Abstract
Purpose
Fractionated peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE is increasingly applied as an effective treatment for patients with disseminated neuroendocrine tumors. In parallel to dose planning before external beam radiation therapy, dosimetry is also needed to optimize PRRT to the individual patient. Accordingly, absorbed doses to organs at risk need to be calculated during PRRT, based on serial measurements of radioactivity distribution utilizing SPECT/CT. The dosimetry should be based on as few measurements as possible, while still retaining reliable results. The main aim of the present work was to calculate the fractional contribution of the extrapolations of the curve fits for the absorbed dose calculations to the kidneys. The secondary aim was to study agreement between absorbed dose (AD) and the effective half-life (teff) for the kidneys, estimated by means of measurements at one or two time points, in comparison to our current method employing three time points.
Methods
In 777 patients with disseminated neuroendocrine tumors undergoing PRRT, SPECT/CT over the abdomen was acquired at 1, 4, and 7 days after 177Lu-DOTATATE infusion. The absorbed dose to the kidneys was calculated from SPECT/CT radioactivity distribution data, and the teff and fractional contributions of the extrapolations were estimated, utilizing data from one, two, and three time points, respectively.
Results
The fractional contributions from extrapolations before day 1 measurement and after day 7 measurement were approximately 26% and 11%, respectively. The mean differences in absorbed dose, based on one, two, and three time points were small, but with high method dependence for individual patients. The differences in estimated teff were small when it was based on measurements at days 1 and 7, but high for days 1 and 4 time points.
Conclusion
When assessing simplifications of methods for calculation of the absorbed dose to the kidneys, it was of the uttermost importance to incorporate the fractional contribution for the extrapolations included in the reference method. Measurements at an early and a late time point were found most important. An intermediate measurement contributes with an idea of the goodness of the fit.
Similar content being viewed by others
Introduction
During the last decade, peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTA-D-Phe1-Tyr3-octreotate (177Lu-DOTATATE) has evolved as an effective treatment option for patients with disseminated somatostatin receptor positive neuroendocrine tumors (NET) [1,2,3,4,5,6,7,8]. In external beam radiation therapy, dose planning ensures that the target receives a predefined absorbed dose without inducing severe side effects on surrounding tissues. It is also well established that a higher absorbed dose on a tissue introduces a larger damage on the tissue, even if the response is not linear and with possible thresholds both for normal organ and tumor response. There is no evidence that this would not be advantageous also in the setting of targeted radiotherapy, where the radiation originates from a radiotracer. On the contrary, accumulating scientific evidence supports such a dose-response relationship in PRRT. For example, Pauwels et al. [9] showed a correlation between the absorbed dose and tumor shrinkage for PRRT with 90Y-DOTATOC, and for treatment with 177Lu-DOTATATE, Ilan et al. [10] showed a good correlation between absorbed dose and shrinkage of pancreatic neuroendocrine tumors, and Jahn et al. [11] showed a significant correlation between the injected activity and tumor shrinkage for small intestinal neuroendocrine tumors.
The current standard in 177Lu-DOTATATE therapy, based on the original Rotterdam protocol, is to administer four 7.4 GBq cycles (29.6 GBq in total), which is considered safe for the organs at risk in the majority of patients. Moreover, in recent 177Lu-DOTATATE trials, only occasional patients have experienced significant renal toxicity (grades 3–4) [12]. This may be illustrated by a dosimetry-tailored dose escalation study in 200 patients receiving 22.2–74 GBq [8] and a study in 74 patients receiving 14.8–37.8 GBq [13], in both of which only one patient showed renal toxicity grades 3–4. The administered activity to the individual patient could therefore most probably be increased in order to increase the absorbed dose to the tumors. This is also supported by the results in 51 patients who received median 5 (range 3–7) cycles of 7.4 GBq 177Lu-DOTATATE up to 27 Gy biological effective dose (BED) to the kidneys and in 5 patients in whom this was further increased to 40 Gy BED, allowing for administering median 7 (range 5–8) cycles [14]. GFR decreased in most patients, but no grades 3–4 renal toxicity were observed in this study. Similarly, this is supported by the results in a quite recent Canadian study by Del Prete et al. [15]. Neither salvage treatment, administering two additional PRRT cycles up to 60.5 GBq cumulative activity, shown any increase in kidney and bone marrow-related side effects [16]. However, there are no published data for the patient outcome and radiation-related side effects for dosimetry-guided PRRT, allowing for > 4 initial cycles, in comparison to a treatment-re-treatment regimen. Neither is it known how the PRRT protocol is best adapted to benefit the outcome for the different types of NETs. It is obvious that the once established 23 Gy upper limit for absorbed dose to the kidneys is too low, and in order to establish the true upper limit, dosimetry-guided PRRT is needed. By taking advantage of quantitative imaging, this would not only allow for tailoring the thresholds for normal organs, but also to perform tumor dosimetry and optimize the absorbed dose to tumor tissue, and thereby provides possibilities to individualize the subsequent treatment cycles.
