Abstract
Background
Although the link between brain aging and Alzheimer’s disease (AD) is a matter of debate, processes hallmarking cellular and tissue senescence have been repeatedly associated with its pathogenesis. Here, we have studied X chromosome aneuploidy (a recognized feature of aged cell populations) in the AD brain.
Results
Extended molecular neurocytogenetic analyses of X chromosome aneuploidy in 10 female AD as well as 10 age and sex matched female control postmortem brain samples was performed by multiprobe/quantitative FISH. Additionally, aneuploidy rate in the brain samples of 5 AD and as 5 age and sex matched control subjects were analyzed by interphase chromosome-specific multicolor banding (ICS-MCB). Totally, 182,500 cells in the AD brain and 182,500 cells in the unaffected brain were analyzed. The mean rate of X chromosome aneuploidy in AD samples was approximately two times higher than in control (control: mean - 1.32%, 95% CI 0.92- 1.71%; AD: mean - 2.79%, 95% CI 1.88-3.69; P = 0.013). One AD sample demonstrated mosaic aneuploidy of chromosome X confined to the hippocampus affecting about 10% of cells. ICS-MCB confirmed the presence of X chromosome aneuploidy in the hippocampal tissues of AD brain (control: mean - 1.74%, 95% CI 1.38- 2.10%; AD: mean - 4.92%, 95% CI 1.14-8.71; P < 0.001).
Conclusions
Addressing X chromosome number variation in the brain, we observed that somatically acquired (post-zygotic) aneuploidy causes large-scale genomic alterations in neural cells of AD patients and, therefore, can be involved in pathogenesis of this common neurodegenerative disorder. In the context of debates about possible interplay between brain aging and AD neurodegeneration, our findings suggest that X chromosome aneuploidy can contribute to both processes. To this end we conclude that mosaic aneuploidy in the brain is a new non-heritable genetic factor predisposing to AD.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) represents one of the most common age-related neurodegenerative disorders with a strong genetic basis. Currently, a widely accepted model of AD genetics proposes that this devastating pathology is associated with several genetic defects including single-gene mutations, risk-enhancing single nucleotide polymorphisms (SNP) and copy number variations (CNV), genetic instabilities (at chromosomal or sub-chromosomal level), and a complex pattern of genetic-environmental interactions [1–3]. Parallelly, a debate questioning the existence of interplay between brain aging and AD pathology does take place in order to critically address the contribution of cell senescence and related phenomena to AD neurodegeneration [4, 5]. Nonetheless, there is a consensus about AD genetic background, which is hypothesized, among others, to result in alteration to neuronal cell cycle.
A series of studies has provided indirect evidences that abnormal behavior of chromosome X during the cell division is observed in AD patients [6–8]. Furthermore, some late-onset AD cases were linked to chromosome X [9]. On the other hand, mosaic numerical chromosome imbalances (somatic aneuploidy) have been repeatedly observed in the AD and unaffected (aged) human brain and are now considered as an integrated part of the pathogenic cascade mediating progressive neurodegeneration in this devastating neurological disease [10–20]. Since X chromosome loss is one of the most prominent hallmarks of aging in human females [21] and an association between aging and aneuploidy in the murine brain has been shown [22], it is attractive to test whether X chromosome aneuploidy can be an element of AD pathogenic cascade at least in cases of neurodegeneration in the diseased brain of females. Here, we used a set of molecular cytogenetic techniques [23–25] providing high-resolution analysis of interphase chromosomes to detect genome variations manifesting at chromosomal level in small cell populations for a molecular neurocytogenetic analysis of the AD brain and control samples.
