Abstract
In recent decades, special attention has been given to the potential association between the gut ecosystem and chronic diseases. Several features and complications of chronic kidney disease (CKD) may induce an unbalanced gut environment, leading to unfavorable consequences for a patient’s health. The first section of this review is dedicated to a description of some aspects of gut microbiota and intestinal barrier physiology. The following section explores the impact of CKD on the gut ecosystem and intestinal barrier, particularly the association with uremic toxins, inflammation, and immunodeficiency. Finally, the review describes the state of the art of potential therapies with prebiotics, probiotics, and synbiotics employed to modulate the gut environment and to reduce the generation of colon-derived uremic toxins in CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
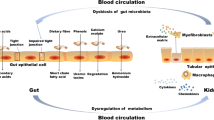
The human gut harbors trillions of bacteria that live in a symbiotic relationship and produce important metabolic and immunologic effects in the host [1,2,3]. Those effects not only are achieved by complex interactions among commensal bacteria and different cell types from the human body but can also be mediated by products derived from microbiota metabolism [4]. Higher carbohydrate to protein bacterial fermentation contributes to the integrity of intestinal barrier, homeostasis of the gut ecosystem, and human health. It has been postulated that chronic kidney disease (CKD) induces negative changes in both the gut environment and the microbiome profile [5]. Intestinal barrier disruption [6, 7], inflammation [8], impairment of the immune system [9], and higher generation of colon-derived uremic toxins p-cresyl sulfate (PCS) and indoxyl sulfate (IS) [10] seem to be some of the consequences of an unbalanced gut ecosystem. Furthermore, the progressive accumulation of PCS and IS has been associated with cardiovascular disease [11], progression of CKD [12], and mortality [13, 14]. Thus, it is important to understand the complex interactions within and between the gut and other body systems in healthy individuals and in those with CKD.
The gut microbiota
The human microbiota consists of trillions of bacteria distributed in complex and specific communities in different parts of the body, such as the skin, respiratory tract, urogenital tract, oral cavity, and gastrointestinal tract. The human gut harbors an average of 100 trillion bacteria, and approximately 3 million bacterial genes are found only in the intestinal microbiome, which is 150-fold more genes than the human genome [1, 15]. Approximately 90% of bacterial species that make up the human intestinal flora belong to the phyla Bacteriodetes (Bacteroides, Prevotella, and Xylanibacter) and Firmicutes (Ruminococcus, Clostridium, Lactobacillus, Eubacterium, and Faecalibacterium). In lower proportions, species belonging to the phyla Actinobacteria, Proteobacteria, and Verrucomicrobia are also found. Methanogenic archaea, yeast, and viruses also appear in the composition of the intestinal microbiota [1, 16]. Recently, it has been suggested that a greater proportion of Proteobacteria could be the microbial signature of dysbiosis in gut microbiota [17].
The gut microbiota is vastly diverse. Each individual has a single microbiota that fluctuates over time [16]. Several factors can influence the development, composition, and activity of the intestinal microbiota, such as host genetics, early colonization, age, lifestyle and environment, diet, medications, and the health status of the host [18, 19].
This complex and specific gut community has been considered a “hidden organ” due to its important physiological effects on the body by interacting with different systems. The gut microbiota produces energy from fermentation of nondigested compounds, synthesizes vitamins (K and B groups) [20], activates bioactive food components (isoflavanoids, flavonoids, and plant lignans) [21], contributes to intestinal barrier integrity [22], upregulates mucin genes [23], stimulates secretion of antimicrobial peptides [24], produces bacteriocins that contribute to resistance or resilience to infection by pathogens [25], competes with pathogenic bacteria for binding to intestinal epithelial cells [26], and influences host metabolism [2] and the immunological system [3, 27]. More recently, it has been suggested that the gut microbiota modulates the functionality of the central nervous system. Indeed, a multidirectional dialog between the gut ecosystem (microbiota, mucosal immune system, and neuroendocrine system) and the central nervous system has been proposed [2, 28].
The bacterial fermentation of undigested carbohydrates contributes to several benefits, including the maintenance of appropriate intestinal pH, by generating lactic acid, acetic acid, and short-chain fatty acids (acetate, propionate, and butyrate). In addition to being an energy source for colonocytes, butyrate is also an essential factor regulating the differentiation of colonic T regulatory cells (Treg). These cells have an important role in the tolerance of the gut lumen towards microorganisms by modulating inflammatory responses [29,30,31]. In addition, the colonic microbiota can catabolize proteins and amino acids that have not been digested or absorbed in the upper portions of the gut. The end products of the proteolytic activity include short-chain fatty acids, branched-chain fatty acids, ammonia, amines, phenols, indoles, thiols, CO2, hydrogen, and hydrogen sulfide (H2S) [29]. Most of these metabolites are normally excreted in feces and urine. Among these products are biogenic amines, immunomodulatory compounds, and other signaling molecules, which seem to be involved in regulating the gut mucosal immune system through pro-inflammatory and anti-inflammatory mechanisms [4]. Although considered potentially toxic, these metabolites occur in the colon at such a concentration range that no toxic effects are expected either locally or systemically [32].
Among the bacterial species living in the human gut, there are saccharolytic bacterial species, which preferentially ferment carbohydrates and use proteins and amino acids to grow, and proteolytic bacterial species, which preferentially ferment proteins and amino acids to produce energy by deamination. The type and amount of substrate, and especially the ratio between undigested carbohydrates and proteins, are the key modulators of bacterial composition and metabolism. When the carbohydrate/protein ratio is reduced, the fermentation of proteins is favored over carbohydrates, resulting in an overgrowth of proteolytic species and a decrease in the growth of saccharolytic bacterial species, resulting in changes in the pattern of metabolites produced [33,34,35,36]. In addition to dietary components, other factors can influence gut microbiota composition and metabolism, such as intestinal pH, medication, time of intestinal transit, host genetics, innate and adaptive immunity, and presence of diseases. Therefore, the amount and type of metabolites produced by bacteria as well as the composition of gut microbiota are the result of complex interactions between the microbiota, the host, and the intestinal environment [29, 37].