Although the kidney toxicity with 177Lu-DOTATATE is less than for 90Y-labeled preparations [1, 17, 18], as pointed out above, the maximum tolerable absorbed dose remains to be defined [4, 19], and a reliable and preferably not too extensive dosimetry is required for the individual patient. For PRRT with 177Lu-DOTATATE, calculation of the absorbed dose is based on the activity distribution and kinetics over time for the relevant organs and tissues, generally obtained by gamma camera measurements repeated over time. To calculate the entire time course of activity distribution from injection time out to infinity, interpolations between the measurements and extrapolations before the first and after the last measurement points are required. To avoid errors in time-integrated activity and absorbed dose keeping these errors as small as possible, the area under the curve of the extrapolations should be as small as possible and preferably include less than 20% of the decays in the volume [20]. This is important from two perspectives. The first is that large extrapolations of a constant function in a curve fit increases the uncertainty. The second is that we cannot presume that the biological conditions, affecting the biodistribution, are the same over time.
Because of logistical and financial reasons and for patient comfort, estimation of absorbed doses should be performed with as few measurements as possible, while still achieving reliable results of the absorbed dose calculations. A clinically applicable and robust dosimetry protocol for solid organs, based on 3D imaging by SPECT, has been developed and applied in our center since 2005 [21,22,23].
The main aim of the present work was to calculate the fractional contributions of the extrapolations of the curve fits for the absorbed dose calculations to the kidneys. The secondary aim was to study how well kidney-absorbed dose and effective half-life (teff) estimations, using methods based on measurements at one or two time points, agree with the current method employing three time points.
Materials and methods
Patients
In this retrospective study, 777 patients (333 females and 444 males) with metastatic somatostatin receptor-positive neuroendocrine tumors treated with 177Lu-DOTATATE were included, and all of them met previously described inclusion criteria [21]. Dosimetry on these patients was performed during the years 2006 to 2019. Both the left and the right kidneys were included in the analysis, with the exception of the right kidney in 12 patients and the left kidney in 11 patients, in whom the kidneys had been resected or had very impaired function.
177LuCl3 was purchased from IDB Radiopharmacy bv, Baarle-Nassau, The Netherlands, and DOTATATE was a generous gift from Erasmus Medical Centre, Rotterdam, The Netherlands.
Compliance with ethical standards
The study received no external funding, and all authors declare no conflict of interest.
Since September 2010, all patients were included into a prospective study (EudraCT no. 2009-012260-14) approved by the Regional Ethical Review Board in Uppsala. Before that, from 2005, the patients were admitted on a single-patient basis for compassionate use with individual permission from the Swedish Medical Products Agency. All patients gave their written informed consent before study inclusion.