Results
Using multiprobe/quantitative FISH and interphase chromosome-specific multicolor banding (ICS-MCB) (Figure 1), we have assessed the rate of aneuploidy involving different chromosomes in postmortem brain tissues of 10 control and 10 AD patients analyzed in a double-blinded study. Chromosome enumeration probes for six different autosomes (1, 7, 11, 16, 17, and 18) and chromosome X were applied. 2000 cells were scored for each DNA probe/brain tissue sample. Following combination of chromosome-enumeration probes were used: chromosomes 1, X, Y (as internal control for the sex of subjects analyzed/stringency of hybridization); chromosomes 1, 7, 11 and chromosomes 16, 17 and Y. In total, scoring more than 140,000 cells in control samples and comparable amount of cells (140,000) in the AD brain has demonstrated aneuploidy rates (losses + gains) to vary in a wide range between chromosomes and individuals (Table 1). In average, aneuploid cell populations demonstrated chromosome gains in 10-20% of cells, whereas 80-90% of cells exhibited chromosome losses. Cells affected by multiple aneuploidies were not detected. Interphase nucleus morphology and numbers have not significantly differed between AD and control samples. In control samples, the mean frequency of autosomal aneuploidy ranged between 0.51 and 0.82 with an “autosomal” mean determined as 0.66 (95% CI, 0.57-0.7%). The mean of X chromosome aneuploidy rates in controls was 1.32% (95% CI, 0.92-1.71%). In the AD brain, the mean autosomal aneuploidy frequency was in a range of 0.86-1.22% and “autosomal” mean was 0.93% (95% CI, 0.78-1.07%) The mean frequency of X chromosome aneuploidy rates was 2.78% (95% CI, 1.88-3.68). We have compared the frequency of aneuploidy between each homologous chromosome pair by scoring 20000 cells in control and 20000 cells in AD groups (Table 1) using nonparametric statistical tests (Mann-Whitney U test for independent groups). Insignificant interindividual differences between autosomal aneuploidy rates were obtained in controls and AD (P value ranged between 0.053 and 0.733). The increase of X chromosome aneuploidy rates in the AD cerebrum was significantly (P value = 0.013).
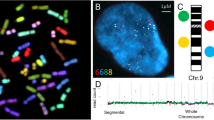
Molecular neurocytogenetic analyses of the AD brain. (A) multiprobe (two-probe) and quantitative FISH using DNA probes for chromosomes 1 (two red signals/D1Z1) and X (one green signal DXZ1/relative intensity is 2120 pixels) demonstrating true X chromosome monosomy; (B) multiprobe (two-probe) and quantitative FISH using DNA probes for chromosomes 1 (two red signals/D1Z1) and X (one green signal DXZ1/relative intensity is 4800 pixels) demonstrating overlapping of two X chromosome signals, but not a chromosome loss; (C) ICS-MCB with a probe set for chromosome X showing one nucleus bearing two chromosomes X and another nucleus bearing single chromosome X.
To get further insight into the contribution of somatic gonosomal aneuploidy to the AD brain pathology, we have compared X chromosome aneuploidy rates in different brain areas (hippocampus and cerebrum) of 5 AD patients (22500 cells) and 5 controls (22500 cells). ICS-MCB and multiprobe/quantitative FISH have shown a dramatic increase of X chromosome aneuploidy rates in hippocampal cells of AD patients. The mean rate of chromosome X aneuploidy was 1.74% (95% CI, 1.38-2.10%) in controls and 4.92% (95% CI, 1.14-8.71%) in AD (P value <0.001).
X chromosome aneuploidy levels detected in 5 AD patients were verified in the prefrontal cortex (the second brain tissue affected by neurodegeneration). The mean rate of chromosome X aneuploidy in the cerebrum was 1.16% (95% CI, 0.56-1.76%) in controls and 2.84% (95% CI, 1.78-3.90%) in AD (P value = 0.009). Surprisingly, one sample derived from the hippocampus of an AD patient (Figure 2 A, B) was found to exhibit low-level somatic chromosomal mosaicism (about 10% of cells were affected by X chromosome aneuploidy) almost exclusively confined to the hippocampus (Figure 2B). To our knowledge, cases of AD demonstrating brain-specific (brain-area-specific) chromosomal mosaicism involving chromosome X have been never reported.
X chromosome aneuploidy in the hippocampus of the AD brain and controls analyzed by ICS-MCB (AD: n = 5, red bars; control: n = 5, blue bars); AD: mean 4.92%, 95% CI 1.14-8.71; control: mean 1.74%, 95% CI 1.38-2.1; X axis: number of samples’ pairs: AD patient—age-matched control; Y axis: rates of aneuploidy (given in %).
