Abstract
Sr isotope ratios of hydrothermal fluids were observed at five sediment-associated sites in the Okinawa Trough to investigate the diversity of subseafloor fluid–rock–sediment interactions. The estimated 87Sr/86Sr ratios of the hydrothermal endmember fluids at the five sites were all higher than those at the sediment-starved sites. The endmember Sr isotopic ratios of hydrothermal fluids were diverse within the Okinawa Trough, ranging from 0.7077 at the Iheya North Knoll site to 0.712 at the Yonaguni Knoll IV site. To our knowledge, 0.712 is the highest value reported to date for seafloor hydrothermal fluids. This variation is likely attributable to the relative contributions of multiple subseafloor Sr reservoirs, which are 87Sr-poor volcanic rock and 87Sr-rich hemipelagic sediments containing clay minerals of terrestrial origin. These data support a model based on the carbon isotope ratio of CH4, which indicates whether volcanic rocks or terrestrial sediments are distributed in the high-temperature reaction zone of the hydrothermal system.

Key points
-
The Sr isotopic ratios of hydrothermal fluids from the Okinawa Trough are exceptionally high.
-
The enrichment of 87Sr at Yonaguni Knoll IV cannot be explained without the influence of terrestrial clay minerals in sediments.
-
High Sr isotopic ratios are a common feature of sediment-associated hydrothermal systems.
Similar content being viewed by others
1 Introduction
Deep-sea hydrothermal activity in sedimentary basins (Von Damm et al. 1985b; Butterfield et al. 1994; Kawagucci 2015; Baumberger et al. 2016) involves fluid chemistry that differs from sediment-starved site activity (Von Damm et al. 1985a; Bonatti 1975) because of hydrothermal fluid–sediment interactions. As deep-sea sediments consist of various materials, such as organic matter, clay minerals, and biogenic carbonate, constituents of these compounds are released and characterize the fluid chemistry at sediment-associated sites. For example, strontium (Sr), boron (B), and lithium (Li) are released into fluids through high-temperature alteration of sedimentary clay minerals (Araoka et al. 2016; Chiba et al. 1993; Yamaoka et al. 2015; You and Gieskes 2001). Furthermore, methane (CH4) and ammonia (NH4+) enrichment of fluids at sediment-associated sites is considered to be a result of sedimentary organic matter decomposition (Ishibashi et al. 1995; Gamo et al. 1991).
Isotopic ratios are powerful geochemical tools for tracing the origins and fates of environmental constituents. Recent studies have reported significant variations in CH4 isotopic ratios in sediment-associated fluids (e.g., Kawagucci et al. 2013). This variation strongly suggests two styles of fluid–sediment interaction on the subseafloor. Kawagucci et al. (2011) hypothesized that microbes inhabiting sedimentary environments in low-temperature fluid recharge zones generate 13C-depleted CH4, whereas thermal decomposition of sedimentary organic matter in high-temperature fluid discharge zones generates 13C-rich CH4. However, this model can only be applied when sediments contain sufficient carbon. Some sediments lack carbon; hence, variation in carbon isotopes cannot be applied to assess the style of subsurface sediment-fluid interactions.
87Sr/86Sr ratios are widely used as tracers to identify the origin of Sr, because terrestrial (continental) and marine (volcanic) Sr reservoirs have distinct signatures (Chiba et al. 1993; Zhang et al. 2022, 2020; Noguchi et al. 2011). The Sr isotopic ratios of hydrothermal endmember fluids occurring at sediment-starved vent sites exhibit values within a specific range on a global scale (0.7029–0.7058; n = 87; Diehl and Bach 2020). In contrast, the Sr isotopic ratios of hydrothermal endmember fluids in the Okinawa Trough (0.7089 for Izena Hole: Chiba et al. 1992; 0.7100 for Minami-Ensei Knoll: Chiba et al. 1993; Kawagucci et al. 2013), a sediment-covered backarc basin containing more than 20 hydrothermal vent sites (e.g., Kawagucci 2015), have been found to be higher than those of hydrothermal endmember fluids occurring at sediment-poor vent sites. The high Sr isotopic ratios are likely related to subseafloor fluid–sediment interactions, because Sr is present in sedimentary organic matter, clay minerals, and biogenic carbonate (e.g., Yoshimura et al. 2020). This study systematically discusses the causes of the diversity of hydrothermal endmember fluids that exhibit high Sr isotopic ratios or Sr isotopic ratios comparable to those of surrounding volcanic rocks and contributes to our understanding of the mechanisms that produce such diversity.
In the present study, we determined the Sr isotopic ratios in addition to the major and trace element concentrations in hydrothermal fluids collected from five vent sites in the Okinawa Trough, particularly in the central and southern regions. The Sr isotope ratios of the vent fluids at the five sites were considerably higher than those reported previously for sediment-poor seafloor hydrothermal fluids. The possible mechanism generating this high Sr isotopic ratio at the Okinawa Trough was explored based on the Sr isotopic ratios of hydrothermal fluids from the Izena Hole and Minami-Ensei Knoll in the middle Okinawa Trough (MOT), as reported previously (Chiba et al. 1992, 1993).