Image acquisition
All 777 patients underwent SPECT/CT of the abdomen 1, 4, and 7 days after administration of the first cycle of 7.4 GBq 177Lu-DOTATATE. For the first 69 patients, imaging was performed on a Hawkeye Millennium VG (GE Healthcare) dual-head camera equipped with 5/8” NaI(Tl) crystals and MEGP (medium energy general purpose) collimators. A 20% energy window around the 2 dominant γ-ray energies of 177Lu, 113.0 and 208.4 keV, was applied. SPECT/CT, applying 60 frames with a 60-s exposure time per frame (total acquisition time for SPECT is then 30 min), was performed over the upper abdomen including organs at risk (kidneys, liver, and spleen). In the next 400 patients, imaging was performed on an Infinia (International General Electric, General Electric Medical Systems, Haifa, Israel) dual-headed gamma camera with 3/8” NaI(Tl)-crystals equipped with MEGP collimators. The measurements employed a 20% energy window around the dominant 208.4 keV gamma ray energy of 177Lu. SPECT/CT of the upper abdomen included the organs at risk (kidneys, liver, and spleen), applying 120 frames with a 30-s exposure time per frame. In the last 308 patients, SPECT/CT was performed on a Discovery 670 PRO (International General Electric, General Electric Medical Systems, Haifa, Israel) dual-headed gamma camera with 3/8” NaI(Tl)-crystals equipped with MEGP collimators with the same settings as for the Infinia. For reconstruction, the ordered subset expectation maximization (OSEM) algorithm included in the Xeleris 3.0 workstation (International General Electric, General Electric Medical Systems, Haifa, Israel) was used with previously determined default settings (iterative reconstruction with eight subsets and four iterations followed by a Hann filtering with a cutoff of 0.85). In all the systems above, the images were attenuation corrected with the concomitantly acquired CT-based attenuation map but were not corrected for scatter, collimator/response, or PVE. The small VOI method is sensitive to artefacts, and because of the Gibbs artefacts, no collimator response/resolution recovery was included. Scatter correction was also omitted since the only available methods for us are the triple or dual energy window methods, which for us have generated more problems than they have solved.
Absorbed dose calculations
All volumes of interests (VOIs) were defined using in-house-developed software within the Hermes platform on a Hermes HNAC workstation with the Gold 2.9 software (HERMES, Stockholm, Sweden).
In the SPECT images, small spherical volumes of interests (VOIs; 4 ml) were placed in both kidneys to include the renal cortex as described previously [21]. Activity concentrations were determined for each time point (1, 4, and 7 days after 177Lu-administration), and time-integrated activity concentration was calculated as the area under the curve of a single exponential fit (from infusion start to infinity) to the time-activity concentration curve (A(t)).
In the MIRD 21 pamphlet [24], the mean absorbed dose D(rT,TD) to a target structure rT in the time period from time 0 to time TD is defined as:
where A(rS,t) is the activity of the radiopharmaceutical in source tissue rS at time t, and S(rT ← rS,t) is the radionuclide-specific quantity representing the mean absorbed dose rate to target tissue rT at time t after administration per unit activity present in source tissue rS.
It has been shown [25] that the absorbed dose from surrounding organs to kidneys in therapy with 177Lu-DOTATATE does not add much to the absorbed dose. This general formula can be rewritten to only include the absorbed dose originating from the target structure itself:
To simplify further, Eq. 2 can be rewritten again to work with concentrations instead.
where C(rS,t) is the activity concentration of the radiopharmaceutical in target tissue rS at time t, and ACDF(rT ← rT,t) (activity concentration dose factor) is the radionuclide-specific quantity representing the mean absorbed dose rate to target tissue rT at time t after administration per unit activity concentration present in target tissue rT. ACDF is a multiplication of S-factor with the volume for the S-factor. The ACDF does not change much with the volume, and using dose factors (DF) from the spherical model in OLINDA [26] gives an ACDF of 86.0 mGy*g/MBq*h for a 100-g sphere and 86.7 mGy*g/MBq*h for a 300-g sphere leading to a difference of less than 1%.
Calculating the time-integrated activity concentration (\( \overset{\sim }{C} \)) from time of administration to infinity and assuming a density of 1 means that the final equation for calculation of the absorbed dose to the kidneys ends up with a simple multiplication:
This procedure has previously been described in more detail in the following references [21, 23].
Fractional contributions
For the absorbed dose to the kidneys, several fractional contributions (f) (Eq. 5) were calculated for each therapy cycle of 7.4 GBq.
In this equation, the fractional contribution (f) is defined as the area under the curve of the expression (C(t)) between the time of the first measurement point (t1) and the time of the last measurement point (t2) divided by the total area under the curve from the start time (ts) to the end time (te). The expression (C(t)) is in this case the single exponential fit to the measurements of radioactivity concentration in the kidneys.