Discussion
The hypothesis suggesting aneuploidy (trisomy of chromosome 21) to be involved in AD pathogenesis has long been explored through addressing mitotic tissues [26–28]. However, until recently, no consensus was reached regarding the true occurrence of somatic aneuploidy in the AD brain [12–20, 27, 28]. The intercellular genomic variations affecting the AD brain were assumed to be limited to chromosome 21 aneuploidy because of neurological parallels between AD and Down syndrome [12, 26, 29, 30]. This assumption was further supported by reports showing that molecular dysfunctions in AD neural cells are likely to predispose to formation of chromosome 21-specific aneuploidy [19, 29, 30]. Alternatively, brain-specific chromosome instability manifesting as aneuploidy involving different chromosomes was detected in AD [11–15, 17, 18], but low-level mosaic chromosome 21-specific aneuploidy was found to feature the AD brain [12]. Thus, chromosomal (genomic instability) seems to mediate neurodegeneration in AD regardless of chromosomes involved in aneuploidy [31]. Interestingly, mosaic aneuploidy affecting the human brain is probably a general genetic mechanism for neurodegenerative and psychiatric diseases [10, 12, 20, 32–37]. Only rarely, however, X chromosome aneuploidy is observed in the diseased brain of AD patients. Since genetic brain diseases (apart from AD and ataxia telangiectasia) studied according to molecular neurocytogenetic paradigm are not usually associated with aging (i.e. autism and schizophrenia [32–37]), one can speculate that such a genetic hallmark of human aging as X chromosome aneuploidy [15, 21, 34] should be observed as in the healthy aged brain as in the diseased brain. Furthermore, several evaluations of X chromosome aneuploidy in the AD brain have yielded contradictory results [12, 17, 38]. Nevertheless, the analysis of X chromosome number variations was not the focus of these studies. The present report demonstrates that X chromosome aneuploidy affects neural cells more significantly in the AD brain when compared to unaffected individuals. Therefore, it is to address an important issue concerning the origins and pathogenic value of mosaic aneuploidy in the AD brain.
The human brain is mainly populated by postmitotic cells. Since somatic aneuploidy is likely to occur during mitotic division, it is difficult to propose a universal mechanism for an increase of aneuploidy rates in the aging human brain. Though, there are a couple of possible solutions for this paradox:
-
(i)
chromosome instability in the human developmental central nervous system is a source for aneuploid cells in postnatal brain [39–41]; failed clearance of abnormal cells during prenatal brain development has to lead to persistence of abnormal neural cell populations; this mechanism seems to be appropriate not only for brain diseases mediated by somatic genome variations in neural cells, but also for somatic mosaicism, as a whole [42]; the persistence of abnormal cells is hypothesized to arise from risk-enhancing SNP or CNV frequently found in AD patients affecting genome stability and programmed cell death pathways [3]. Therefore, mosaic (postzygotic) aneuploidy acquiring during early brain development may be considered as a new non-heritable genetic factor predisposing to late onset AD.
-
(ii)
adult neurogenesis (gliogenesis) during ontogeny can also be considered as a mechanism explaining accumulation of aneuploid cells in the aging brain. It was proposed that neural cell cycle is prone to errors (i.e. abortive cell cycle due to reentering of quiescent neurons into the cell cycle and replication stress) [43] that leads to genome/chromosome instability similar to cancer manifesting as aneuploidy or chromosomal double-strand breaks, resulting, however, in neurodegeneration [44]; the idea is also supported by observations that ectopic cell cycle re-entry of neurons is an element of AD pathogenic cascade [3, 4, 18, 43, 45–47].
In the light of the AD cell cycle theory, the presence of X chromosome aneuploidy in the diseased brain does not seem to be unexplainable. Hence, this genetic marker evidence for pathological brain aging as susceptibility factor for AD. Moreover, a case exhibiting appreciable increase of X chromosome aneuploidy confined to the hippocampus (a brain area that is severely affected in AD [1, 4, 5]) allows speculations about possible predisposition of females affected by low-level mosaic X chromosome aneuploidy to AD. In total, X chromosome aneuploidy appears to play a role in both brain aging and neurodegeneration, whereas aging-related processes are unlikely to cause aneuploidy in the AD brain.
Conclusions
Molecular neurocytogenetic analysis has shown that X chromosome aneuploidy is a cause of large-scale genomic variation in neural cells of AD patients and unaffected controls. The AD brain demonstrates a two-fold increase of X chromosome aneuploidy rates in neural cells of the hippocampus and cerebrum, which are the brain areas dramatically affected by neurodegeneration. Brain-specific X chromosome aneuploidy can be considered an element of AD pathogenesis, bearing in mind that it results from a series of molecular and cellular events more critical for neurodegeneration. Finally, X chromosome aneuploidy is likely to contribute to both pathological and natural brain aging in humans.