2 Geological setting
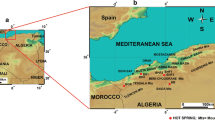
The Okinawa Trough is a backarc basin formed by subduction of the Philippine Sea plate beneath the Eurasian Plate (Lee et al. 1980; Fig. 1a). The trench-arc-back-arc system is ~ 1200 km long; the northern end extends to the west of Kyushu, and the southern end extends to northeastern Taiwan (Letouzey and Kimura 1986). Particles transported from Eurasia and Taiwan Island were deposited in the Okinawa Trough (Dou et al. 2010). The Okinawa Trough is divided into northern, middle, and southern regions bordered by the deep-sea canyon of the Tokara and Kerama gaps (Fig. 1b). The sediment layers in the northern and southern regions of the trough are 8 km and ~ 2 km thick, respectively (Sibuet et al. 1987).
a Location of the Okinawa Trough. The box denotes the area shown in Fig. 1b. b Locations of the hydrothermal fields in the Okinawa Trough (stars). Triangles indicate Quaternary volcanoes. Bathymetric contours are indicated every 200 m (thin lines) and 1000 m (thick lines). Dashed lines represent depth contours (100 and 200 km) in the Wadati-Benioff zone (Letouzey and Kimura 1986; Pezzopane and Wesnousky 1989). Solid circles indicate the volcanic rock sampling sites: red for those in Shinjo et al. (1999), blue for those in Shinjo and Kato (2000), and yellow for those in this study. MOT and SOT represent the middle Okinawa Trough and southern Okinawa Trough, respectively. Boundaries between NOT and MOT and between MOT and SOT are indicated by light blue dashed lines
The Minami-Ensei Knoll is located at the bottom of the MOT at a water depth of ~ 1200 m, with a specific height of 600 m and a diameter of ~ 10 km (Chiba et al. 1993; Kawagucci et al. 2013). The top of the knoll has four depressions, among which only the “C depression” exhibits submarine hydrothermal activity, venting hydrothermal fluids at temperatures of up to 278 °C (Chiba et al. 1993; Kawagucci et al. 2013). Studies of multichannel seismic profiles and P-wave velocity models in the surrounding area have reported that the MOT is covered by a 2–3-km-thick Shimajiri Formation (Nishizawa et al. 2019). Ishibashi et al. (2015) speculated that the Minami-Ensei Knoll intrudes through the thick sediments of the Shimajiri Group of the Okinawa Trough. Kawagucci et al. (2013) and Kawagucci (2015) inferred that hydrocarbon decomposition occurs through CH4 formation and interacts with sediments outside the knoll on the recharging side of the reaction zone.
The Iheya North Knoll is a large volcanic complex with a specific height of ~ 130 m and diameter of ~ 10 km and is located at the bottom of the MOT at a water depth of ~ 1100 m. Three hydrothermally active zones have been described: the Original, Natsu, and Aki sites (Monma et al. 1996; Kasaya et al. 2015; Nakamura et al. 2015). The Original site was drilled in the Integrated Ocean Drilling Program (IODP) Expedition 331 (Takai et al. 2011; Yeats et al. 2017), whereas the CK14-04 and CK16-01 cruises drilled the Aki site in expeditions 907 (Takai et al. 2015) and 908 (Kumagai et al. 2017), respectively. Based on the results of drilling the Original site, the seafloor in this region is covered by low-permeability hydrothermally altered clay mineral layers with a thickness of tens of meters. Mineral layers confine the horizontal hydrothermal flow (Miyoshi et al. 2015; Takai et al. 2011; Masaki et al. 2011). The surrounding trough floor is covered by the 2–3-km-thick Shimajiri Formation, and the knoll itself has no significant cover (Ishibashi et al. 2015). Kawagucci et al. (2011) reported that the contribution of microbial CH4 to hydrothermal fluids is large. Microbial CH4 is assumed to be produced in the sedimentary layers of the recharge zone surrounding the knoll body and incorporated into hydrothermal fluids (Kawagucci et al. 2011; Kawagucci 2015). The Pb isotopic ratios in galena collected from hydrothermal vent chimneys suggest that the sediment influence on hydrothermal fluids at the Iheya North Knoll is lower than that at the Izena Hole (Totsuka et al. 2019).
The Izena Hole is also located in the MOT ~ 300 m above the surrounding seafloor at a depth of 1610 m, and it has a rectangular caldera of approximately 6 km × 4 km on the western slope of a collapsed knoll body. The Izena Hole hydrothermal field has two hydrothermal sites: the HAKUREI and JADE sites (Ishibashi et al. 2014). Both sites have distinct fluid compositions (Ishibashi et al. 2014) and thus, were treated as individual sites in this study. The JADE site is located on the northeast wall of the caldera, and the HAKUREI site is situated in a depression on the southwest side of the caldera floor (Ishibashi et al. 2014; Kawagucci et al. 2010; Halbach et al. 1989). During the CK16-05 cruise (Exp. 909), the area around the HAKUREI site was drilled, and pumice was found several tens of meters below the seafloor. Additionally, hydrothermally altered clay minerals are distributed up to 180 m below the seafloor (Nozaki et al. 2021). The carbon isotopic ratios of CH4 in the JADE and HAKUREI hydrothermal fluids suggest that both microbial and thermogenic CH4 were supplied in the recharge area (Kawagucci et al. 2010; Kawagucci 2015). However, even if hydrogen is produced in the recharge zone during thermogenesis, it is decomposed by magmatic volatiles from oxic silicic magma in the reaction zone (Kawagucci et al. 2013). The reason for the hydrogen enrichment only at the HAKUREI site could be the influence of sediment cover around the discharge zone, and organic matter in the sediment could be decomposed to generate hydrogen (Ishibashi et al. 2014). This theory also explains ethane enrichment only at the HAKUREI site.
The Yonaguni Knoll IV is located at the foot of the western slope of the Yonaguni Knoll in the southern Okinawa Trough (SOT) at a water depth of 1385–1336 m. Hydrothermal fluids emanate from nearly 10 m high chimneys, and the surrounding area is covered by muddy sediments (Suzuki et al. 2008). Studies of seismic structures in the vicinity have reported that the SOT is covered with 2–3-km-thick submarine sediments (Nishizawa et al. 2019). The origin of CH4 is based on the thermogenic decomposition of organic matter, and the high hydrogen concentration suggests that the knoll body, with little organic matter, acts as a recharge zone, with the thermal breaking of organic matter in the sediments around the vent (Kawagucci 2015).