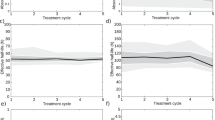
The following fractional contributions were calculated on the single exponential curve fit on days 1, 4, and 7 measurements for the right and left kidney: (fc0-24) the extrapolated portion of the curve from time = 0 (start of 177Lu-DOTATATE infusion) to the measurement at day 1, (fc168-∞) the portion of the curve after day 7 measurement (to infinity), (fc0-24+168-∞) the sum of fc0-24 and fc168-∞, (fc96-∞) the portion of the curve after day 4 measurement (to infinity), and (fc0-24+96-∞) the sum of fc0-24 and fc96-∞; each of these extrapolations were calculated as a fraction of the total area under the curve from time zero to infinity. In addition, in the light of the reports of Guerriero et al. [27] and Delker et al. [28], indicating that there is a short rapid washout phase with elimination of radioactivity from the kidneys, chiefly affecting the first hours of the time activity curve but with a small influence present after 8 h, we also calculated (fc0-8) the fractional contribution of the first 8 h to the total area under the curve. Examples of all these fractional contributions are shown on a typical curve for the kidney in Fig. 1.
Examples of fractional contributions on a typical curve for the kidney for a (fc0-24) the extrapolated portion of the curve from time = 0 to the measurement at day 1, b (fc168-∞) the portion of the curve after the day 7 measurement (to infinity), c (fc0-24 + 168-∞) the sum of fc0-24 and fc168-∞, d (fc96-∞) the portion of the curve after the day 4 measurement (to infinity), e (fc0-24 + 96-∞) the sum of fc0-24 and fc96-∞, and (fc0-8) the fractional contribution of the first 8 h
Simplification of the measurements
As a standard, absorbed dose (AD147) and teff (teff147) are calculated using single exponential fit to data from 3 measurements 1, 4, and 7 days after start of the therapy. As a first attempt to simplify the dosimetry measurements, two calculations of the absorbed dose (AD14 and AD17) and teff (teff14 and teff17) were performed, using the single exponential functions crossing only two points (1 and 4 days, and 1 and 7 days, respectively). In addition, absorbed doses were calculated using a single measurement (at 4 days), assuming the median of our 777 patients teff 52 h (AD4/52) and using Eq. 7 (adapted to activity concentration) in the paper by Hänscheid et al. [29] (AD4/H). Since the standard method in the paper by Hänscheid et al. [29] is based on 2D measurements ending at day 4, we also performed a comparison between AD14 versus AD4/52 and AD4/H, based on the 3D measurements from our cohort of 777 patients.
Statistical methods
Bland-Altman analyses for absorbed dose and teff values were performed for the comparisons between the different simplified methods, detailed above, relative to those based on the three-point measurement. Bland-Altman analyses of absorbed dose values relative to those based on days 1 and 4 measurement were calculated for the one time point method. Median, minimum, and maximum of the results were also calculated.
Results
Fractional contributions
The contributions to the absorbed dose to the left kidneys, as a fraction of the total absorbed dose from infusion start to infinity, are presented as a box-whiskers plot in Fig. 2. For the left kidneys, the extrapolation before the measurement at day 1 generally represented a little more than 25% of the absorbed dose, while the extrapolation after the measurement at day 7 contributed approximately 10%. This means that the total fractional contribution of extrapolations for the reference method was a little more than 35%. However, about 10% originates from the extrapolation during the first 8 h, during which the uptake and the 1st rapid elimination phase occur. The fractional contribution before day 1 and after day 4 was about 60%. For both the right and left kidneys, the fractional contributions from start to infinity are shown in Table 1. The difference in fractional contribution between the right and left kidney was generally small, even if they sometimes exceeded 20% in the individual patients. This small difference between the results in the right and left kidneys would not affect the conclusions.
Absorbed dose
The absorbed dose to both the left and right kidneys, calculated with our standard method, is presented as histograms in Fig. 3. The results of the absorbed doses for the right and left kidneys are seen to be similar. During each 7.4 GBq PRRT cycle, the median absorbed doses were approximately 4 Gy, with a range from less than 1 Gy up to more than 10 Gy. The calculated median and range for all five methods are presented in Table 2.