Methods
Tissue collection and sample preparation
Postmortem brain tissues (10 AD female and 10 age- and sex matched samples) were obtained from the Postmortem Brain Tissue Bank of Mental Health Research Center, Russian Academy of Medical Sciences. The review board of Ethical Committee at Russian Academy of Medical Sciences (Moscow, Russia) approved all the research procedures. Informed consents or waivers of consents were not required as all case subjects were deceased and anonymously diagnosed. AD diagnosis was based on the results of postmortem evaluations. All control cases were free of mental illness and malignant brain pathology. Death causes were not associated with brain diseases, injuries or conspicuous morphological abnormalities of the brain. In 5 AD cases and 5 controls, hippocampal and cerebral tissues were also acquired. The processing of frozen post-mortem tissues for molecular cytogenetic analyses was performed as described earlier in details [48].
Multiprobe FISH
Multiprobe FISH with chromosome-enumeration DNA probes for chromosomes 1 (D1Z1/Cy3-labeled), 7 (D7Z1/FITC-labeled), 11 (D11Z1/FITC-labeled), 16 (D16Z2/FITC-labeled), 17 (D17Z1/Cy3-labeled), 18 (D18Z1/Cy3-labelled) and X (DXZ1/FITC-labeled) were performed according to previous protocols [12, 32, 33, 36, 39–41, 44, 49].
Qantitative FISH
Nuclei showing single signals were digitalized and evaluated by an original quantitative FISH technique described previously [50]. Similarly, a quantification of ICS-MCB signals was done for differing between chromosome overlapping and monosomies [51].
ICS-MCB
ICS-MCB patterns were generated with a set of human microdissection probes for chromosome X on interphase nuclei isolated from the human brain following previously developed protocols [41, 51, 52]. This method represents a three-to-five-color FISH-based approach producing a reproducible fluorochrome profile along interphase chromosomal axes for determination of the number and structure of interphase chromosomes [51, 52].
Data and image analysis
The whole intermixed population of nuclei consisted of both neural (different types of neurons and glia) and non-neural cells have been analyzed. As aneuploidy was defined to represent an exceedingly rare event in brain cell populations >2000 nuclei per chromosome per sample were analyzed. No fewer than 500 interphase nuclei per chromosome per sample were analyzed using ICS-MCB. The procedure of cell scoring and image analysis including digitalization of wild-type ICS-MCB images for differentiation between “overlapped” chromosomes and true monosomies has been previously described step-by-step in detail [12, 36, 40, 41, 44, 49, 51, 52].
Statistics
Mean frequency and 95% confidence interval for aneuplody rates were determined. To compare stochastic (background) aneuploidy in two groups (AD and age-matched controls), nonparametric statistics (Mann-Whitney U-test for independent groups) was used. P values less than 0.05 were considered as significant. Shapiro-Wilk’s W test was used for descriptive statistical analysis of distributions for normality in two ways: with outliers and without outliers (distributions were considered normal at P > 0.050). An independent sample T-test was used in cases of normally distributed parameters after exclusion of outliers. P values less than 0.05 was considered as significant.
Abbreviations
- AD:
-
Alzheimer’s disease
- CNV:
-
Copy number variations
- FITC:
-
Fluorescein-isothiocyanate
- FISH:
-
Fluorescence in situ hybridization
- ICS-MCB:
-
Interphase chromosome-specific multicolor banding
- SNP:
-
Single nucleotide polymorphisms.
