Abstract
Purpose
Multiple measurements have been required to estimate the radiation dose to the kidneys resulting from [177Lu]DOTATATE therapy for neuroendocrine tumors. The aim of this study was to investigate the influence of early time-point measurement in the renal dose calculation.
Procedures
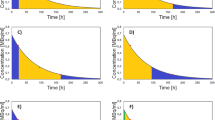
Anterior/posterior whole-body planar scintigraphy images were acquired at approx. 1, 24, 48, and 72 h after administration of [177Lu]DOTATATE. Furthermore, we acquired planar 1-bed dynamic recordings in 12 frames (5 min each) during the first hour. We assessed kidney exposure with a three-phase model consisting of a linear increase to the maximum within the initial minutes p.i., followed a bi-exponential decline. This three-phase-model served as reference for evaluating accuracy of dose estimates in 105 kidneys calculated by conventional mono-exponential fitting of the final three and four whole-body images.
Results
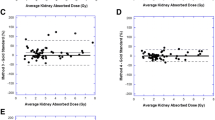
Mean effective half-life times for the reference model were 25.8 ± 12.0 min and 63.9 ± 17.6 h, predicting a mean renal dose of 5.7 ± 2.1 Gy. The effective half-life time was 46.3 ± 15.4 h for the last four and 63.3 ± 17.0 h for the last three data points. The mean start of the first whole-body measurement was 1.2 ± 0.1 h p.i. The ratio of fast to slow phases was 28.1 ± 23.9 % at this time point, which caused a mean absolute percentage dose deviation of 12.4 % for four data points, compared to 3.1 % for three data points. At a mean time of 2.4 h p.i. (max 5.1 h), the ratio of fast to slow phase declined below 5 %.
Conclusions
Kinetic analysis of renal uptake using dynamic planar scans from the first hour after injection revealed a fast and a slow washout phase. Although the fast phase did not contribute substantially to the estimated renal dose, it could influence planar measurements performed within the first hours. We found that the presence of two clearance phases can hamper accurate dose estimation based on a single-phase model, resulting in approximately 12.4 % dose underestimation, thus potentially resulting in overtreatment. In the absence of dynamic initial recordings, the first dosimetry measurements should therefore be obtained later than 3–5 h after [177Lu]DOTATATE injection. Omitting the early whole-body image reduced the dose estimation error to 3.1 %.






Similar content being viewed by others
References
Pavel M, Baudin E, Couvelard A et al (2012) ENETS consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 95:157–176
Bodei L, Mueller-Brand J, Baum RP et al (2013) The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 40:800–816
van der Zwan WA, Bodei L, Mueller-Brand J, et al. (2014) GEP-NETS update: radionuclide therapy in neuroendocrine tumors. European journal of endocrinology / European Federation of Endocrine Societies
Koch W, Auernhammer CJ, Geisler J et al (2014) Treatment with octreotide in patients with well-differentiated neuroendocrine tumors of the ileum: prognostic stratification with Ga-68-DOTA-TATE positron emission tomography. Mol Imaging 13:1–10
Bodei L, Cremonesi M, Grana CM et al (2012) Yttrium-labelled peptides for therapy of NET. Eur J Nucl Med Mol Imaging 39(Suppl 1):S93–S102
Kam BL, Teunissen JJ, Krenning EP et al (2012) Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging 39(Suppl 1):S103–S112
Konijnenberg M, Melis M, Valkema R et al (2007) Radiation dose distribution in human kidneys by octreotides in peptide receptor radionuclide therapy. J Nuclear Med : Off Public Soc Nuclear Med 48:134–142
Emami B, Lyman J, Brown A et al (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21:109–122
Larsson M, Bernhardt P, Svensson JB et al (2012) Estimation of absorbed dose to the kidneys in patients after treatment with 177Lu-octreotate: comparison between methods based on planar scintigraphy. EJNMMI Res 2:49
Breeman WA, De Jong M, Visser TJ et al (2003) Optimising conditions for radiolabelling of DOTA-peptides with 90Y, 111In and 177Lu at high specific activities. Eur J Nucl Med Mol Imaging 30:917–920
Ichihara T, Ogawa K, Motomura N et al (1993) Compton scatter compensation using the triple-energy window method for single- and dual-isotope SPECT. J Nuclear Med : Off Public Soc Nuclear Med 34:2216–2221
Fleming JS (1979) A technique for the absolute measurement of activity using a gamma camera and computer. Phys Med Biol 24:176–180
Siegel JA, Thomas SR, Stubbs JB et al (1999) MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nuclear Med : Off Public Soc Nuclear Med 40:37S–61S