The intestinal barrier
The gastrointestinal tract functions as a barrier between the external environment and the internal milieu of the body. As mentioned previously, the surface of the intestinal mucosa is constantly exposed to a complex and dynamic community of commensal microorganisms. To maintain a symbiotic relationship between host and microbiota, the gastrointestinal immune system is involved in several activities. The epithelial lining acts as a first line of defense for the mucosal immune system, both to protect against bacterial invasion and to establish tolerance of the gut lumen towards commensal microorganisms. Through cell adhesion and junction structures, the intestinal epithelium constitutes a physical barrier against bacteria from the lumen and a selectively permeable barrier allowing the passage of nutrients, ions, and water [38,39,40]. Numerous physiological and pathological stimuli dynamically regulate the permeability of the gut epithelium by changing structures involved in the mechanisms of cell adhesion and formation of cellular junctions [41]. In addition to enterocytes and colonocytes, other cell types constitute the intestinal epithelium and contribute to barrier function. Among them there are goblet cells (mucus secreting), Paneth cells (secreting antimicrobial peptides), M cells (antigen presenting) and enteroendocrine cells (producing and secreting peptide hormones). The activity and the communication of these cells with immune cells allow the gut to identify intestinal luminal contents and signals and to regulate and modulate several activities, both in the intestine and in other parts of the body, such as mucus secretion, intestinal motility, and aspects of host energy metabolism [42]. Furthermore, it has been suggested that the mucosal immune system plays an important role in the host-controlling microbiota composition [27].
The intestinal epithelial cells express numerous receptors, pro-inflammatory mediators, and other factors, such as B cell-activating factor (BAFF) and transforming growth factor (TGF)-β, allowing them to communicate with the immune system and contribute to the control of inflammatory responses and intestinal barrier integrity [4, 43, 44]. Through Toll-like receptors (TLRs), the intestinal epithelial cells identify bacterial cell components. These receptors have the ability to recognize specific pathogen-associated molecular patterns (PAMPs). For example, TLR-2 recognizes peptidoglycan, and TLR-4 recognizes lipopolysaccharide (LPS) [45, 46].
Dynamic control over TLR expression and activation in intestinal cells has an important role in tolerance towards commensal microorganisms and intestinal homeostasis. The activation of TLRs promotes several important activities, such as epithelial cell proliferation, immunoglobulin A (IgA) secretion in the intestinal lumen, production of antimicrobial peptides and pro-inflammatory cytokines, and reorganization of tight junction structures, which favors intercellular junctions, reducing intestinal permeability [47, 48]. However, if these receptors are over-activated, the symbiotic relationship between host and microbiota might be disrupted, resulting in damage to the intestinal homeostasis. Thus, under normal physiological conditions, the recognition of LPS via TLR-4 by intestinal epithelial cells is suppressed; continuous exposure to LPS induces desensitization of these cells against this molecule. This process is mediated by expression of inhibitory molecules of the signaling triggered by TLR-4 [49, 50]. The inflammatory cytokines also regulate the expression of TLRs in intestinal epithelial cells. Studies have shown that interferon-γ (IFN-γ) and tumor necrosis factor (TNF) stimulate TLR-4 gene transcription, while interleukin-4 (IL-4) and interleukin-13 (IL-13), secreted by type 2 helper cells (Th2), seem to reduce the expression of TLR-4 in intestinal epithelial cells, since these cytokines lead to a reduction in the responsiveness of intestinal epithelial cells to LPS [51,52,53,54]. Both dendritic cells and macrophages of the intestinal mucosa also appear to have reduced responsiveness to LPS when compared to similar cells from other body tissues [42, 55].
Since the maintenance of homeostatic interactions with a diverse resident microbiota is essential to maintaining a strict balance of pro- and anti-inflammatory responses, the activation of anti-inflammatory cells, such as regulatory T cells (Treg), is required to control the immune response in the gut and consequently avoid impairment of intestinal homeostasis. IL-10 secretion from Treg cells acts on different cell types, such as dendritic cells, effector T cells, macrophages, and intestinal epithelial cells, to attenuate pro-inflammatory responses [40, 56]. In addition, as previously mentioned, microbial molecules have a profound effect on host mucosal and systemic immune system development. These effects may occur, for example, by inducing the secretion of antimicrobial peptides and acting on the composition of T lymphocyte subsets, which in turn influences the balance between potentially pro- and anti-inflammatory cells in the intestinal mucosa [27].
In summary, the maintenance of a symbiotic relationship between host and microbiota occurs through broad and complex interrelationships among different cell types and the intestinal microbiota (Fig. 1).
Synbiotic relationship between host and microbiota. To maintain a symbiotic relationship between host and microbiota, the intestinal immune system is involved in several activities. The epithelial lining acts as the first line of defense of the mucosal immune system, both to protect against bacterial invasion and to establish tolerance of the gut lumen towards commensal microorganisms [39, 40]. Through Toll-like receptors (TLRs), epithelial cells recognize bacterial molecules and produce B cell-activating factor (BAFF), a proliferation-inducing ligand (APRIL), and transforming growth factor (TGF)-β; these cytokines promote immunoglobulin A (IgA) secretion in the intestinal lumen [149]. A strict balance between the function of CD4+ effector T cells and Treg cells is essential for homeostasis. Dendritic cell (DC) activation by bacterial molecules and/or epithelial cell-derived factors induces CD4+ T cell differentiation [150]. T helper 17 (TH17) cells secrete pro-inflammatory cytokines (IL-17A and IL-17F), IL-22 (which stimulates the secretion of antimicrobial peptides), and IL-21 (which inhibits Treg cell generation). T helper 1 (TH1) cells secrete interferon-γ (IFNγ), which activates subepithelial macrophages. Together, these cells sustain the intestinal barrier, in general, by protecting against bacterial invasion. On the other hand, in order to modulate the immune response and avoid acute inflammation, Treg cells are induced and these cells secrete IL-10 and/or TGF-β. These cytokines act on different cell types, such as dendritic cells, effector T cells, macrophages, and intestinal epithelial cells to attenuate pro-inflammatory responses. Simultaneously, Treg cells can stimulate IgA induction [40, 151]
The gut in CKD
CKD is a condition characterized by a gradual loss of kidney function over time with consequent retention of a number of compounds collectively termed uremic toxins. Complications such as anemia, metabolic acidosis, bone and mineral metabolism disorders, malnutrition, acquired immunodeficiency, systemic inflammation, and cardiovascular disease are common in patients with CKD [57,58,59]. Recently, it has emerged that alterations observed in the composition of the gut microbiota and in intestinal permeability, as a consequence of CKD, may also contribute to the immunological disorders, uremic toxicity, and potentially to the progression of the disease. It is out of the scope of the current review to describe the mechanisms involved in kidney-gut related disturbances in depth. A comprehensive review of this topic can be found elsewhere [60].
Gut barrier function in CKD
There is indirect evidence linking CKD with damage to the barrier function of the intestinal mucosa (increased intestinal permeability), such as the presence of endotoxemia in the absence of infection [7, 61, 62], bacterial translocation [8], and detection of intestinal bacteria DNA in the blood [63].
Experimental studies have investigated the possible mechanisms and structures involved in gut barrier dysfunction in CKD. Vaziri et al. [6] conducted an in vivo study with uremic rats and observed a large reduction in the expression of tight junction proteins (claudin-1, occludin, and zonula occludens (ZO)-1) in the colonic mucosa, indicating marked damage to the intestinal barrier. In contrast, the amount of mRNA for these proteins was unchanged or even elevated, indicating that the reduction in tight junction proteins may have occurred in the mRNA translation and/or post-translation steps. Similar findings in the expression of those proteins were also described by the same group of researchers in an in vitro study of human colonocytes. These cells were incubated with uremic plasma and showed significant reductions in the expression of tight junction proteins. Thus, it is possible that the compounds present in uremic plasma might be involved in the process of intestinal damage caused by uremia [64].