References
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E: Alzheimer’s disease. Lancet 2011, 377: 1019–1031. 10.1016/S0140-6736(10)61349-9
Tanzi RE: The genetics of Alzheimer disease. Cold Spring Harb Perspect Med 2012., 2:
Iourov IY, Vorsanova SG, Yurov YB: Somatic cell genomics of brain disorders: a new opportunity to clarify genetic-environmental interactions. Cytogenet Genome Res 2013, 139: 181–188. 10.1159/000347053
Herrup K: Reimagining Alzheimer’s disease — an age-based hypothesis. J Neurosci 2010, 30: 16755–16762. 10.1523/JNEUROSCI.4521-10.2010
Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, Abner EL, Smith CD, Van Eldik LJ, Kryscio RJ, Scheff SW: Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol 2011, 121: 571–587. 10.1007/s00401-011-0826-y
Spremo-Potparević B, Zivković L, Djelić N, Plećas-Solarović B, Smith MA, Bajić V: Premature centromere division of the X chromosome in neurons in Alzheimer’s disease. J Neurochem 2008, 106: 2218–2223. 10.1111/j.1471-4159.2008.05555.x
Bajić VP, Spremo-Potparević B, Zivković L, Bonda DJ, Siedlak SL, Casadesus G, Lee HG, Smith MA: The X-chromosome instability phenotype in Alzheimer’s disease: a clinical sign of accelerating aging? Med Hypotheses 2009,73(6):917–920. 10.1016/j.mehy.2009.06.046
Zivković L, Spremo-Potparević B, Siedlak SL, Perry G, Plećaš-Solarović B, Milićević Z, Bajić VP: DNA damage in Alzheimer disease lymphocytes and its relation to premature centromere division. Neurodegener Dis 2013, 12: 156–163. 10.1159/000346114
Zubenko GS, Stiffler JS, Hughes HB, Hurtt MR, Kaplan BB: Initial results of a genome survey for novel Alzheimer’s disease risk genes: association with a locus on the X chromosome. Am J Med Genet 1998, 81: 196–205. 10.1002/(SICI)1096-8628(19980328)81:2<196::AID-AJMG14>3.0.CO;2-C
Iourov IY, Vorsanova SG, Yurov YB: Chromosomal variation in mammalian neuronal cells: known facts and attractive hypotheses. Int Rev Cytol 2006, 249: 143–191.
Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T: Aneuploidy and DNA replication in the normal human brain and Alzheimer’s disease. J Neurosci 2007, 27: 6859–6867. 10.1523/JNEUROSCI.0379-07.2007
Iourov IY, Vorsanova SG, Liehr T, Yurov YB: Aneuploidy in the normal, Alzheimer’s disease and ataxia-telangiectasia brain: differential expression and pathological meaning. Neurobiol Dis 2009, 34: 212–220. 10.1016/j.nbd.2009.01.003
Arendt T, Mosch B, Morawski M: Neuronal aneuploidy in health and disease: a cytomic approach to understand the molecular individuality of neurons. Int J Mol Sci 2009, 10: 1609–1627. 10.3390/ijms10041609
Yurov YB, Vorsanova SG, Iourov IY: GIN‘n’CIN hypothesis of brain aging: deciphering the role of somatic genetic instabilities and neural aneuploidy during ontogeny. Mol Cytogenet 2009, 2: 23. 10.1186/1755-8166-2-23
Yurov YB, Vorsanova SG, Iourov IY: Ontogenetic variation of the human genome. Curr Genomics 2010, 11: 420–425. 10.2174/138920210793175958
Fischer HG, Morawski M, Brückner MK, Mittag A, Tarnok A, Arendt T: Changes in neuronal DNA content variation in the human brain during aging. Aging Cell 2012, 11: 628–633. 10.1111/j.1474-9726.2012.00826.x
Iourov IY, Vorsanova SG, Yurov YB: Genomic landscape of the Alzheimer’s disease brain: chromosome instability — aneuploidy, but not tetraploidy — mediates neurodegeneration. Neurodegener Dis 2011,8(1–2):35–37.
Arendt T: Cell cycle activation and aneuploid neurons in Alzheimer’s disease. Mol Neurobiol 2012, 46: 125–135. 10.1007/s12035-012-8262-0
Granic A, Potter H: Mitotic spindle defects and chromosome mis-segregation induced by LDL/cholesterol-implications for Niemann-Pick C1, Alzheimer’s disease, and atherosclerosis. PLoS One 2013, 8: e60718. 10.1371/journal.pone.0060718
Iourov IY, Vorsanova SG, Yurov YB: Interphase chromosomes of the human brain: the biological and clinical meaning of neural aneuploidy. In Human Interphase Chromosomes. Edited by: Yurov YB, Vorsanova SG, Iourov IY. New York, Heidelberg, Dordrecht, London: Springer; 2013:53–83.