Comparison of the simplified absorbed dose calculations
Bland-Altman plots of the agreement between the simplified methods and our standard method are presented in Fig. 4. Differences between the simplified methods and our standard method are also summarized in Table 3 showing the median and range absorbed dose calculated by each method. The difference between methods was in general quite small (merely a few percent) although individual values varied considerably. For both AD14 and AD17, the differences were in general less than 20% but occasionally up to 50% and 30% for AD14 and AD17, respectively. For the methods employing only one measurement point (AD4/52 and AD4/H), the overestimation of the absorbed dose was generally less than 10% but was occasionally as high as 30% while the underestimations were greater, usually less than 20% but sometimes 40%, or even 50% in some cases.
The single time point methods (AD4/52 and AD4/H) are compared to the method excluding the day 7 measurement (AD14) in the Bland-Altman plot in Fig. 5. Numerical values of the differences between AD14 versus AD4/52 and AD4/H are presented as median and range in Table 3. The AD4/52 and AD4/H did not overestimate the absorbed dose versus AD14 by more than a few percent (< 6%). However, the underestimates were occasionally 40% and in rare cases as much as 60%.
Effective half-life
Histograms of the estimated effective half-life teff147, for the left and right kidneys, are presented in Fig. 6. A median value of 52 h was observed for both the left and right kidneys. In 90% of the patients, the teff147 values ranged from 41 to 68 h for the left kidneys and 41 to 75 h for the right kidneys. However, in 5% of patients (both kidneys), teff147 was lower than 41 h and occasionally as low as 30 h, and also, in 5% of the patients, teff147 exceeded 68 h for the left kidney and 75 h for the right kidney, and sometimes reached almost 100 h.
The results of the teff17 were almost the same as teff147, differing less than 0.5 h in more than 90% of the kidneys. The only exception was the maximum value for the right kidney that in a single case exceeded 100 h.
The teff14 showed a median of 48 h with a range from 20 h to the physical half-life of 6.7 days, and in 90% of the observed patients, the teff14 ranged 34 to 69 h for the left kidneys and 35 to 73 h for the right kidneys.
Comparison of the simplified effective half-life calculations
Figure 7 shows a Bland-Altman plot comparing teff147 versus teff14 and teff17 for the left kidney. In Table 3, the numerical values for both left and right kidneys are presented as median (range).
For both the left and right kidney, the median difference between teff147 and teff17 was 0%, in more than 90% of the kidneys less than ± 0.5%, and all within ± 10%.
The median difference between teff147 and teff14 was − 9% for both the left and right kidneys, and in 90% of the patients, the difference ranged from − 27 to 11% and − 29 to 14% for the left and right kidneys, respectively. However, in 5% of the patients, the difference between teff147 and teff14 was larger and ranged from − 27 to − 79% and − 29 to − 88% for the left and right kidneys, respectively. In 5% of the patients, these differences were larger still and ranged from 10 to 89% and 14 to 66% for the left and right kidneys, respectively.
Discussion
In this study, both extrapolations and simplifications in kidney dosimetry during 177Lu-DOTATATE therapy were analyzed. To ensure reliable results, extrapolations should be as small as possible and preferably not exceed 20% of the total decays in the volume [20]. In this study, the extrapolations for the time period following day 7 measurement were fairly low and present no problem in compliance with these criteria. This was not the case for the extrapolation before day 1 measurement, corresponding to approximately 25% of the total dose from time zero to infinity. Thus, it is of importance to consider the dose delivered in the early phase during the first hours. Even if the extrapolation between 8 h and 1 day represents more than 10%, this does generally not exceed 20%. Furthermore, reduction of the extrapolation time would involve SPECT/CT imaging during nighttime, which is not feasible in the clinical daily practice. The total approximately 30% extrapolation is not optimal but challenging to reduce. Further investigations regarding the effect of early time point measurements on the absorbed dose calculation are warranted. The objective is to simplify the dosimetry procedures as much as possible, mainly by reducing the number of measurements, without jeopardizing the accuracy of the absorbed dose calculations. Hänscheid et al. [29] in their paper concluded that the absorbed dose can be deduced with reasonable accuracy from a single measurement 4 days after the administration. They also concluded that deviations from the monoexponential function may introduce additional errors. Their study cohort consisted of 29 patients who underwent scintigraphy by planar imaging up to 4 days after activity administration. In the present study, we analyzed our data from 777 patients who had undergone SPECT/CT imaging at three time points at 1, 4, and 7 days following 177Lu-DOTATE infusion and compared these results with those based on measurements at a single time point, according to Hänscheid et al. For a more thorough assessment of the differences between dosimetry protocols, we compared the proposed