The breakdown of intestinal epithelial tight junctions leads to entry of microbial components into the underlying tissue compartments, which triggers a local inflammatory process, which in turn contributes to perpetuating gut barrier damage [65, 66]. Indeed, in uremic rats, disruptions in tight junctions were associated with local inflammation in the gastrointestinal tissue [6, 67]. It has been proposed that the impaired nuclear factor-erythroid-2-related factor 2 (Nrf2) system is involved in uremia-associated intestinal inflammation and breakdown of intestinal epithelial tight junctions [68]. Nrf2 is a transcription factor that activates genes encoding antioxidant and phase II detoxifying enzymes and related proteins. This nuclear factor plays a central role in cellular defense against oxidative stress [69, 70]. The contribution of the impaired Nrf2 system to CKD-induced damage to the intestinal barrier was demonstrated when Lau et al. [68], using a potent Nrf2 activator, observed attenuation of colonic inflammation and restoration of epithelial tight junction proteins in the colon of CKD rats.
Edema and ischemia in the intestinal mucosa may also contribute to intestinal barrier damage. Therefore, hypervolemia, excessive use of diuretics, and hypotensive episodes during or after dialysis can aggravate CKD-induced intestinal barrier dysfunction [7, 71, 72].
Assessment of intestinal permeability disruption with non-invasive methods (serum metabolites) in clinical studies represents a challenge in CKD. A recently published systematic review that included 15 studies noted significantly increased permeability in the late stages of CKD despite the variety of methods used among the different studies. This result should, however, be interpreted with caution due to the possible influence of decreased renal clearance of the serum markers tested and to the lack of non-invasive validated method in CKD [73].
Gut microbiota in CKD
Studies have shown that CKD has been associated with altered colonic microbiota composition [5, 10, 74, 75]. Significant differences in the abundance of numerous microbial operational taxonomic units (OTUs) were observed when feces of CKD patients were compared with that of normal renal functional subjects. An overgrowth of bacteria species belonging to the phyla Proteobacteria, Firmicutes, and Actinobacteria was found in CKD patients. Among the 19 bacterial families that were dominant in individuals with CKD, 12 are urease producers, five are uricase producers, and three are producers of indole and p-cresol-forming enzymes. Among the four bacterial families that were reduced in these patients, two are producers of butyrate (short-chain fatty acid) forming enzymes [5, 10]. Similar results have been found recently. Compared with controls, CKD patients had a significant reduction in two butyrate-producing species (Roseburia spp. and Faecalibacterium prausnitzii) [76]. In general, these changes suggest potentially upregulated microbiota in the generation of specific uremic toxins and downregulated microbiota in the generation of beneficial products.
Changes in the composition of the microbiota do not necessarily implicate in alterations in the microbial metabolism. It is well known that several bacterial species can share similar functional gene profiles, so different microbiota profiles can perform the same functions [16]. In addition to the microbiota composition, the gut environment significantly influences the production of microbial metabolites [77]. As mentioned earlier, the gut environment plays an important role in both the composition and activities of the gut microbiota. Therefore, CKD-induced changes in the intestinal environment may potentially influence colonic microbial metabolism and play a pivotal role in alterations of the microbial community. The high concentration of urea in intracellular and extracellular compartments, due to reduced renal function, generates high influx of this compound into the gastrointestinal tract where microbial ureases catalyze the hydrolysis of urea and generate large amounts of ammonia. Ammonium hydroxide, a by-product of ammonia, increases the intestinal pH, which leads to mucosal irritation and interferes with the growth of commensal bacteria, favoring the establishment of intestinal dysbiosis [78, 79]. The negative impact of urea conversion by microbial urease over intestinal epithelial cells was demonstrated in an in vitro study when colonocytes were incubated with urea plus urease. After incubation, a large reduction in the expression of tight junction proteins was found [80]. In addition to urea, other compounds accumulated in the blood are also secreted in larger amounts into the intestinal lumen, such as uric acid and oxalate, which may also influence microbial composition and metabolism [81, 82].
Other CKD-related factors may also promote changes in the intestinal environment. These factors include low fiber intake due to restricted fruit and vegetable consumption to control serum potassium [83, 84], constipation [85,86,87], abnormal digestive function [88], impairment of digestion and absorption of protein [89, 90], oral iron supplementation [91], and use of phosphate binders to control serum phosphorus and antibiotics to treat infections [92, 93].
However, despite the presence of these CKD-related factors, the influence of CKD on colonic microbial metabolism remains poorly understood. As far as we know, there is only one study in humans on microbial metabolism in individuals with CKD. Poesen et al. [94] investigated fecal metabolite profiles of hemodialysis patients and healthy individuals and observed distinct colonic microbial metabolism between these two groups. They found upregulated generation of p-cresol, indoles, aldehydes, benzenes, furans, and branched-chain, medium-chain, and short-chain fatty acids and a reduced generation of ketones in patients on hemodialysis. The clinical impact of these alterations is unknown for the majority of these fecal metabolites. Studies with uremic rats also demonstrated significant differences in fecal metabolite profiles, indicating an important influence of loss of renal function [94, 95]. However, in humans, in contrast to rats, when the researchers compared the patients on hemodialysis with household contacts on the same diet, the influence of decreased renal function was inferior to the external factors, such as the diet [94].
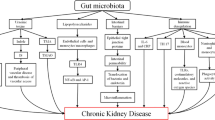
In summary, the impact of CKD on the gut is complex and seems to involve a dynamic interplay between intestinal cells and microbiota, which are heavily influenced by the gut environment (Fig. 2). As a consequence, it has been proposed that CKD-induced intestinal changes may also influence the accumulation of specific uremic toxins and disturbances in the immune system described in the following sections.