Russell LM, Strike P, Browne CE, Jacobs PA: X chromosome loss and ageing. Cytogenet Genome Res 2007, 116: 181–185. 10.1159/000098184
Faggioli F, Wang T, Vijg J, Montagna C: Chromosome-specific accumulation of aneuploidy in the aging mouse brain. Hum Mol Genet 2012, 21: 5246–5253. 10.1093/hmg/dds375
Vorsanova SG, Yurov YB, Iourov IY: Human interphase chromosomes: a review of available molecular cytogenetic technologies. Mol Cytogenet 2010, 3: 1. 10.1186/1755-8166-3-1
Iourov IY, Vorsanova SG, Yurov YB: Single cell genomics of the brain: focus on neuronal diversity and neuropsychiatric diseases. Curr Genomics 2012, 13: 477–488. 10.2174/138920212802510439
Yurov YB, Vorsanova SG, Iourov IY: Human Interphase Chromosomes: Biomedical Aspects. New York, Heidelberg, Dordrecht, London: Springer; 2013.
Potter H: Review and hypothesis: Alzheimer disease and down syndrome — chromosome 21 nondisjunction may underlie both disorders. Am J Hum Genet 1991, 48: 1192–1200.
Hultén MA, Jonasson J, Nordgren A, Iwarsson E: Germinal and somatic trisomy 21 mosaicism: how common is it, what are the implications for individual carriers and how does it come about? Curr Genomics 2010, 11: 409–419. 10.2174/138920210793176056
Iourov IY, Vorsanova SG, Yurov YB: Somatic genome variations in health and disease. Curr Genomics 2010, 11: 387–396. 10.2174/138920210793176065
Borysov SI, Granic A, Padmanabhan J, Walczak CE, Potter H: Alzheimer Aβ disrupts the mitotic spindle and directly inhibits mitotic microtubule motors. Cell Cycle 2011, 10: 1397–1410. 10.4161/cc.10.9.15478
Granic A, Padmanabhan J, Norden M, Potter H: Alzheimer Abeta peptide induces chromosome mis-segregation and aneuploidy, including trisomy 21: requirement for tau and APP. Mol Biol Cell 2010, 21: 511–520. 10.1091/mbc.E09-10-0850
Arendt T, Brückner MK, Mosch B, Lösche A: Selective cell death of hyperploid neurons in Alzheimer’s disease. Am J Pathol 2010, 177: 15–20. 10.2353/ajpath.2010.090955
Yurov YB, Vostrikov VM, Vorsanova SG, Monakhov VV, Iourov IY: Multicolor fluorescent in situ hybridization on post-mortem brain in schizophrenia as an approach for identification of low-level chromosomal aneuploidy in neuropsychiatric diseases. Brain Dev 2001,23(1):S186-S190.
Yurov YB, Vorsanova SG, Iourov IY, Demidova IA, Beresheva AK, Kravetz VS, Monakhov VV, Kolotii AD, Voinova-Ulas VY, Gorbachevskaya NL: Unexplained autism is frequently associated with low-level mosaic aneuploidy. J Med Genet 2007, 44: 521–525. 10.1136/jmg.2007.049312
Iourov IY, Vorsanova SG, Yurov YB: Chromosomal mosaicism goes global. Mol Cytogenet 2008, 1: 26. 10.1186/1755-8166-1-26
Iourov IY, Vorsanova SG, Yurov YB: Molecular cytogenetics and cytogenomics of brain diseases. Curr Genomics 2008, 9: 452–465. 10.2174/138920208786241216
Yurov YB, Iourov IY, Vorsanova SG, Demidova IA, Kravetz VS, Beresheva AK, Kolotii AD, Monakchov VV, Uranova NA, Vostrikov VM, Soloviev IV, Liehr T: The schizophrenia brain exhibits low-level aneuploidy involving chromosome 1. Schizophr Res 2008, 98: 139–147.
