/JNEUROSCI.1526-14.2014
Westendorp, R. G. J., & Kirkwood, T. B. L. (1998). Human longevity at the cost of reproductive success. Nature, 396(6713), 743–746. https://doi.org/10.1038/25519
Xia, Y., Dai, R., Wang, K., Jiao, C., Zhang, C., Xu, Y., Li, H., Jing, X., Chen, Y., Jiang, Y., Kopp, R. F., Giase, G., Chen, C., & Liu, C. (2021). Sex-differential DNA methylation and associated regulation networks in human brain implicated in the sex-biased risks of psychiatric disorders. Molecular Psychiatry, 26(3), 835–848. https://doi.org/10.1038/s41380-019-0416-2
Yurov, Y. B., Vorsanova, S. G., Liehr, T., Kolotii, A. D., & Iourov, I. Y. (2014). X chromosome aneuploidy in the Alzheimer’s disease brain. Molecular Cytogenetics, 7(1), Article 1. https://doi.org/10.1186/1755-8166-7-20
Yusipov, I., Bacalini, M. G., Kalyakulina, A., Krivonosov, M., Pirazzini, C., Gensous, N., Ravaioli, F., Milazzo, M., Giuliani, C., Vedunova, M., Fiorito, G., Gagliardi, A., Polidoro, S., Garagnani, P., Ivanchenko, M., & Franceschi, C. (2020). Age-related DNA methylation changes are sex-specific: A comprehensive assessment. Aging, 12(23), 24057–24080.
Zarulli, V., Barthold Jones, J. A., Oksuzyan, A., Lindahl-Jacobsen, R., Christensen, K., & Vaupel, J. W. (2018). Women live longer than men even during severe famines and epidemics. Proceedings of the National Academy of Sciences, 115(4), E832–E840. https://doi.org/10.1073/pnas.1701535115
Zeng, Y., Nie, C., Min, J., Chen, H., Liu, X., Ye, R., Chen, Z., Bai, C., Xie, E., Yin, Z., Lv, Y., Lu, J., Li, J., Ni, T., Bolund, L., Land, K. C., Yashin, A., O’Rand, A. M., Sun, L., et al. (2018). Sex Differences in genetic associations with longevity. JAMA Network Open, 1(4), e181670. https://doi.org/10.1001/jamanetworkopen.2018.1670
Zhao, W., Ammous, F., Ratliff, S., Liu, J., Yu, M., Mosley, T. H., Kardia, S. L. R., & Smith, J. A. (2019). Education and lifestyle factors are associated with DNA methylation clocks in older African Americans. International Journal of Environmental Research and Public Health, 16(17), Article 17. https://doi.org/10.3390/ijerph16173141
Acknowledgements
Not applicable.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Study conception and design: CG, MGB. Data and studies collection: CG, VI, MGB, CF. Draft manuscript preparation: VI, CG. Final version of the manuscript VI, CG, MGB, CF. All authors reviewed, read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iannuzzi, V., Bacalini, M.G., Franceschi, C. et al. The role of genetics and epigenetics in sex differences in human survival. Genus 79, 1 (2023). https://doi.org/10.1186/s41118-023-00181-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41118-023-00181-1