Schematic representation of the potential impact of CKD on the gut ecosystem. The CKD-induced changes in the intestinal environment may potentially influence the colonic microbial metabolism and play a pivotal role in alterations in the microbial community. As shown in the figure, several CKD-related factors contribute to alterations in the intestinal environment. These factors may lead to increased gut pH and alterations in gut composition and microbial metabolism, favoring the establishment of intestinal dysbiosis [10, 79]. Together, these changes may impair the gut barrier, leading to entry of microbial components and uremic toxins into the underlying tissue compartments, which triggers a local inflammatory process. The increase of microbial components in the lamina propria consequently promotes secretion of pro-inflammatory cytokines by increasing the activation of dendritic cells (DCs), macrophages, and CD4+ effector T cells (Th1 and Th17), disrupting the strict balance between the function of CD4+ effector T cells and Treg cells. This inflammatory process contributes to perpetuating gut barrier damage [40, 120]. The continuous entry of these components (endotoxins and uremic toxins) into blood circulation may lead to a persistent systemic inflammation that links the gut with CKD-associated inflammation and immunodeficiency [9]
Gut ecosystem in CKD and uremic toxins
The numerous metabolites that normally compose the mammalian blood metabolome originate from the colonic microbiota [96]. Among them, p-cresol and indole are generated from bacterial fermentation of tyrosine and tryptophan, respectively. p-Cresol is a phenolic compound that, by sulfate conjugation in the colonic mucosa and in the liver, generates p-cresyl sulfate, whereas indole generates indoxyl sulfate after sulfate conjugation in the liver [97].
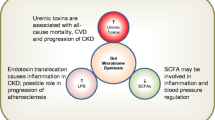
The decrease in the excretion of p-cresyl sulfate and indoxyl sulfate, due to the loss or reduction of renal function, leads to blood retention of these compounds. Indeed, it has already been shown in clinical studies that the concentration of these uremic toxins is markedly elevated in all stages of CKD, with a gradual increase as renal function decreases [11, 98, 99]. Given the importance of colonic metabolism to the composition of the mammalian blood metabolome, it has been proposed that alterations in the colonic metabolism of CKD patients, such as upregulation of both p-cresol and indole generation, can amplify the accumulation of these uremic toxins [94].
The harmful effect of these uremic toxins on different cell types, such as kidney tubular cells [100,101,102], endothelial cells [103,104,105], immunological cells [106, 107], and bone cells [108,109,110], has been demonstrated in experimental studies. Moreover, the accumulation of these toxins in individuals with CKD has been associated with progression of CKD [12], inflammation [98, 111], cardiovascular disease [11], vascular calcification [13], and mortality from cardiovascular disease and from all causes [13, 14, 112, 113].
Gut ecosystem in CKD and immunity
CKD is simultaneously associated with systemic inflammation and acquired immunodeficiency [59, 114]. Persistent systemic inflammation contributes to atherogenesis, cardiovascular disease, anemia, cachexia, and bone loss, whereas acquired immunodeficiency leads to impaired response to vaccination and to microbial infections, increasing frequency and severity of microbial infections in these patients, which in turn contributes to systemic inflammation [115]. Together, these immunological disorders have a strong negative impact on the survival of CKD patients [59, 116, 117].
Functional and structural alterations in immune cell populations contribute to CKD-associated systemic inflammation and acquired immunodeficiency [118]. Several factors may contribute to these immunological disorders, including uremic toxicity, oxidative stress, metabolic acidosis, vitamin D deficiency, impaired protein catabolism, chronic and recurrent infection, decreased clearance of pro-inflammatory cytokines and the dialysis process itself [115, 119]. More recently, it has been proposed that CKD-induced changes in the gut microbiome and consequent dysfunction in the gut barrier may have potential effects on systemic immunity [9, 115].
Gut barrier dysfunction promotes leakage of bacterial components into the bloodstream, such as lipopolysaccharide (LPS) [120]. In fact, as previously mentioned, CKD patients are frequently exposed to significant endotoxemia in the absence of infection. This entry of bacterial components from the gut to the bloodstream could contribute to or explain, at least in part, the persistent systemic inflammation in patients with CKD by activation of the innate immune response [7, 9]. Given the current understanding of sepsis, which involves concomitant inflammation and immunodeficiency, it is supposed that persistent TLR stimulation by endotoxins derived from intestinal bacteria may lead to a subsequent refractory state in CKD, a phenomenon named “compensatory anti-inflammatory syndrome,” suppressing innate and adaptive immunity, which could explain the simultaneous association of CKD with systemic inflammation and acquired immunodeficiency [9].
A recent study with animals supports this hypothesis that uremic dysbiosis and intestinal barrier dysfunction are significantly implicated in CKD-related systemic inflammation. The eradication of facultative anaerobic microbiota with antibiotics in uremic rats prevented bacterial translocation and reduced serum endotoxin levels and markers of systemic inflammation [121]. Thus, the gut seems to be a source of inflammation in CKD. Since inflammation is a major mediator of CKD progression, alterations in the gut and gut microbiome are potentially involved in CKD progression. In addition, CKD progression will perpetuate and amplify alterations in the gut microbiome and intestinal barrier, forming a vicious circuit [120]. Indeed, butyrate-producing species (Roseburia spp. and Faecalibacterium prausnitzii) seem to be negatively related to renal function and microinflammation of CKD patients, suggesting a relation of these bacteria with CKD-associated inflammation and CKD progression [76]. As previously mentioned, butyrate is a short-chain fatty acid resulting from bacterial fermentation, and it is an important nutrient for colonocytes and has anti-inflammatory properties. The short-chain fatty acids seem to also have beneficial effects on the kidney. In a study of acute kidney injury, the three main short-chain fatty acids (acetate, propionate, and butyrate) improved renal dysfunction caused by injury and were associated with reduced local and systemic inflammation [122].
As previously mentioned, the gut environment has an in important role in the composition and metabolism of gut microbiota, and CKD induces changes in the intestinal environment. The contribution of the intestinal environment and potentially of microbial metabolism to the pathogenesis of CKD-induced damage in gut barrier function and consequent inflammation and CKD progression was shown in a recent study with animals. A significant attenuation in the alteration of tight junction proteins in colonic tissue, oxidative stress and inflammation, and CKD progression delay was found when uremic rats were treated with high fermentable fiber, which has a potential beneficial effect on enzyme activity and on microbiota composition [123]. These benefits are associated with reduced cecal pH and alterations in gut microbiota composition (reduced microbial diversity) and in metabolomic profiles [124].
It is important to highlight, however, that most of these studies are in animals. Future studies are needed to confirm and explore the relations between intestinal dysbiosis, barrier dysfunction, CKD-related systemic inflammation, and CKD progression in humans, which are very complex with several influencing factors.
Potential dietetic approaches targeting the gut environment and colon-derived uremic toxins in CKD
As previously described, the potential involvement of gut disorders in CKD has encouraged the investigation of alternatives to modulate colonic imbalance and its potential negative effects on the disease. Dietary management and supplementation with prebiotics, probiotics, and synbiotics have been highlighted in this scenario. These approaches have focused on the modulation of the carbohydrate/protein ratio delivered into the colon and/or on the composition and metabolism of microbiota. Despite the paucity of high-quality studies, promising data have been generated.