3 Samples and sample preparation
3.1 Sample collection
Fluid samples were collected from the Iheya North Knoll (during the KY11-02, NT11-16, and NT12-06 cruises), Izena Hole (during the NT10-17 cruise at the JADE site and NT10-17 and NT11-15 cruises at the HAKUREI site), Hatoma Knoll (during the YK07-04, NT07-12, NT08-13, and NT09-11 cruises), and Yonaguni Knoll IV (during the YK03-05 cruise). KY, NT, and YK in the cruise names indicate the vessels KAIYO, NATSUSHIMA, and YOKOSUKA, respectively, all of which are owned by the Japan Agency for Marine-Earth Science and Technology (JAMSTEC); the two-digit numbers preceding and following the hyphen indicate the post-2000 year and the serial number of the voyage, respectively. The sample IDs provided information on the cruise, dive, sampler type, and sample number (Table 1). The samplers used included the water and hydrothermal fluid Atsuryoku Tight Sampler (WHATS) (Tsunogai et al. 2003; Saegusa et al. 2006; Miyazaki et al. 2017), Ti-syringe sampler (Von Damm et al. 1985a), Cheap-WHATS sampler (Kawagucci et al. 2016), vacuum sampler (Sedwick et al. 1994), bag sampler (a 2-L plastic bag connected to an impeller pump), and Niskin sampler. The samplers were loaded onto the JAMSTEC ROV Hyper Dolphin and DSV Shinkai 6500. The sampling locations (latitude, longitude, and depth) and the maximum temperature of the fluid observed during sampling are shown in Table 1.
After the samples were brought on board, the liquid was immediately distributed to a polyethylene bottle for pH measurement, followed by a polyethylene syringe with a mixed cellulose ester membrane filter (mesh size: 0.45 μm) as described by Toki et al. (2016). The samples were filtered and divided into two polyethylene bottles for onboard NH4+ concentration analysis and laboratory Cl– concentration analysis and for on-land cation (Na, Mg, Ca, K, B, and Sr) concentrations and Sr isotopic ratio analyses. The samples in polyethylene bottles for cation concentration and Sr isotopic ratio analyses were further acidified (pH < 2) with 3 M HNO3 and transported to the laboratory.
3.2 Analytical procedure
Analyses of various chemical components of the fluid were performed as previously described (Toki et al. 2016). The pH of the fluid was analyzed using a glass electrode at room temperature, ~ 20 to 25 °C. Fluid Cl– concentrations were analyzed by Mohr titration, and values were determined using standard seawater (IAPSO) to within 1% precision. Fluid NH4+ concentrations were analyzed using the colorimetric method, and a calibration curve was constructed using an ammonium chloride solution to determine the concentration. One sample was measured three times for each analysis to determine the analytical precision. Analytical precision was within 1%. Cations in the fluid were analyzed using inductively coupled plasma optical emission spectroscopy (ICP-OES; Model 5100, Agilent Technology, Santa Clara, CA, USA) at Kyushu University. Concentrations were determined using standard solutions prepared by mixing standard reagents for atomic absorption with an analytical precision within 3%.
Sr was separated from fluid samples with a Sr-Spec resin (mesh size: 50–100 µm; Eichrom Industries), as described by Pin et al. (1994). A 0.15-mL aliquot was placed into a precleaned vial made of perfluoroalkoxy alkane, dried at ~ 100 °C and then, dissolved in 0.15 mL 2 M HNO3 (EL grade). A mini-column with a 0.1 mL Sr-Spec resin bed was first washed with 1 mL ultrapure water (Resistivity at 25 °C:18.2 MΩ·cm) and then, conditioned with 0.5 mL 2 M HNO3. Next, 0.15 mL of the sample solution was loaded onto the column, and the major ions were washed out with 0.15 mL 2 M HNO3. Barium was eluted with 1.2 mL of 8 M HNO3. Next, we washed the column with 0.4 mL 2 M HNO3 and eluted the Sr fraction on the column by adding 1 mL of 0.05 M HNO3. The Sr fraction was collected in a precleaned vial made of perfluoroalkoxy alkane and dried at ~ 100 °C on a hot plate.
The Sr isotopic ratio (87Sr/86Sr) of the samples from the Hatoma Knoll in the SOT was measured using thermal ionization mass spectrometry (MAT262; Finnigan). The dried eluent was dissolved in 2 µL of 1 M H3PO4, and the solution was loaded onto a Ta filament. The drop was heated and transferred to a thermal ionization mass spectrometer. The measured 87Sr/86Sr ratio was normalized to an 86Sr/88Sr value of 0.1194. We obtained an average value of 0.710235 ± 0.000028 (1σ, n = 9) for the National Institute of Standards and Technology (NIST) SRM-987 standard.
The 87Sr/86Sr ratios of the other samples were measured via multiple collector-inductively coupled plasma-mass spectrometry (Neptune Plus) with a solution sample of 200 ppb Sr prepared with 0.3 M HNO3. The values were standardized to NIST SRM-987 (87Sr/86Sr = 0.71025). The precision is the standard deviation of the measured SRM-987 sample: 0.710252 ± 0.000025 (2σ; n = 16).