single time point method not only to our standard three time point protocol, AD147, but also to one that excludes the last time point, AD14. This resulted in an extrapolation after day 4 measurement point of about 30% and a total extrapolation of about 50% for the AD14 method, which is considerably more than the accepted 20%. Consequently, with a simplified absorbed dose calculation, it is crucial to determine the extrapolations in the reference method because the effect of using a reference with large extrapolations will be several unaccounted uncertainties. The absorbed doses in kidneys in our study were in good agreement with those reported by others [30]. All the tested methods for kidney dosimetry produced a median difference of 5.2% or less, an entirely acceptable difference in absorbed dose calculations in PRRT. However, the problem is that since PRRT is a radiation treatment, a good agreement on average is not sufficient to determine whether the accuracy is good enough. Thus, the absorbed dose calculated for an individual patient must yield the same result independent of the method applied. To comply with this requirement, the range of differences between the methods must be low. With our standard method, AD147 and the AD17 protocol, the differences were generally less than 20%, but in occasional cases nearly 30%. Although this may be considered sufficient, one must question if it is indeed good enough in the specific context of targeted radiotherapy whereby the amount of administered activity is tailored for the individual patient [8, 31].
Further, if the teff will be used to perform dosimetry based on one SPECT/CT at 24 h for subsequent treatments, according to the method of Garske et al. [31], then, the accuracy of teff is also highly important to avoid increased errors in the absorbed dose estimations in the subsequent treatment cycles. As expected, teff17 agreed well with teff147, indicating that simplification of kidney dosimetry by means of a two time points (1 and 7 days) SPECT/CT protocol is feasible. The differences in absorbed doses between the AD14 and AD147 protocols were in general less than 20%. However, in individual patients, this difference was 30% and occasionally as high as 50% for the left kidneys and 70% for the right kidneys. The differences in teff between protocols was much larger and was as high as ± 90% when teff147 and teff14 was compared, making the latter protocol unreliable. The differences between the two single time point methods (AD4/52 and AD4/H) versus AD147 were found too high to be recommended, according to the calculations based on our data in the present patient cohort. Because this contradicted the results of an earlier study [29], we further investigated the difference between these one-point methods (AD4/52 and AD4/H) and the simplified AD14 method, based on SPECT/CT at two time points. It was particularly interesting to note that the AD4/52 and AD4/H protocols never overestimated the absorbed dose by more than approximately 6%, as compared to the AD14 method. The underestimation for AD4/52 and AD4/H was generally less than 30%, which may explain the favorable results reported in a relatively small sample of patients using planar imaging [29]. However, with less extrapolation, SPECT/CT measurements, and the considerably larger number of patients in the present study, these methods yielded too uncertain results. There may be several reasons for our deviating results, as compared to those reported previously [29]. It is reasonable that data from measurements at only one time point will introduce uncertainties in the calculations. Another factor, pointed out by Hänscheid et al. [29], is that deviations from the monoexponential decay in the slow elimination phase from the kidneys may be encountered. Notably, no evaluation of the uncertainties for these data was performed, since much data needed on the early measurements were not available. Thus, the results of the method comparisons need to be further assessed including an analysis of the uncertainties of the methods. Further, the true value of teff may in some patients be below 29 h or above 96 h. Probably, also other factors may impact the calculations. The uptake kinetics before the day 1 measurement is hitherto less studied and needs to be further explored to improve our knowledge regarding this early phase/phases.
Conclusion
When simplifying the dosimetry protocol for estimation of the absorbed dose to the kidneys, it was of the uttermost importance to incorporate a calculation of the fractional contribution for the extrapolations included in the reference method. Calculations of the absorbed dose based on measurements at only one time point were unreliable. Measurements at an early and a late time point produce more reliable results, and an intermediate measurement is preferable to get an idea of the goodness of the fit.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 177Lu-DOTATATE:
-
177Lu-DOTA-D-Phe1-Tyr3-octreotate
- ACDF:
-
Activity concentration dose factor
- AD:
-
Absorbed dose
- BED:
-
Biological effective dose
- DF:
-
Dose factor
- MEGP:
-
Medium energy general purpose
- NET:
-
Neuroendocrine tumor
- OSEM:
-
Ordered subset expectation maximization
- PRRT:
-
Peptide receptor radionuclide therapy
- t eff :
-
Effective half-life
- VOI:
-
Volume of interest
References
Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–23. https://doi.org/10.1200/JCO.2010.33.7873.
Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with (1)Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–35. https://doi.org/10.1007/s00259-011-1902-1.
Gabriel M, Andergassen U, Putzer D, Kroiss A, Waitz D, Von Guggenberg E, et al. Individualized peptide-related-radionuclide-therapy concept using different radiolabelled somatostatin analogs in advanced cancer patients. Q J Nucl Med Mol Imaging. 2010;54:92–9.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30.
Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754–62.
Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with (1)(7)(7)Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–35. https://doi.org/10.1007/s00259-011-1902-1.
Bodei L, Cremonesi M, Grana C, Rocca P, Bartolomei M, Chinol M, et al. Receptor radionuclide therapy with 90Y-[DOTA]0-Tyr3-octreotide (90Y-DOTATOC) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2004;31:1038–46. https://doi.org/10.1007/s00259-004-1571-4.
Garske-Roman U, Sandstrom M, Fross Baron K, Lundin L, Hellman P, Welin S, et al. Prospective observational study of (177)Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging. 2018;45:970–88. https://doi.org/10.1007/s00259-018-3945-z.
Pauwels S, Barone R, Walrand S, Borson-Chazot F, Valkema R, Kvols LK, et al. Practical dosimetry of peptide receptor radionuclide therapy with (90)Y-labeled somatostatin analogs. J Nucl Med. 2005;46(Suppl 1):92S–8S.
Ilan E, Sandstrom M, Wassberg C, Sundin A, Garske-Roman U, Eriksson B, et al. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J Nucl Med. 2015;56:177–82. https://doi.org/10.2967/jnumed.114.148437.
Jahn U, Ilan E, Sandstrom M, Garske-Roman U, Lubberink M, Sundin A. 177Lu-DOTATATE peptide receptor radionuclide therapy: dose response in small intestinal neuroendocrine tumors. Neuroendocrinology. 2019. https://doi.org/10.1159/000504001.
Bergsma H, Konijnenberg MW, van der Zwan WA, Kam BL, Teunissen JJ, Kooij PP, et al. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging. 2016;43:1802–11. https://doi.org/10.1007/s00259-016-3382-9.
Sabet A, Ezziddin K, Pape UF, Reichman K, Haslerud T, Ahmadzadehfar H, et al. Accurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with (177)Lu-octreotate. Eur J Nucl Med Mol Imaging. 2014;41:505–10. https://doi.org/10.1007/s00259-013-2601-x.
Sundlov A, Sjogreen-Gleisner K, Svensson J, Ljungberg M, Olsson T, Bernhardt P, et al. Individualised (177)Lu-DOTATATE treatment of neuroendocrine tumours based on kidney dosimetry. Eur J Nucl Med Mol Imaging. 2017;44:1480–9. https://doi.org/10.1007/s00259-017-3678-4.
Del Prete M, Buteau FA, Arsenault F, Saighi N, Bouchard LO, Beaulieu A, et al. Personalized (177)Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: initial results from the P-PRRT trial. Eur J Nucl Med Mol Imaging. 2019;46:728–42. https://doi.org/10.1007/s00259-018-4209-7.
van der Zwan WA, Brabander T, Kam BLR, Teunissen JJM, Feelders RA, Hofland J, et al. Salvage peptide receptor radionuclide therapy with [(177)Lu-DOTA,Tyr(3)]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2019;46:704-717. doi:https://doi.org/10.1007/s00259-018-4158-1.
Barone R, Borson-Chazot F, Valkema R, Walrand S, Chauvin F, Gogou L, et al. Patient-specific dosimetry in predicting renal toxicity with (90)Y-DOTATOC: relevance of kidney volume and dose rate in finding a dose-effect relationship. J Nucl Med. 2005;46(Suppl 1):99S–106S.
Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging. 2008;35:1847–56. https://doi.org/10.1007/s00259-008-0778-1.