Bushman DM, Chun J: The genomically mosaic brain: aneuploidy and more in neural diversity and disease. Semin Cell Dev Biol 2013, 24: 357–369. 10.1016/j.semcdb.2013.02.003
Westra JW, Barral S, Chun J: A reevaluation of tetraploidy in the Alzheimer’s disease brain. Neurodegener Dis 2009, 6: 221–229. 10.1159/000236901
Yurov YB, Iourov IY, Monakhov VV, Soloviev IV, Vostrikov VM, Vorsanova SG: The variation of aneuploidy frequency in the developing and adult human brain revealed by an interphase FISH study. J Histochem Cytochem 2005, 53: 385–390. 10.1369/jhc.4A6430.2005
Yurov YB, Iourov IY, Vorsanova SG, Liehr T, Kolotii AD, Kutsev SI, Pellestor F, Beresheva AK, Demidova IA, Kravets VS, Monakhov VV, Soloviev IV: Aneuploidy and confined chromosomal mosaicism in the developing human brain. PLoS One 2007, 2: e558. 10.1371/journal.pone.0000558
Iourov IY, Liehr T, Vorsanova SG, Kolotii AD, Yurov YB: Visualization of interphase chromosomes in postmitotic cells of the human brain by multicolour banding (MCB). Chromosome Res 2006, 14: 223–229. 10.1007/s10577-006-1037-6
Iourov IY, Vorsanova SG, Yurov YB: Intercellular genomic (chromosomal) variations resulting in somatic mosaicism: mechanisms and consequences. Curr Genomics 2006, 7: 435–446. 10.2174/138920206779116756
Yurov YB, Vorsanova SG, Iourov IY: The DNA replication stress hypothesis of Alzheimer’s disease. Sci World J 2011, 11: 2602–2612.
Iourov IY, Vorsanova SG, Liehr T, Kolotii AD, Yurov YB: Increased chromosome instability dramatically disrupts neural genome integrity and mediates cerebellar degeneration in the ataxia-telangiectasia brain. Hum Mol Genet 2009, 18: 2656–2669. 10.1093/hmg/ddp207
Kanungo J: DNA-dependent protein kinase and DNA repair: relevance to Alzheimer’s disease. Alzheimers Res Ther 2013, 5: 13. 10.1186/alzrt167
Katsel P, Tan W, Fam P, Purohit DP, Haroutunian V: Cycle checkpoint abnormalities during dementia: a plausible association with the loss of protection against oxidative stress in Alzheimer’s disease. PLoS One 2013, 8: e68361. 10.1371/journal.pone.0068361
Migliore L, Coppedè F, Fenech M, Thomas P: Association of micronucleus frequency with neurodegenerative diseases. Mutagenesis 2011, 26: 85–92. 10.1093/mutage/geq067
Iourov IY, Vorsanova SG, Pellestor F, Yurov YB: Brain tissue preparations for chromosomal PRINS labeling. Methods Mol Biol 2006, 334: 123–132.
Iourov IY, Vorsanova SG, Soloviev IV, Yurov YB: Interphase FISH: detection of intercellular genomic variations and somatic chromosomal mosaicism. In Fluorescence In Situ Hybridization (FISH)—Application Guide. Edited by: Liehr T. Berlin-Heidelberg: Springer; 2009:301–311.
Iourov IY, Soloviev IV, Vorsanova SG, Monakhov VV, Yurov YB: An approach for quantitative assessment of fluorescence in situ hybridization (FISH) signals for applied human molecular cytogenetics. J Histochem Cytochem 2005, 53: 401–408. 10.1369/jhc.4A6419.2005
Iourov IY, Liehr T, Vorsanova SG, Yurov YB: Interphase chromosome-specific multicolor banding (ICS-MCB): a new tool for analysis of interphase chromosomes in their integrity. Biomol Eng 2007, 24: 415–417. 10.1016/j.bioeng.2007.05.003
Iourov IY, Liehr T, Vorsanova SG, Yurov YB: Interphase chromosome-specific multicolor banding. In Human Interphase Chromosomes. Edited by: Yurov YB, Vorsanova SG, Iourov IY. New York, Heidelberg, Dordrecht, London: Springer; 2013:161–169.
Acknowledgments
The study was supported by the by BMBF/DLR (Deutsches Luft- und Raumfahrtszentrum/Bundesministerium für Bildung und Forschung) BLR 11/002, the Grant of the President of the Russian Federation (MD-4401.2013.7), and RFBR 12-04-00215-а.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YBY, SGV, TL, and IYI conceived the research, designed the study, and wrote the manuscript. YBY, ADK and IYI performed the experiments. YBY, SGV, TL, and IYI conceived the project and obtained the funding. YBY and TL contributed important reagents. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yurov, Y.B., Vorsanova, S.G., Liehr, T. et al. X chromosome aneuploidy in the Alzheimer’s disease brain. Mol Cytogenet 7, 20 (2014). https://doi.org/10.1186/1755-8166-7-20
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1755-8166-7-20