3.3 Endmember calculation
The sample may inevitably be mixed with seawater from the sampling inlet, or the hydrothermal fluids may be combined with seawater below the seafloor before venting. Therefore, it is customary to determine the chemical composition of pure hydrothermal fluids by assuming that they do not contain Mg (Von Damm 1995). This is because laboratory experiments have shown that when basalt, andesite, and rhyolite react with seawater, most of the Mg is removed from the seawater (Bischoff and Dickson 1975; Mottl and Holland 1978; Seyfried Jr and Bischoff 1981; Hajash and Chandler 1982; Shiraki et al. 1987). The endmember Sr concentration for each sample was estimated using the following equations:
where subscripts indicate the sample, endmember hydrothermal fluid (EHF), and seawater (SW). [Mg]EHF, [Mg]SW, and [Sr]SW were 0, 53 mmol/kg, and 87 µmol/kg, respectively (Von Damm et al. 1985a); therefore, [Sr]EHF can be calculated from the analytical results of the [Mg]sample and [Sr] sample. Because Mg-poor hydrothermal fluids are obtained from most hydrothermal systems (Table 1; Fig. 2), pure hydrothermal fluids are assumed to be Mg-free to obtain the endmembers. However, this study did not receive Mg-free hydrothermal fluids from the Yonaguni Knoll IV hydrothermal system. Suzuki et al. (2008) also collected samples from the Yonaguni Knoll IV hydrothermal system and obtained almost Mg-free hydrothermal fluids. Therefore, even in the Yonaguni Knoll IV hydrothermal system, pure hydrothermal water was assumed to be Mg-free, and endmembers were obtained. Only by standardization will it be possible to compare hydrothermal waters and chemical compositions worldwide (Von Damm 1995).
Relationship between Mg and Sr concentrations in hydrothermal fluids from the Iheya North Knoll, HAKUREI, JADE, Hatoma Knoll, and Yonaguni Knoll IV sites in the Okinawa Trough. Light blue stars indicate the value of seawater. Red lines are regression lines through all data and seawater for each site. No such line was drawn for the Yonaguni Knoll IV because of poor fit
To estimate the 87Sr/86Sr ratio of the pure hydrothermal fluid at each sampling site, the 87Sr/86Sr ratio in the fluid sample was plotted against the [Mg]sample/[Sr]sample ratio (Fig. 3). In this plot, mixing between pure fluid and seawater is represented by a straight line. The Mg-free y-intercept of the regression line for a hydrothermal site represents the 87Sr/86Sr ratio of the pure hydrothermal fluid for the hydrothermal site (Albarède et al. 1981).
Relationship between Mg/Sr and 87Sr/86Sr in hydrothermal fluids from the Iheya North Knoll, HAKUREI, JADE, Hatoma Knoll, and Yonaguni Knoll IV sites in the Okinawa Trough. For an explanation of insets, refer to Fig. 2
4 Results
Table 1 lists the analytical results, including the Mg and Sr concentrations and the 87Sr/86Sr ratios. The endmember Sr concentrations of each sample were calculated using Eq. (1) and (2), which yielded concentrations of 75.5–81.8 µmol/kg (Iheya North Knoll), 120.3–129.1 µmol/kg (HAKUREI), 98.7–99.3 µmol/kg (JADE), 34.7–67.4 µmol/kg (Hatoma Knoll), and 83.6–95.1 µmol/kg (Yonaguni Knoll IV) (Table 1). As the error for endmember extrapolation increases with seawater entrainment, we limited the discussion to samples with Mg < 30 mmol/kg (Table 1).
The Sr isotopic ratios of the fluid samples collected at Iheya North Knoll, HAKUREI, JADE, Hatoma Knoll, and Yonaguni Knoll IV were 0.70764–0.70770, 0.70920–0.70924, 0.70891–0.70895, 0.70850–0.70922, and 0.70917–0.71126, respectively (Table 1). The 87Sr/86Sr ratios of the pure hydrothermal fluid at each hydrothermal site were estimated based on the 87Sr/86Sr–[Sr]/[Mg] plot (see Sect. 3.3, Endmember calculation) and are listed in Table 2. The estimated Sr isotopic ratios of the endmembers ranged from 0.7077 (Iheya North Knoll) to 0.7120 (Yonaguni Knoll IV). The endmember Sr isotopic ratio of the hydrothermal fluid at Yonaguni Knoll IV was the highest among all Sr isotopic ratios reported to date from hydrothermal sites worldwide (Table 2 and Fig. 4). The endmember Sr isotopic ratios differed between the HAKUREI (0.7092) and JADE (0.7089) sites. However, both sites are located at the Izena Hole (Ishibashi et al. 2014). The Sr isotopic ratios of the endmembers did not correlate with the Sr concentrations of the endmembers. The Okinawa Trough exhibited no geographic trends in Sr isotopic ratio (Fig. 1 and Table 2).
Endmember Sr isotopic ratios of hydrothermal fluids from the Okinawa Trough and sites worldwide. Black represents data from MOR hydrothermal systems, blue represents data from backarc systems, and red represents data from arc volcanoes. Open symbols indicate sediment-starved systems, and closed symbols indicate sediment-associated systems. The Sr isotopic ratios of solids (MORB, volcanic rocks from the Okinawa Trough, and sediments from the East China Sea) are also shown. A light blue star and dash-dotted line indicate the Sr isotopic ratios of seawater
5 Discussion
5.1 Sr isotopic ratios of venting fluids and subseafloor reservoirs
To understand the diverse fluid–sediment interactions through interpretation of the Sr isotopic ratios, we compared the Sr isotopic ratios of hydrothermal fluids observed to date with those of representative subseafloor Sr reservoirs worldwide (Fig. 4), classified based on tectonic setting and sediment occurrence at the sites. The Sr isotopic ratios of hydrothermal fluids in sediment-starved mid-ocean ridge systems fell within a specific range of 0.7030–0.7042 at EPR21° N and EPR13° N (Albarède et al. 1981; Michard et al. 1984), Kairei (Gamo et al. 2001), and the Kane Fracture Zone and the Endeavor Segment of the Juan de Fuca Ridge (Campbell et al. 1988; Jamieson et al. 2016). The Sr isotopic ratios of sediment-starved backarc systems were somewhat higher and within a certain range (0.7036–0.7056) in the Lau Basin (Mottl et al. 2011), Manus Basin (Reeves et al. 2011), North Fiji Basin (Grimaud et al. 1991), and Mariana Trough (Kusakabe et al. 1990). The Sr isotopic ratios of sediment-associated MOR (Mid-ocean ridge) systems are 0.7043 in the Middle Valley (Butterfield et al. 1994), 0.7056 in the Guaymas Basin in the Gulf of California (Piepgras and Wasserburg 1985), 0.7081 at Loki’s Castle on the Arctic MOR (Baumberger et al. 2016), and 0.7099 at the Escanaba Trough of the Juan de Fuca Ridge (Campbell et al. 1994), which are relatively high compared with those of sediment-starved hydrothermal systems. Sr isotopic ratios of volcanic rocks were 0.704–0.706 in the Okinawa Trough (Appendix) and 0.7023–0.7030 at the MOR (MOR basalt, MORB; White et al. 1987). The Sr isotopic ratios of bulk sediment on the continental shelf of the East China Sea were virtually identical to those of bulk sediment in the Okinawa Trough (0.713–0.719; Asahara et al. 1995).