The prescription of a low-protein diet is an approach widely employed for NDD-CKD patients to control metabolic disturbances and uremic symptoms and to slow the progression of the disease [125]. Studies suggest that this dietary intervention may also exert some influence on the gut environment. Reduction of serum urea is generally achieved with dietary protein restriction, which in turn may limit the influx of urea into the gut and potentially contribute to preventing harmful changes in the gut ecosystem [126]. Moreover, the decrease in protein intake may reduce the delivery of amino acids into the colon and the consequent proteolytic fermentation that generates p-cresol and indole. The benefits of decreasing protein intake on colon-derived uremic toxins, especially on indoxyl sulfate (IS), were demonstrated in healthy subjects [36] and CKD patients [127]. Most importantly, the colonic balance between dietary protein and fiber appears to be more relevant in the reduction of serum p-cresyl sulfate (PCS) and IS than the protein intake alone [127,128,129]. Therefore, consuming diets with higher fiber in relation to protein might represent an important alternative to reduce the production and to attenuate the accumulation of PCS and IS in CKD, particularly in hemodialysis patients who require larger amounts of protein to maintain their nitrogen balance.
Fibers are non-digestible carbohydrates that, along with some nitrogen compounds, represent the sources of energy of the gut microbiota. As previously mentioned, although fibers are the preferable substrate for most bacteria, the colonic balance between fiber and protein determines whether the energy supply will derivate from saccharolytic or proteolytic fermentation. In the presence of enough fiber, nitrogen is used to the growth and development of microbiota, instead of being deviated to proteolytic fermentation, avoiding, at least in part, the generation of colon-derived uremic toxins [33, 97]. In addition, products of fiber fermentation stimulate intestinal transit time, enhancing nitrogen fecal excretion and contributing to the integrity and function of the intestinal barrier [123, 130, 131]. In CKD, a meta-analysis of controlled feeding trials showed that fermentable fibers, namely, prebiotics [132], were able to promote a reduction in serum urea and creatinine [133]. The potential of prebiotics to reduce colon-derived uremic toxins has been attributed to their bifidogenic effect and the consequent inhibition of the growth of uremic toxin-producing bacteria [134]. However, to date, no clinical trial has evaluated the association between changes in the microbiota and the decrease in colon-derived uremic toxins after prebiotic therapy in CKD. The few short-term prebiotic interventions performed so far have reported mixed effects on PCS and IS [131, 135,136,137,138]. The reasons for the heterogeneity in the results may be related to differences in type and amount of prebiotic used, factors that might be relevant for modulation of serum PCS and/or IS in CKD (Table 1).
Probiotics have also been described as an alternative approach to promote the balance of the gut microbiota. They are defined as live microorganisms, which, when administered in adequate amounts, confer benefits to the host [139]. The benefits of probiotics are mediated by their interaction with the host’s microbiota, gut epithelial cells, and gut immune system, potentially providing improvement in gut barrier integrity and function, in the activity of the gut immune system and inflammatory response, and in the control of photobiont overgrowth [140]. However, the body of evidence supporting the use of probiotics in CKD is still insipient, and the effectiveness of the treatment over the reduction of colon-derived uremic toxins and inflammation is so far inconclusive (Table 1). Variability in bacterial strains, doses, and administration form hinder comparison of the results. In addition, since the genesis of “dysbiose” in CKD seems to involve the imbalance of the entire colonic ecosystem, the greater availability of probiotic bacteria per se (without adequate availability of colonic substrate), might not be sufficient to recover the gut milieu. Therefore, the association of probiotics with prebiotics, composing synbiotics, might promote better modulation of the gut ecosystem. Despite the differences in the combination and doses of probiotic and prebiotics and the short-term follow-up, studies with synbiotics seem to be better quality, in terms of methodological aspects compared to those with probiotics or prebiotics alone. Moreover, the overall result has been more consistent showing a reduction in PCS [141,142,143] or an increase in the relative abundance of fecal Bifidobacterium (Table 1) [141, 144]. However, the benefits of probiotics and synbiotics in the reduction of PCS and IS appear to be associated with the sustained use of the therapy, since an increase in those toxins was observed when the supplementation was discontinued [134, 141, 145]. In addition, potential harmful effects of probiotic supplementation, such as bacterial translocation in conditions of increased intestinal permeability and immunodeficiency [146], generally found in advanced stages of CKD have not been investigated so far.
The effectiveness of prebiotics, probiotics, and synbiotics in reducing colon-derived uremic toxins in CKD patients was tested in a meta-analysis. Combining those therapies, a decrease of approximately 6.4 mg/L was found in IS in hemodialysis patients. The study, unfortunately, did not evaluate the effectiveness on PCS or the differences among the treatments due to the limited number of applicable clinical trials at that time [134].
In summary, the dietary manipulation of the gut ecosystem with prebiotics, probiotics, and synbiotics seems to be a promising strategy in the management of uremic toxins in CKD. The studies conducted so far have large variability with regard to the type and dose of fiber/prebiotic, bacterial strain, or their combination as synbiotics, and most of them have relevant limitations such as short follow-up, small sample sizes, and/or the absence of dietary control. Therefore, the evidence for such strategies is still incipient to establish the most appropriate therapeutic approach for CKD. Until high-quality evidence is obtained in this field, a healthy dietary pattern that includes larger amounts of whole grains, fruits, and vegetables should be encouraged among CKD patients since there are additional benefits other than the decrease in uremic toxin production [147, 148].
Conclusion
It is becoming clear that the chronic loss of renal function can negatively impact the gut ecosystem and vice versa, and this bidirectional relationship contributes with uremic syndrome, inflammation, immunodeficiency, kidney damage, cardiovascular risk, and mortality among patients with CKD. In this view, the gut has been described as an important target to modify the burden of CKD, and interventions with prebiotics, probiotics, and synbiotics have been emerging as an attractive strategy in this field. However, since the evidence about the impact of those approaches is still incipient, this constitutes an important field of research in CKD.
References
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65.
Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology. 2014;146(6):1525–33.
Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014;146(6):1477–88.
Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology. 2014;146(6):1449–58.
Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83(2):308–15.
Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H, Subramanian VS. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol Dial Transplant. 2012;27(7):2686–93.
McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(1):133–41.
Shi K, Wang F, Jiang H, Liu H, Wei M, Wang Z, et al. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig Dis Sci. 2014;59(9):2109–17.
Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83(6):1010–6.
Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39(3):230–7.
Rossi M, Campbell K, Johnson D, Stanton T, Pascoe E, Hawley C, et al. Uraemic toxins and cardiovascular disease across the chronic kidney disease spectrum: an observational study. Nutr Metab Cardiovasc Dis. 2014;24(9):1035–42.
Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26(3):938–47.
Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(10):1551–8.
Poesen R, Evenepoel P, de Loor H, Kuypers D, Augustijns P, Meijers B. Metabolism, protein binding, and renal clearance of microbiota-derived p-cresol in patients with CKD. Clin J Am Soc Nephrol. 2016;11(7):1136–44.
Relman DA. The human microbiome and the future practice of medicine. JAMA. 2015;314(11):1127–8.