Berger MJ, Hubbell JH, Seltzer SM (2010) XCOM: photon cross section database (version 1.5). http://physics.nist.gov/xcom. National Institute of Standards and Technology, Gaithersburg
Kojima A, Takaki Y, Matsumoto M et al (1993) A preliminary phantom study on a proposed model for quantification of renal planar scintigraphy. Med Phys 20:33–37
Loevinger R, Berman M (1968) A formalism for calculation of absorbed dose from radionuclides. Phys Med Biol 13:205–217
Stabin M, Siegel J, Hunt J et al (2001) RADAR: the radiation dose assessment resource. J Nucl Med 42
Williams LE, Liu A, Yamauchi DM et al (2002) The two types of correction of absorbed dose estimates for internal emitters. Cancer 94:1231–1234
Stabin MG, Xu XG, Emmons MA et al (2012) RADAR reference adult, pediatric, and pregnant female phantom series for internal and external dosimetry. J Nuclear Med : Off Public Soc Nuclear Med 53:1807–1813
Glatting G, Kletting P, Reske SN et al (2007) Choosing the optimal fit function: comparison of the Akaike information criterion and the F-test. Med Phys 34:4285–4292
Kletting P, Schimmel S, Kestler HA et al (2013) Molecular radiotherapy: the NUKFIT software for calculating the time-integrated activity coefficient. Med Phys 40:102504
Kletting P, Kull T, Reske SN, Glatting G (2009) Comparing time activity curves using the Akaike information criterion. Phys Med Biol 54:N501–N507
Guerriero F, Ferrari ME, Botta F et al (2013) Kidney dosimetry in (1)(7)(7)Lu and (9)(0)Y peptide receptor radionuclide therapy: influence of image timing, time-activity integration method, and risk factors. BioMed Res Int 2013:935351
Baechler S, Hobbs RF, Prideaux AR et al (2008) Extension of the biological effective dose to the MIRD schema and possible implications in radionuclide therapy dosimetry. Med Phys 35:1123–1134
Cremonesi M, Botta F, Di Dia A et al (2010) Dosimetry for treatment with radiolabelled somatostatin analogues. a review. Quart J Nuclear Med Molec Imag : Off Public Italian Assoc Nuclear Med 54:37–51
Garkavij M, Nickel M, Sjogreen-Gleisner K et al (2010) 177Lu-[DOTA0, Tyr3] octreotate therapy in patients with disseminated neuroendocrine tumors: analysis of dosimetry with impact on future therapeutic strategy. Cancer 116:1084–1092
Sandstrom M, Garske U, Granberg D et al (2010) Individualized dosimetry in patients undergoing therapy with (177)Lu-DOTA-D-Phe (1)-Tyr (3)-octreotate. Eur J Nucl Med Mol Imaging 37:212–225
Beauregard JM, Hofman MS, Pereira JM et al (2011) Quantitative (177)Lu SPECT (QSPECT) imaging using a commercially available SPECT/CT system. Cancer Imag : official Public Int Cancer Imag Soc 11:56–66
Sanders JC, Kuwert T, Hornegger J, Ritt P (2014) Quantitative SPECT/CT Imaging of Lu with in vivo validation in patients undergoing peptide receptor radionuclide therapy. Molec Imag Biol: MIB : Off Public Acad Molec Imag
Kwekkeboom DJ, Bakker WH, Kooij PP et al (2001) [177Lu-DOTAOTyr3]octreotate: comparison with [111In-DTPAo]octreotide in patients. Eur J Nucl Med 28:1319–1325
Wehrmann C, Senftleben S, Zachert C et al (2007) Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother Radiopharm 22:406–416
Helisch A, Forster GJ, Reber H et al (2004) Pre-therapeutic dosimetry and biodistribution of 86Y-DOTA-Phe1-Tyr3-octreotide versus 111In-pentetreotide in patients with advanced neuroendocrine tumours. Eur J Nucl Med Mol Imaging 31:1386–1392
Spiess AN, Neumeyer N (2010) An evaluation of R2 as an inadequate measure for nonlinear models in pharmacological and biochemical research: a Monte Carlo approach. BMC Pharmacol 10:6
Lassmann M, Chiesa C, Flux G et al (2011) EANM dosimetry committee guidance document: good practice of clinical dosimetry reporting. Eur J Nucl Med Mol Imaging 38:192–200
Acknowledgments
The authors would like to thank the colleagues from the Department of Nuclear Medicine for their participation in data collection. Especially, we would like to thank the nuclear medicine technicians for performing the imaging studies and the nurses of the nuclear medicine therapy ward for urine collection. We note professional editing of the manuscript provided by Inglewood Biomedical Editing.
Conflict of Interest
The authors declare that they have no conflict of interest.
Human Rights Statement
For this type of study, formal consent is not required.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delker, A., Ilhan, H., Zach, C. et al. The Influence of Early Measurements Onto the Estimated Kidney Dose in [177Lu][DOTA0,Tyr3]Octreotate Peptide Receptor Radiotherapy of Neuroendocrine Tumors. Mol Imaging Biol 17, 726–734 (2015). https://doi.org/10.1007/s11307-015-0839-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-015-0839-3