5.2 Relationships of Sr isotopic ratios with representative chemical and isotopic compositions of hydrothermal fluids
To assess the co-variation between Sr isotopic ratios and other chemical and isotopic components from the global vent dataset, we plotted several representative parameters of the endmember hydrothermal fluids against the Sr isotopic ratios (Fig. 5a‒g). No clear correlation between Sr/Cl and Sr isotopic ratios was detected among global sediment-associated sites, including the Okinawa Trough sites (Fig. 5a), whereas Sr/Cl ratios varied even within the sediment-starved sites. This finding indicates that the Sr isotopic ratios of sediment-associated sites are determined not by a single Sr reservoir or the degree of Sr leaching from a reservoir but by multiple Sr reservoirs. Moreover, no clear correlation was observed between the Ca/Cl and Sr isotopic ratios in the global hydrothermal fluids (Fig. 5b). Reeves et al. (2010) conducted a series of observations in the Manus backarc basin. They found that the Ca/Cl ratio variation among hydrothermal fluids in this basin is related to the host rock type. For example, the Vienna Wood site is basalt-hosted with a Ca/Cl ratio of approximately 0.12. In contrast, the felsic rock-hosted sites have Ca/Cl ratios lower than 0.06 (Fig. 5b). The relatively low Ca/Cl ratios and variable Sr isotopic ratios in the Okinawa Trough fluids may be attributable to a lower contribution from the Ca-rich mafic rock and comparable contributions from multiple Sr reservoirs (Table 3). Similarly, the large variation in the Ca/Cl ratio but constant Sr isotopic ratios similar to the mid-ocean ridge basalt (MORB) value in sediment-starved hydrothermal fluids may be explained by Ca leaching from plagioclase in basalt (partly substituted by Sr) via albitization (Rosenbauer et al. 1988; Von Damm and Bischoff 1987). No correlation was observed between the K/Cl and Sr isotopic ratios in the hydrothermal fluids analyzed (Fig. 5b and c). Because felsic rocks are more enriched in K than mafic rocks (Sakai et al. 1990), felsic rock-hosted hydrothermal systems, such as the Okinawa Trough and Manus Basin (Reeves et al. 2011), have higher K/Cl ratios than basalt-hosted hydrothermal systems (Fig. 5c). The K/Cl ratios at the sediment-associated sites were not always high, and we found no clear correlation between the K/Cl and Sr isotopic ratios. This finding suggests that the Sr isotopic ratios in hydrothermal fluids are regulated by factors other than the host rock type (Fig. 5a‒c).
The B, NH4+, CH4, and H2 concentrations were higher in fluids at sediment-associated hydrothermal sites and were relatively strongly correlated with Sr isotopic ratios (Fig. 5d‒g), suggesting that 87Sr-rich Sr reservoirs in the fluids were mainly derived from the same B, NH4+, CH4, and H2 reservoir sources at sediment-associated hydrothermal sites. NH4+, CH4, and H2 in fluids are thought to be generated by the decomposition of sedimentary organic matter, represented by (CH2O)106 (NH3)16 H3PO4 (Redfield 1934), as follows (Piepgras and Wasserburg 1985):
These ideal equations assume a reaction that generates CO2 and an intermediate product, H2 (Eq. 1), followed by methanogenesis with 4:1 H2:CO2 stoichiometry (Eq. 4). In these equations, the NH3 generated was equilibrated with water as follows:
\({\text{NH}}_{{3}} + {\text{ H}}_{{2}} {\text{O }} \leftrightarrow {\text{ NH}}_{{4}}^{ + } + {\text{ OH}}^{ - }\) (5).
B in the fluid is considered to originate from B in the sediment. Sedimentary B is generally abundant because of its accumulation by adsorption onto the surfaces of suspended particles, including organic particles (Spivack et al. 1987). Sedimentary B is bound to the lattice of clay minerals during burial (Williams et al. 2001) and is released after exposure to high temperatures (> 300 °C; You and Gieskes 2001). Although B enrichment in fluids at sediment-associated sites is not directly related to the decomposition of organic matter (unlike CH4 and NH3), sedimentary B and its release at high temperatures are responsible for the abundance of B in fluids.