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–30.
Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33(9):496–503.
Ottman N, Smidt H, de Vos WM, Belzer C. The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol. 2012;2:104.
Graf D, Di Cagno R, Fåk F, Flint HJ, Nyman M, Saarela M, et al. Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis. 2015;26:26164.
Cummings JH, Macfarlane GT. Role of intestinal bacteria in nutrient metabolism. JPEN J Parenter Enteral Nutr. 1997;21(6):357–65.
Blaut M, Clavel T. Metabolic diversity of the intestinal microbiota: implications for health and disease. J Nutr. 2007;137(3 Suppl 2):751S–5S.
Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G1025–34.
Mattar AF, Teitelbaum DH, Drongowski RA, Yongyi F, Harmon CM, Coran AG. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int. 2002;18(7):586–90.
Schlee M, Harder J, Köten B, Stange EF, Wehkamp J, Fellermann K. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin Exp Immunol. 2008;151(3):528–35.
O'Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189–205.
Sherman PM, Johnson-Henry KC, Yeung HP, Ngo PS, Goulet J, Tompkins TA. Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect Immun. 2005;73(8):5183–8.
Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–73.
El Aidy S, Dinan TG, Cryan JF. Gut microbiota: the conductor in the Orchestra of Immune-Neuroendocrine Communication. Clin Ther. 2015;37(5):954–67.
Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95(1):50–60.
Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–25.
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73.
Verbeke KA, Boobis AR, Chiodini A, Edwards CA, Franck A, Kleerebezem M, et al. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr Res Rev. 2015;28(1):42–66.
Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69(1):52–60.
Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70(6):443–59.
Birkett A, Muir J, Phillips J, Jones G, O'Dea K. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am J Clin Nutr. 1996;63(5):766–72.
Poesen R, Mutsaers HA, Windey K, van den Broek PH, Verweij V, Augustijns P, et al. The influence of dietary protein intake on mammalian tryptophan and phenolic metabolites. PLoS One. 2015;10(10):e0140820.
Hawrelak JA, Myers SP. The causes of intestinal dysbiosis: a review. Altern Med Rev. 2004;9(2):180–97.
Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100(2):123–36.
Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31(3):368–76.
Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10(3):159–69.
Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279(5):G851–7.
Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140(6):859–70.
Fiocchi C. Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Phys. 1997;273(4 Pt 1):G769–75.
Pitman RS, Blumberg RS. First line of defense: the role of the intestinal epithelium as an active component of the mucosal immune system. J Gastroenterol. 2000;35(11):805–14.
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5.
Winkler P, Ghadimi D, Schrezenmeir J, Kraehenbuhl JP. Molecular and cellular basis of microflora-host interactions. J Nutr. 2007;137(3 Suppl 2):756S–72S.
Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10(2):131–44.
Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132(4):1359–74.
Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126(4):1054–70.
Lotz M, Gütle D, Walther S, Ménard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203(4):973–84.
Abreu MT, Arnold ET, Thomas LS, Gonsky R, Zhou Y, Hu B, et al. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem. 2002;277(23):20431–7.
Suzuki M, Hisamatsu T, Podolsky DK. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect Immun. 2003;71(6):3503–11.
Mueller T, Terada T, Rosenberg IM, Shibolet O, Podolsky DK. Th2 cytokines down-regulate TLR expression and function in human intestinal epithelial cells. J Immunol. 2006;176(10):5805–14.
Lotz M, König T, Ménard S, Gütle D, Bogdan C, Hornef MW. Cytokine-mediated control of lipopolysaccharide-induced activation of small intestinal epithelial cells. Immunology. 2007;122(3):306–15.
Steimle A, Frick JS. Molecular mechanisms of induction of tolerant and tolerogenic intestinal dendritic cells in mice. J Immunol Res. 2016;2016:1958650.
Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31(3):401–11.
Kaysen GA. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001;12(7):1549–57.
Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:S4–9.
Girndt M, Sester U, Sester M, Kaul H, Köhler H. Impaired cellular immune function in patients with end-stage renal failure. Nephrol Dial Transplant. 1999;14(12):2807–10.
Felizardo RJ, Castoldi A, Andrade-Oliveira V, Câmara NO. The microbiota and chronic kidney diseases: a double-edged sword. Clin Transl Immunol. 2016;5(6):e86.
Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, et al. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3(2):431–6.
Feroze U, Kalantar-Zadeh K, Sterling KA, Molnar MZ, Noori N, Benner D, et al. Examining associations of circulating endotoxin with nutritional status, inflammation, and mortality in hemodialysis patients. J Ren Nutr. 2012;22(3):317–26.
Wang F, Jiang H, Shi K, Ren Y, Zhang P, Cheng S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology (Carlton). 2012;17(8):733–8.
Vaziri ND, Goshtasbi N, Yuan J, Jellbauer S, Moradi H, Raffatellu M, et al. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am J Nephrol. 2012;36(5):438–43.
Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci (Landmark Ed). 2009;14:2765–78.
Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5(6):685–94.
Vaziri ND, Yuan J, Nazertehrani S, Ni Z, Liu S. Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Am J Nephrol. 2013;38(2):99–103.
Lau WL, Liu SM, Pahlevan S, Yuan J, Khazaeli M, Ni Z, et al. Role of Nrf2 dysfunction in uremia-associated intestinal inflammation and epithelial barrier disruption. Dig Dis Sci. 2015;60(5):1215–22.
Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10(11):549–57.
Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic Res. 2010;44(11):1267–88.
Gonçalves S, Pecoits-Filho R, Perreto S, Barberato SH, Stinghen AE, Lima EG, et al. Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant. 2006;21(10):2788–94.
Chang M, Kistler EB, Schmid-Schönbein GW. Disruption of the mucosal barrier during gut ischemia allows entry of digestive enzymes into the intestinal wall. Shock. 2012;37(3):297–305.
Terpstra ML, Singh R, Geerlings SE, Bemelman FJ. Measurement of the intestinal permeability in chronic kidney disease. World J Nephrol. 2016;5(4):378–88.
Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74(2):349–55.
Wang IK, Lai HC, Yu CJ, Liang CC, Chang CT, Kuo HL, et al. Real-time PCR analysis of the intestinal microbiotas in peritoneal dialysis patients. Appl Environ Microbiol. 2012;78(4):1107–12.
Jiang S, Xie S, Lv D, Zhang Y, Deng J, Zeng L, et al. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Van Leeuwenhoek. 2016;109(10):1389–96.
Smith EA, Macfarlane GT. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol. 1996;81(3):288–302.
Vaziri ND, Dure-Smith B, Miller R, Mirahmadi MK. Pathology of gastrointestinal tract in chronic hemodialysis patients: an autopsy study of 78 cases. Am J Gastroenterol. 1985;80(8):608–11.
