Abstract
Introduction
Uremic syndrome consists of nitrogenous waste retention, deficiency in kidney-derived hormones, and reduced acid excretion, and, if untreated, may progress to coma and eventual death. Previous experience suggests that oral administration of a probiotic formulation of selected microbial strains may extend renoprotection via intraintestinal extraction of toxic waste solutes in patients with chronic kidney disease (CKD)stages 3 and 4. This report presents preliminary data from a pilot study.
Methods
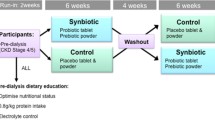
This was a 6-month prospective, randomized, double-blind, placebo-controlled crossover trial of a probiotic bacterial formulation conducted in four countries, at five institutions, on 46 outpatients with CKD stages 3 an nd 4: USA (n=10), Canada (n=113), Nigeria (n=115), and Argentina (n=8). Outcomes were compared using biochemical parameters:blood urea nitrogen (BUN), serum creatinine, and uric acid. General well-being was assessed as a secondary parameter by a quality of life (QQOL) questionnaire on a subjective scale of 1–10.
Results
Oral ingestion of probiotics (90 billion colony forming units [CFUs]/day) was well tolerated and safe during the entire trial period at all sites. BUN levels decreased in 29 patients (63%, P<0.05), creatinine levels decreased in 20 patients (43%, no statistical significance), and uric acid levels decreased in 15 patients (33%, no statistical significance). Almost all subjects expressed a perceived substantial overall improvement in QOL (86%, P<0.05).
Conclusion
The main outcomes of this preliminary trial include a significant reduction of BUN, enhanced well-being, and absence of serious adverse effects, thus supporting the use of the chosen probiotic formulation for bowel-based toxic solute extraction. QOL and BUN levels showed statistically significant differences in outcome (P<0.05) between placebo and probiotic treatment periods at all four sites (46 patients). A major limitation of this trial is the small sample size nd elated inconsistencies.
Similar content being viewed by others
References
Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food London, Ontario, Canada, April 30–May 1, 2002.
Sherman M. Probiotics and microflora. US Pharmacist. 2009;34:42–44.
Lee Y-K, Salminen S. The coming of age of probiotics. TIFST. 1995;6:241–245.
Murthy M. Delineation of beneficial characteristics of effective probiotics. JAMA. 2000;3:38–43.
Reddy SB. Possible mechanisms by which proand prebiotics influence colon carcinogenesis and tumor growth. J Nutr. 1999;129(7 suppl.):1478S–1482S.
USRDS 2009 Annual Data Report. United States Renal Data System web site. Available at: www.usrds.org/adr.htm. Accessed November 1, 2009.
Ayodele OE, Alebiosu CO. Burden of chronic kidney disease: an international perspective. Adv Chronic Kidney Dis. 2010;17:215–224.
Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins: classification, concentration, and inter-individual variability. Kidney Int. 2003;63:1934–1943.
Ranganathan N. Probiotic dietary supplementation in patients with stage III and IV chronic kidney disease: a 6-month pilot scale trial in Canada. Curr Med Res Opin. 2009;25:1919–1930.
Drasar BS, Roberts AK. Chapter 3: Control of the large bowel bowel microflora. The adult climax microflora. In: Human Microbial Ecology. Hill MJ, Marsh PD, editors. Boca Raton, FL: CRC Press, Inc.; 1990:93–103.
Reuter G. Lactobacilli and Bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbial. 2001;2:43–53.
Stig Benchmark: reviews - immunomodulation by pro- and prebiotics. Japan Bifidus Foundation. 2001;120:9–18.
Sparks RE. Review of gastrointestinal perfusion in the treatment of uremia. Clin Nephrol. 1979;2:81–85.
Fuller R. Probiotics in human medicine. Gut. 1991;32:439–442.
Speck ML. Contributions of microorganisms to foods and nutrition. Nutr News. 1975;38:13.
Alm L. The effect of Lactobacillus acidophilus administration upon survival of salmonella in randomly selected human carriers. Prog Ed Nutr Sci. 1983;7:13–17.
Clements ML. Exogenous lactobacilli fed to mantheir fate and ability to prevent diarrheal disease. Prog Ed Nutr Sci. 1983;7:29–37.
Barbero GJ, Runge G, Fischer D, Crawford MN, Torres FE, Gyorgy P. Investigations of bacterial flora, pH and sugar content in the intestinal tract of infants. J Pediat. 1952;40:152–163.
Mata LJ. Intestinal colonization of breast-fed children in a rural area of low socioeconomic level. Ann N Y Acad Sci. 1971;176:93–109.
Wynder EL. Colon cancer prevention. Cancer. 1977;40:2565–2571.