5.3 Virtually complete removal of seawater-derived Sr suggested from sediment-starved system observations
The Sr isotopic ratios of hydrothermal endmember vent fluids were comparable to those of major subseafloor Sr reservoirs, such as volcanic rocks, recharged seawater, and bulk sediment (Fig. 4). Thus, the Sr isotopic ratios of the hydrothermal endmember fluids can be explained by the Sr influx/efflux of these reservoirs during subseafloor hydrothermal-fluid circulation. The Sr isotopic ratios of hydrothermal fluids in the sediment-starved MOR and backarc systems were within a certain range (0.7034–0.7056), and the Sr isotopic ratios of hydrothermal endmember fluids in the sediment-starved setting were much lower than the seawater value (0.7092) and close to the value for volcanic rocks (0.7023–0.7056). The low Sr isotopic ratios and consistency between fluids and volcanic rocks demonstrate that virtually all seawater-derived 87Sr recharged into the subseafloor was removed, and 87Sr-depleted Sr was leached from volcanic rocks into the fluid. This observation-based model is consistent with experimental simulation studies on hydrothermal fluid–mineral interactions, in which Sr in the liquid phase (i.e., recharged seawater) is removed through co-precipitation with anhydrite when heated to 200 °C (Albarède et al. 1981; Wilckens et al. 2019), whereas Sr in some minerals (e.g., from volcanic rock and sediment) is released into fluids at temperatures above 200 °C (Wei 2007; Berndt et al. 1988; James et al. 2003). The removal of seawater-derived Sr through co-precipitation with anhydrite is also expected in sediment-associated high-temperature hydrothermal fluids because of its sulfate-depleted nature at temperatures higher than 200 °C (Albarède et al. 1981; Wilckens et al. 2019). This suggests that Sr derived from recharged seawater accounts for a negligible proportion of Sr in the hydrothermal vent fluids in the Okinawa Trough.
5.4 Possible contribution of 87Sr-rich terrestrial minerals in sediment to Sr isotopic ratios of hydrothermal fluid in the Okinawa Trough
Because the Sr isotopic ratios of volcanic rocks in the Okinawa Trough are low and fall within a specific range (Appendix), the high Sr isotopic ratios of fluids at the Okinawa Trough sites (Fig. 4) are attributable to reactions with 87Sr-rich sediments. To understand how sedimentary Sr reservoirs contribute to the venting fluid at sediment-associated sites, we considered the compositional characteristics of the Okinawa Trough sediments and the possible Sr isotopic ratios of each Sr-bearing component.
The sediments of the Okinawa Trough contain several Sr-bearing components, such as organic matter originating from primary production in the ocean surface layer, biological skeletons, and clastic debris weathered from the continental crust. Organic matter occupies < 10% of the bulk sediment, and the Sr content of sedimentary organic matter is approximately 10 ppm (Gao et al. 2008). The Sr content in the organic matter can be as low as ppb in bulk sediments and is negligible as a Sr source for hydrothermal fluids (Gao et al. 2008). The 88Sr/86Sr ratios of hydrothermal fluids suggest a significant contribution of Sr derived from biogenic carbonates to hydrothermal fluid Sr (Yoshimura et al. 2020). The Sr isotopic ratio of sedimentary biogenic carbonate produced by foraminifera or coccoliths in seawater columns is identical to that of seawater at the time of formation (Stevenson et al. 2009). Throughout the history of the Okinawa Trough, the variation in seawater 87Sr/86Sr isotopic ratios has remained virtually constant at 0.7092 (Glasby and Notsu 2003; Elderfield and Schultz 1996; McArthur et al. 2001; Mokadem et al. 2015). Some data in the hydrothermal fluid at Yonaguni Knoll IV fall into the seawater value, not on the simple mixing line between the 87Sr-poor endmember and seawater (Figs. 2 and 3), which may be because of the contribution of sedimentary biogenic carbonate.
Because of their terrestrial origin, sedimentary clay minerals and quartz in the Okinawa Trough have high 87Sr/86Sr ratios. Clay minerals and quartz deposited in the Okinawa Trough are transported in large quantities from Eurasia via the Yangtze and Yellow Rivers (Yuan et al. 2008). The Sr isotopic ratios of bulk terrestrial sands on the Loess Plateau, one of the largest mineral sources for rivers, ranged from 0.71418 to 0.72045 (Yokoo et al. 2004). Specifically, the Sr isotopic ratios in the clay mineral- and quartz-bearing fractions, estimated by processing bulk sand with HCl, were 0.71379–0.71563 and 0.71867–0.72157, respectively (Yokoo et al. 2004). The Sr contents of clay minerals and quartz in the Okinawa Trough have been found to be 100–300 ppm and 100–150 ppm, respectively (Yokoo et al. 2004). Because high 87Sr/86Sr isotopic ratios (0.71418–0.72045) of sedimentary clay minerals and quartz were expected in the Okinawa Trough, the 87Sr/86Sr isotopic ratio of 0.7120 at the Yonaguni Knoll IV site cannot be explained without the Sr contribution from these terrestrial components. The 87Sr/86Sr isotopic ratios observed in this study suggest that in addition to volcanic rocks, sedimentary biogenic carbonate, terrestrial clay minerals, and quartz are likely to be Sr sources for the fluid.
5.5 Involvement of sediment in the high-temperature reaction zone
The higher concentrations of B, NH4+, CH4, and H2 in hydrothermal fluids among sediment-associated systems, including the Okinawa Trough sites, suggest the involvement of sediments in the high-temperature reaction zone. To explain the variation in Sr isotopes among the hydrothermal fluids, we developed a simple hypothesis (Fig. 6). The subseafloor hydrothermal fluid loses seawater-derived Sr during heating by anhydrite precipitation. It monotonously accumulates Sr through Sr leaching from the solid phase. Sr removal, excluding anhydrite precipitation, did not occur until venting. In this hypothesis, the critical parameter is the temperature of the fluid–sediment interaction. For example, at the Yonaguni IV site, which is characterized by high Sr isotopic ratios, high-temperature (> 150 °C; Wei 2007) fluid–sediment interactions should be substantial because of the intense leaching of 87Sr-rich components in sedimentary clay minerals and quartz. In the Iheya North Knoll and Hatoma Knoll, characterized by relatively low Sr isotopic ratios in hydrothermal systems in the Okinawa Trough, high-temperature fluid–sediment interactions are absent, and both sedimentary and volcanic rock Sr reservoirs play comparable roles in controlling the vent Sr isotopic ratios.