Cremonesi M, Botta F, Di Dia A, Ferrari M, Bodei L, De Cicco C, et al. Dosimetry for treatment with radiolabelled somatostatin analogues. A review. Q J Nucl Med Mol Imaging. 2010;54:37–51.
Hindorf C, Glatting G, Chiesa C, Linden O, Flux G, Committee ED. EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry. Eur J Nucl Med Mol Imaging. 2010;37:1238–50. https://doi.org/10.1007/s00259-010-1422-4.
Sandstrom M, Garske U, Granberg D, Sundin A, Lundqvist H. Individualized dosimetry in patients undergoing therapy with (177)Lu-DOTA-D-Phe (1)-Tyr (3)-octreotate. Eur J Nucl Med Mol Imaging. 2010;37:212–25. https://doi.org/10.1007/s00259-009-1216-8.
Sandstrom M, Garske-Roman U, Granberg D, Johansson S, Widstrom C, Eriksson B, et al. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med. 2013;54:33–41. https://doi.org/10.2967/jnumed.112.107524.
Sandstrom M, Ilan E, Karlberg A, Johansson S, Freedman N, Garske-Roman U. Method dependence, observer variability and kidney volumes in radiation dosimetry of (177)Lu-DOTATATE therapy in patients with neuroendocrine tumours. EJNMMI Phys. 2015;2:24. https://doi.org/10.1186/s40658-015-0127-y.
Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet No. 21: a generalized schema for radiopharmaceutical dosimetry--standardization of nomenclature. J Nucl Med. 2009;50:477–84. https://doi.org/10.2967/jnumed.108.056036.
Sandstrom M, Garske-Roman U, Johansson S, Granberg D, Sundin A, Freedman N. Kidney dosimetry during (177)Lu-DOTATATE therapy in patients with neuroendocrine tumors: aspects on calculation and tolerance. Acta Oncol. 2018;57:516–21. https://doi.org/10.1080/0284186X.2017.1378431.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
Guerriero F, Ferrari ME, Botta F, Fioroni F, Grassi E, Versari A, et al. Kidney dosimetry in (1)(7)(7)Lu and (9)(0)Y peptide receptor radionuclide therapy: influence of image timing, time-activity integration method, and risk factors. Biomed Res Int. 2013;2013:935351. https://doi.org/10.1155/2013/935351.
Delker A, Ilhan H, Zach C, Brosch J, Gildehaus FJ, Lehner S, et al. The influence of early measurements onto the estimated kidney dose in [(177)Lu][DOTA(0),Tyr(3)]octreotate peptide receptor radiotherapy of neuroendocrine tumors. Mol Imaging Biol. 2015;17:726–34. https://doi.org/10.1007/s11307-015-0839-3.
Hanscheid H, Lapa C, Buck AK, Lassmann M, Werner RA. Dose mapping after endoradiotherapy with (177)Lu-DOTATATE/DOTATOC by a single measurement after 4 days. J Nucl Med. 2018;59:75–81. https://doi.org/10.2967/jnumed.117.193706.
Cremonesi M, Ferrari M, Bodei L, Tosi G, Paganelli G. Dosimetry in peptide radionuclide receptor therapy: a review. J Nucl Med. 2006;47:1467–75.
Garske U, Sandstrom M, Johansson S, Sundin A, Granberg D, Eriksson B, et al. Minor changes in effective half-life during fractionated 177Lu-octreotate therapy. Acta Oncol. 2012;51:86–96. https://doi.org/10.3109/0284186X.2011.618511.
Acknowledgements
The authors want to thank the staff at the department of medical physics, endocrine oncology, and nuclear medicine at Uppsala University Hospital for their support.
Funding
Open Access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
MS performed the data collection, drafted the manuscript, performed the statistical analysis, and conceived the study design. NF helped with the study design and helped to draft the manuscript. KFB, TK, and AS participated in the anatomical outlining of the organs and helped with the draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
IRB approval was given. The requirement of informed consent was waived for this retrospective anonymized study.
Consent for publication
We agree.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sandström, M., Freedman, N., Fröss-Baron, K. et al. Kidney dosimetry in 777 patients during 177Lu-DOTATATE therapy: aspects on extrapolations and measurement time points. EJNMMI Phys 7, 73 (2020). https://doi.org/10.1186/s40658-020-00339-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40658-020-00339-2