Kang JY. The gastrointestinal tract in uremia. Dig Dis Sci. 1993;38(2):257–68.
Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol. 2013;37(1):1–6.
Hatch M, Vaziri ND. Enhanced enteric excretion of urate in rats with chronic renal failure. Clin Sci (Lond). 1994;86(5):511–6.
Hatch M, Freel RW, Vaziri ND. Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol. 1994;5(6):1339–43.
Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12(1):17–31.
Schena FP. Management of patients with chronic kidney disease. Intern Emerg Med. 2011;6(Suppl 1):77–83.
Wu MJ, Chang CS, Cheng CH, Chen CH, Lee WC, Hsu YH, et al. Colonic transit time in long-term dialysis patients. Am J Kidney Dis. 2004;44(2):322–7.
Cano AE, Neil AK, Kang JY, Barnabas A, Eastwood JB, Nelson SR, et al. Gastrointestinal symptoms in patients with end-stage renal disease undergoing treatment by hemodialysis or peritoneal dialysis. Am J Gastroenterol. 2007;102(9):1990–7.
Strid H, Simrén M, Johansson AC, Svedlund J, Samuelsson O, Björnsson ES. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrol Dial Transplant. 2002;17(8):1434–9.
Grant CJ, Harrison LE, Hoad CL, Marciani L, Gowland PA, McIntyre CW. Patients with chronic kidney disease have abnormal upper gastro-intestinal tract digestive function: a study of uremic enteropathy. J Gastroenterol Hepatol. 2017;32(2):372–7.
Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P. Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int. 2003;64(6):2196–203.
Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Impairment of small intestinal protein assimilation in patients with end-stage renal disease: extending the malnutrition-inflammation-atherosclerosis concept. Am J Clin Nutr. 2004;80(6):1536–43.
Kortman GAM, Reijnders D, Swinkels DW. Oral iron supplementation: potential implications for the gut microbiome and metabolome in patients with CKD. Hemodial Int. 2017;21(Suppl 1):S28–36.
Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5(3):e9836.
Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156(Pt 11):3216–23.
Poesen R, Windey K, Neven E, Kuypers D, De Preter V, Augustijns P, et al. The influence of CKD on colonic microbial metabolism. J Am Soc Nephrol. 2016;27(5):1389–99.
Meinardi S, Jin KB, Barletta B, Blake DR, Vaziri ND. Exhaled breath and fecal volatile organic biomarkers of chronic kidney disease. Biochim Biophys Acta. 2013;1830(3):2531–7.
Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106(10):3698–703.
Mafra D, Barros AF, Fouque D. Dietary protein metabolism by gut microbiota and its consequences for chronic kidney disease patients. Future Microbiol. 2013;8(10):1317–23.
Rossi M, Campbell KL, Johnson DW, Stanton T, Vesey DA, Coombes JS, et al. Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage 3-4 chronic kidney disease. Arch Med Res. 2014;45(4):309–17.
Lin CJ, Chen HH, Pan CF, Chuang CK, Wang TJ, Sun FJ, et al. p-Cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J Clin Lab Anal. 2011;25(3):191–7.
Satoh M, Hayashi H, Watanabe M, Ueda K, Yamato H, Yoshioka T, et al. Uremic toxins overload accelerates renal damage in a rat model of chronic renal failure. Nephron Exp Nephrol. 2003;95(3):e111–8.
Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T. Uraemic toxins induce proximal tubular injury via organic anion transporter 1-mediated uptake. Br J Pharmacol. 2002;135(2):555–63.
Watanabe H, Miyamoto Y, Honda D, Tanaka H, Wu Q, Endo M, et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013;83(4):582–92.
Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65(2):442–51.
Cerini C, Dou L, Anfosso F, Sabatier F, Moal V, Glorieux G, et al. P-cresol, a uremic retention solute, alters the endothelial barrier function in vitro. Thromb Haemost. 2004;92(1):140–50.
Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost. 2007;5(6):1302–8.
Adesso S, Popolo A, Bianco G, Sorrentino R, Pinto A, Autore G, et al. The uremic toxin indoxyl sulphate enhances macrophage response to LPS. PLoS One. 2013;8(9):e76778.
Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, Vanholder R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant. 2007;22(2):592–6.
Nii-Kono T, Iwasaki Y, Uchida M, Fujieda A, Hosokawa A, Motojima M, et al. Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int. 2007;71(8):738–43.
Mozar A, Louvet L, Godin C, Mentaverri R, Brazier M, Kamel S, et al. Indoxyl sulphate inhibits osteoclast differentiation and function. Nephrol Dial Transplant. 2012;27(6):2176–81.
Tanaka H, Iwasaki Y, Yamato H, Mori Y, Komaba H, Watanabe H, et al. p-Cresyl sulfate induces osteoblast dysfunction through activating JNK and p38 MAPK pathways. Bone. 2013;56(2):347–54.
Borges NA, Barros AF, Nakao LS, Dolenga CJ, Fouque D, Mafra D. Protein-bound uremic toxins from gut microbiota and inflammatory markers in chronic kidney disease. J Ren Nutr. 2016;26(6):396–400.
Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69(6):1081–7.
Lin CJ, Wu V, Wu PC, Wu CJ. Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One. 2015;10(7):e0132589.
Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease—what have we learned in 10 years? Semin Dial. 2010;23(5):498–509.
Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13(10):738–53.
Carrero JJ, Stenvinkel P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S49–55.
Stenvinkel P. Inflammation in end-stage renal disease—a fire that burns within. Contrib Nephrol. 2005;149:185–99.
Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22(1):149–56.
Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39(1–3):84–92.
Lau WL, Kalantar-Zadeh K, Vaziri ND. The gut as a source of inflammation in chronic kidney disease. Nephron. 2015;130(2):92–8.
Andersen K, Kesper MS, Marschner JA, Konrad L, Ryu M, Kumar Vr S, et al. Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-related systemic inflammation. J Am Soc Nephrol. 2017;28(1):76–83.
Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol. 2015;26(8):1877–88.
Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One. 2014;9(12):e114881.
Kieffer DA, Piccolo BD, Vaziri ND, Liu S, Lau WL, Khazaeli M, et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Renal Physiol. 2016;310(9):F857–71.
Kovesdy CP, Kalantar-Zadeh K. Back to the future: restricted protein intake for conservative management of CKD, triple goals of renoprotection, uremia mitigation, and nutritional health. Int Urol Nephrol. 2016;48(5):725–9.
Younes H, Alphonse JC, Hadj-Abdelkader M, Rémésy C. Fermentable carbohydrate and digestive nitrogen excretion. J Ren Nutr. 2001;11(3):139–48.
Marzocco S, Dal Piaz F, Di Micco L, Torraca S, Sirico ML, Tartaglia D, et al. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013;35(1–3):196–201.
Patel KP, Luo FJ, Plummer NS, Hostetter TH, Meyer TW. The production of p-cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin J Am Soc Nephrol. 2012;7(6):982–8.