Schematic diagram showing two types of hydrothermal circulation in the Okinawa Trough (modified after Kawagucci 2015). Arrows indicate hydrothermal fluid circulation, with color representing the temperature of hydrothermal fluid. a Hydrothermal circulation in contact with sediments at high temperatures and volcanic rocks at moderate temperatures (> 150 °C). Fluid is in contact with sediments at high temperatures, and the sediments supply Sr (e.g., Yonaguni Knoll IV). b Hydrothermal circulation in contact with sediments at low temperatures and volcanic rocks at high temperatures. Sr from volcanic rocks is supplied to hydrothermal fluids (e.g., Iheya North Knoll and Hatoma Knoll). Thus, CH4 is provided by thermogenic CH4 at high temperatures (a) and microbial sources at low temperatures (b)
Our hypothesis can be incorporated into a previously developed model that describes how sedimentary organic matter interacts with subseafloor hydrothermal fluid from the perspective of carbon isotopic ratios in CH4 (Fig. 6; Kawagucci et al. 2011, 2013; Kawagucci 2015; Toki et al. 2016). According to the model, the 13C-rich thermogenic CH4 and 87Sr-rich terrestrial Sr in the Yonaguni IV and Minami-Ensei sites indicate a substantial contribution to the thermal decomposition of sedimentary organic matter (> 100 °C) in addition to high-temperature leaching of Sr from clay minerals and quartz, representing a high-temperature fluid–sediment interaction in the subseafloor reaction zone (Fig. 6a). In contrast, the model also indicated that the 13C-depleted biogenic CH4 signature and 87Sr-poor volcanic Sr signature found at the Iheya North Knoll and Hatoma Knoll sites suggest metabolic degradation of sedimentary organic matter in the cool fluid recharge zone (< 122 °C), with a supply of volcanic rock-derived Sr following the removal of Sr associated with anhydrite precipitation below 150 °C (Fig. 6b). In this case, 87Sr-poor Sr generated through high-temperature fluid–rock interactions in the reaction and discharge zones is likely the Sr source for the venting fluid. Thus, the model provides a reasonable explanation for the observed isotopic compositions of carbon and Sr in the hydrothermal fluids of sediment-associated systems.
6 Conclusions
Hydrothermal fluids were collected from the Okinawa Trough, and their Sr isotopic ratios were measured. The Sr isotopic ratios of hydrothermal fluids from Yonaguni Knoll IV were extremely high, in agreement with previously published results from the Minami-Ensei Knoll. Such 87Sr-rich isotopic ratios of hydrothermal fluids might be a common feature of sediment-associated hydrothermal systems. These results are consistent with a recent model describing how sedimentary organic matter interacts with fluid within a subseafloor hydrothermal fluid circulation developed from the perspective of the carbon isotopic ratios of CH4. In this model, sediments are distributed around the reaction zone at the Yonaguni Knoll IV and Minami-Ensei sites, where the hydrothermal fluids bear 13C-rich thermogenic CH4 and 87Sr-rich terrestrial Sr. However, in the Iheya North Knoll and Hatoma Knoll sites, where the hydrothermal fluids bear 13C-depleted biogenic CH4 and 87Sr-poor volcanic Sr, sediments are distributed around the recharge zone. Thus, both tracers are an advantageous way to determine whether fluids interact with sediments in the hydrothermal reaction zone. To further justify the model’s ability to clarify how subseafloor fluid–sediment interactions affect fluid chemistry, exploring the isotope systematics of multiple elements such as B and Li will be helpful.
Availability of data and materials
All newly obtained data from this study are presented in the manuscript, and data sharing is not applicable.
Abbreviations
- CK:
-
Drilling vessel Chikyu
- DSV:
-
Deep-submergence vehicle
- EPR:
-
East Pacific Rise
- IAPSO:
-
International Association for the Physical Sciences of the Oceans
- ICP-OES:
-
Inductively coupled plasma optical emission spectroscopy
- IODP:
-
Integrated Ocean Drilling Program
- JAMSTEC:
-
Japan Agency for Marine-Earth Science and Technology
- KY:
-
Research vessel Kaiyo
- MOR:
-
Mid-ocean ridge
- MORB:
-
Mid-ocean ridge basalt
- MOT:
-
Middle Okinawa Trough
- NBS:
-
National Bureau of Standards
- NIST:
-
National Institute of Standards and Technology
- NOT:
-
Northern Okinawa Trough
- NT:
-
Research vessel Natsushima
- ROV:
-
Remotely operated vehicle
- SOT:
-
Southern Okinawa Trough
- SRM:
-
Standard reference material
- SUGAR:
-
Super-cutting-edge Grand and Advanced Research Program
- WHATS:
-
Water and Hydrothermal Fluid Atsuryoku Tight Sampler
- X-star:
-
Institute for Extra-cutting-edge Science and Technology Avant-Garde Research
- YK:
-
Research vessel Yokosuka
References
Albarède F, Michard A, Minster JF, Michard G (1981) 87Sr/86Sr ratios in hydrothermal waters and deposits from the East Pacific Rise at 21°N. Earth Planet Sci Lett 55(2):229–236. https://doi.org/10.1016/0012-821x(81)90102-3