Donaldson RM. Normal bacterial populations of the intestine and their relation to intestinal function. N Engl J Med. 1964;270:1050–1056.
Shahani KM, Ayebo AD. Role of dietary lactobacilli in gastrointestinal microecology. Am J Clin Nutr. 1980;32:2448–2457.
Gilliland SE. Antagonistic action of Lactobacillus acidophilus toward intestinal and food borne pathogens in associative cultures. J Food Prot. 1997;40:820–823.
Sherwood L, Nahas L. Lerner PI, Weinstein L. Studies of intestinal microflora I: effects of diet, age, and periodic sampling on numbers of fecal microorganisms in man. Gastroenterology. 1967;53:845–855.
Costerton JW, Rozee KR, Cheng KJ. Colonization of particulates, mucous, and intestinal tissue. Prog Ed Nutr Sci. 1983;7:91–105.
Tasovac B, Kocic A. Lactobacillus acidophilus flora and its effect in preventing infant entercolitis. Srp Arh Celok Lek. 1970;98:2019–2028. Article in Serbian
Kalouod H, Stogmann W. Clinical experience with a Bifidus milk feed. Arch Kinderheilk. 1968;177:29–35.
Mayer JB. Moglichkeiten einer phsiologischen antiviotischem therapie beim Saugling mit Bacterium bifidum (Lactobacillus Bifidus). Mschr Kinderheilk. 1966;114:67–73.
Mayer JB. Interrelationships between diet, intestinal flora and viruses. Phys Med Rehab. 1969;10:16–23.
Reyed MR. The Role of Bifidobacteria in health. Res J Med Med Sci. 2007;2:14–27.
Schauss AG. Lactobacillus acidophilus: Methods of action, clinical application, and toxicity data. J Adv Med. 1990;3:163–178.
Simon GL, Gorbach SL. Intestinal flora in health and disease. In: Physiology of the Gastrointestinal Tract. New York: Raven Press; 1981:1361–1369.
Rasic JLJ, Kurmann JA. Bifidobacteria and their Role. Basel, Switzerland: Birkhauser Verlag; 1983.
Blom H, Mortvedt C. Anti-microbial substances produced by food associated micro-organisms. Biochem Soc Trans-Food Biotech. 1991;694–698.
Daeschel MA. Applications of bacteriocins in food systems. In: Biotechnology and Food Safety. Boston, MA: Butterworth-Heinemann; 1990:91–104.
Shanai KM. Natural antibiotic activity of Lactobacillus acidophilus and Bulagricus II. Isolation of acidophilin from L. acidophilus. Cult Dairy Prod J. 1977;12:8.
Vincent JG, Veomett RC, Riley RF. Antibacterial activity associated with Lactobacillus acidophilus. J Bacteriol. 1959;78:447–484.
Sabine D. An antibiotic-like effect of lactobacillus acidophilus. Nature. 1963;199:811.
Dahiya RS, Speck ML. Hydrogen peroxide formation by Lactobacilli and its effect on Staphylococcus aureus. J Dairy Sci. 1968;51:1568–1572.
Wheater DM. Lactobacillin, an antibiotic from Lactobacilli. Nature. 1951;168:659.
Dunn S, Simenhoff M, Ahmed K, et al. Effects of oral administration of freeze-dried lactobacillus acidophilus on small bowel bacterial overgrowth in patients with end stage kidney disease: Reducing uremic toxins and Improving Nutrition. Int Dairy J. 1998;8:454–553.
Niwa T. Phenol and p-Cresol accumulated in uremic serum measured by HPLC with fluorescence detection. Clin Chem. 1993;39:108–111.
Meydani SM, Ha W. Immunologic effects of yogurt. Am J Clin Nutr. 2000;71:861–872.
Ranganathan N, Patel BG, Ranganathan P, et al. In vitro and in vivo assessment of intraintestinal bacteriotherapy in chronic kidney disease. ASAIO J. 2006;52:70–79
Ranganathan N, Patel B, Ranganathan P, et al. Probiotic amerlioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. Sci World J. 2005;5:652–660.
Patel B, Marczely J, Ranganathan N, Handa R, Willis LR, Friedman EA. Gut-based uremia therapy: oral bacteriotherapy effectively reduces severity of azotemia in 5/6th nephrectomized mini pigs. Presented at: International Society of Nephrology Conference on Prevention of Progression of Renal Disease, Hong Kong, June 2004. Poster #72111.
Palmquist R. A preliminary clinical evaluation of Kibow Biotics, a probiotic agent, on feline azotemia. J Am Vet Med Assoc. 2006;2:23–27.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
About this article
Cite this article
Ranganathan, N., Ranganathan, P., Friedman, E.A. et al. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Therapy 27, 634–647 (2010). https://doi.org/10.1007/s12325-010-0059-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-010-0059-9