Rossi M, Johnson DW, Xu H, Carrero JJ, Pascoe E, French C, et al. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr Metab Cardiovasc Dis. 2015;25(9):860–5.
Bliss DZ, Stein TP, Schleifer CR, Settle RG. Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr. 1996;63:6.
Younes H, Egret N, Hadj-Abdelkader M, Rémésy C, Demigné C, Gueret C, et al. Fermentable carbohydrate supplementation alters nitrogen excretion in chronic renal failure. J Ren Nutr. 2006;16(1):67–74.
Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17(2):259–75.
Chiavaroli L, Mirrahimi A, Sievenpiper JL, Jenkins DJ, Darling PB. Dietary fiber effects in chronic kidney disease: a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. 2015;69(7):761–8.
Rossi M, Klein K, Johnson DW, Campbell KL. Pre-, pro-, and synbiotics: do they have a role in reducing uremic toxins? A systematic review and meta-analysis. Int J Nephrol. 2012;2012:673631.
Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant. 2010;25(1):219–24.
Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol. 2014;9(9):1603–10.
Poesen R, Evenepoel P, de Loor H, Delcour JA, Courtin CM, Kuypers D, et al. The influence of prebiotic arabinoxylan oligosaccharides on microbiota derived uremic retention solutes in patients with chronic kidney disease: a randomized controlled trial. PLoS One. 2016;11(4):e0153893.
Salmean YA, Segal MS, Palii SP, Dahl WJ. Fiber supplementation lowers plasma p-cresol in chronic kidney disease patients. J Ren Nutr. 2015;25(3):316–20.
Food JFWWGRoDGftEoPi. London, Ontario, Canada2002.
Ciorba MA. A gastroenterologist’s guide to probiotics. Clin Gastroenterol Hepatol. 2012;10(9):960–8.
Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol. 2016;11(2):223–31.
Guida B, Germanò R, Trio R, Russo D, Memoli B, Grumetto L, et al. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutr Metab Cardiovasc Dis. 2014;24(9):1043–9.
Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, et al. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol Dial Transplant. 2011;26(3):1094–8.
Cruz-Mora J, Martínez-Hernández NE, Martín del Campo-López F, Viramontes-Hörner D, Vizmanos-Lamotte B, Muñoz-Valle JF, et al. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J Ren Nutr. 2014;24(5):330–5.
Takayama F, Taki K, Niwa T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis. 2003;41(3 Suppl 1):S142–5.
Vaziri ND. Gut microbial translocation in the pathogenesis of systemic inflammation in patients with end-stage renal disease. Dig Dis Sci. 2014;59(9):2020–2.
Kelly JT, Palmer SC, Wai SN, Ruospo M, Carrero JJ, Campbell KL, et al. Healthy dietary patterns and risk of mortality and ESRD in CKD: a meta-analysis of cohort studies. Clin J Am Soc Nephrol. 2017;12(2):272–9.
Smyth A, Griffin M, Yusuf S, Mann JF, Reddan D, Canavan M, et al. Diet and major renal outcomes: a prospective cohort study. The NIH-AARP diet and health study. J Ren Nutr. 2016;26(5):288–98.
Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28(6):740–50.
Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8(6):435–46.
Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10(10):735–44.
Salmean YA, Segal MS, Langkamp-Henken B, Canales MT, Zello GA, Dahl WJ. Foods with added fiber lower serum creatinine levels in patients with chronic kidney disease. J Ren Nutr. 2013;23(2):e29–32.
Tayebi Khosroshahi H, Habibzadeh A, Khoshbaten M, Rahbari B, Chaichi P, Badiee AH. Lactulose for reduction of nitrogen products in patients with chronic kidney disease. Iran J Kidney Dis. 2014;8(5):377–81.
Xie LM, Ge YY, Huang X, Zhang YQ, Li JX. Effects of fermentable dietary fiber supplementation on oxidative and inflammatory status in hemodialysis patients. Int J Clin Exp Med. 2015;8(1):1363–9.
Tayebi-Khosroshahi H, Habibzadeh A, Niknafs B, Ghotaslou R, Yeganeh Sefidan F, Ghojazadeh M, et al. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial. J Renal Inj Prev. 2016;5(3):162–7.
Elamin S, Alkhawaja MJ, Bukhamsin AY, Idris MAS, Abdelrahman MM, Abutaleb NK, et al. Gum arabic reduces C-reactive protein in chronic kidney disease patients without affecting urea or indoxyl sulfate levels. Int J Nephrol. 2017;2017:9501470.
Taki K, Takayama F, Niwa T. Beneficial effects of Bifidobacteria in a gastroresistant seamless capsule on hyperhomocysteinemia in hemodialysis patients. J Ren Nutr. 2005;15(1):77–80.
Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS, et al. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther. 2010;27(9):634–47.
Miranda Alatriste PV, Urbina Arronte R, Gómez Espinosa CO, MeL EC. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr Hosp. 2014;29(3):582–90.
Wang IK, Wu YY, Yang YF, Ting IW, Lin CC, Yen TH, et al. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: a randomised, double-blind, placebo-controlled trial. Benef Microbes. 2015;6(4):423–30.
Natarajan R, Pechenyak B, Vyas U, Ranganathan P, Weinberg A, Liang P, et al. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int. 2014;2014:568571.
Miraghajani M, Zaghian N, Mirlohi M, Feizi A, Ghiasvand R. The impact of probiotic soy milk consumption on oxidative stress among type 2 diabetic kidney disease patients: a randomized controlled clinical trial. J Ren Nutr. 2017;
Soleimani A, Zarrati Mojarrad M, Bahmani F, Taghizadeh M, Ramezani M, Tajabadi-Ebrahimi M, et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017;91(2):435–42.
Viramontes-Hörner D, Márquez-Sandoval F, Martín-del-Campo F, Vizmanos-Lamotte B, Sandoval-Rodríguez A, Armendáriz-Borunda J, et al. Effect of a symbiotic gel (lactobacillus acidophilus + Bifidobacterium lactis + inulin) on presence and severity of gastrointestinal symptoms in hemodialysis patients. J Ren Nutr. 2015;25(3):284–91.
Dehghani H, Heidari F, Mozaffari-Khosravi H, Nouri-Majelan N, Dehghani A. Synbiotic supplementations for azotemia in patients with chronic kidney disease: a randomized controlled trial. Iran J Kidney Dis. 2016;10(6):351–7.
Acknowledgements
We would like to thank Vanessa Ramos de Oliveira for the excellent work on drawing the figures.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
de Andrade, L.S., Ramos, C.I. & Cuppari, L. The cross-talk between the kidney and the gut: implications for chronic kidney disease. Nutrire 42, 27 (2017). https://doi.org/10.1186/s41110-017-0054-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-017-0054-x