Araoka D, Nishio Y, Gamo T, Yamaoka K, Kawahata H (2016) Lithium isotopic systematics of submarine vent fluids from arc and back-arc hydrothermal systems in the western Pacific. Geochem Geophys Geosyst 17(10):3835–3853. https://doi.org/10.1002/2016gc006355
Asahara Y, Tanaka T, Kamioka H, Nishimura A (1995) Asian continental nature of 87Sr/86Sr ratios in north central Pacific sediments. Earth Planet Sci Lett 133(1–2):105–116. https://doi.org/10.1016/0012-821X(95)00048-H
Baumberger T, Früh-Green GL, Dini A, Boschi C, van Zuilen K, Thorseth IH, Pedersen RB (2016) Constraints on the sedimentary input into the Loki’s Castle hydrothermal system (AMOR) from B isotope data. Chem Geol 443:111–120. https://doi.org/10.1016/j.chemgeo.2016.09.026
Berndt ME, Seyfried WE, Beck JW (1988) Hydrothermal alteration processes at midocean ridges: experimental and theoretical constraints from Ca and Sr exchange reactions and Sr isotopic ratios. J Geophys Res Solid Earth 93(B5):4573–4583. https://doi.org/10.1029/JB093iB05p04573
Bischoff JL, Dickson FW (1975) Seawater-basalt interaction at 200°C and 500 bars: Implications for origin of sea-floor heavy-metal deposits and regulation of seawater chemistry. Earth Planet Sci Lett 25(3):385–397. https://doi.org/10.1016/0012-821X(75)90257-5
Bonatti E (1975) Metallogenesis at oceanic spreading centers. Annu Rev Earth Planet Sci 3(1):401–431. https://doi.org/10.1146/annurev.ea.03.050175.002153
Butterfield DA, McDuff RE, Franklin J, Wheat GC (eds) (1994) Geochemistry of hydrothermal vent fluids from Middle Valley, Juan de Fuca Ridge, vol 139. Proceedings of the Ocean Drilling Program, Scientific Results. Ocean Drilling Program, College Station, Texas
Campbell AC, Palmer MR, Klinkhammer GP, Bowers TS, Edmond JM, Lawrence JR, Casey JF, Thompson G, Humphris S, Rona P, Karson JA (1988) Chemistry of hot springs on the Mid-Atlantic Ridge. Nature 335(6190):514–519. https://doi.org/10.1038/335514a0
Campbell AC, German CR, Palmer MR, Gamo T, Edmond JM (1994) Chemistry of hydrothermal fluids from Escanaba Trough, Gorda Ridge. In: Morton JL, Zierenberg RA, Reiss CA (eds) Geologic, hydrothermal and biologic studies at Escanaba Trough, Gorda Ridge, offshore northern California, vol 2022. US Geological Survey Bulletin, pp 201–221
Chiba H, Nakashima K, Gamo T, Ishibashi J, Tsunogai U, Sakai H (1993) Hydrothermal activity at the Minami-Ensei Knoll, Okinawa Trough: chemical characteristics of hydrothermal solutions (in Japanese with English Abstract). Proc JAMSTEC Sympos Deep Sea Res 9:271–282
Chiba H, Sakai H, Ishibashi J, Urabe T Sr isotopic study of seafloor hydrothermal system in mid-Okinawa trough. In: International Union of Geological Sciences (ed) International Geological Congress, Kyoto, Japan, 24 August-3 September 1992. International Union of Geological Sciences, p 754
Diehl A, Bach W (2020) MARHYS (MARine HYdrothermal solutions) database a global compilation of marine hydrothermal vent fluid, end member, and seawater compositions. Geochem Geophys Geosyst 21(12):e2020GC009385. https://doi.org/10.1029/2020GC009385
Dou Y, Yang S, Liu Z, Clift PD, Shi X, Yu H, Berne S (2010) Provenance discrimination of siliciclastic sediments in the middle Okinawa Trough since 30 ka: Constraints from rare earth element compositions. Mar Geol 275(1–4):212–220. https://doi.org/10.1016/j.margeo.2010.06.002
Elderfield H, Schultz A (1996) Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Annu Rev Earth Planet Sci 24:191–224. https://doi.org/10.1146/annurev.earth.24.1.191
Gamo T, Sakai H, Kim E-S, Shitashima K, Ishibashi J (1991) High alkalinity due to sulfate reduction in the CLAM hydrothermal field, Okinawa Trough. Earth Planet Sci Lett 107(2):328–338. https://doi.org/10.1016/0012-821X(91)90080-2
Gamo T, Chiba H, Yamanaka T, Okudaira T, Hashimoto J, Tsuchida S, Ishibashi J, Kataoka S, Tsunogai U, Okamura K, Sano Y, Shinjo R (2001) Chemical characteristics of newly discovered black smoker fluids and associated hydrothermal plumes at the Rodriguez Triple Junction, Central Indian Ridge. Earth Planet Sci Lett 193(3):371–379. https://doi.org/10.1016/S0012-821X(01)00511-8
Gao XL, Chen SY, Long AM (2008) Chemical speciation of 12 metals in surface sediments from the northern South China Sea under natural grain size. Mar Pollut Bull 56(4):786–792. https://doi.org/10.1016/j.marpolbul.2008.01.004
Glasby GP, Notsu K (2003) Submarine hydrothermal mineralization in the Okinawa Trough, SW of Japan: an overview. Ore Geol Rev 23(3–4):299–339. https://doi.org/10.1016/j.oregeorev.2003.07.001
Grimaud D, Ishibashi J, Lagabrielle Y, Auzende J-M, Urabe T (1991) Chemistry of hydrothermal fluids from the 17°S active site on the North Fiji Basin Ridge (SW Pacific). Chem Geol 93(3):209–218. https://doi.org/10.1016/0009-2541(91)90114-7
Hajash A, Chandler GW (1982) An experimental investigation of high-temperature interactions between seawater and rhyolite, andesite, basalt and peridotite. Contrib Mineral Petrol 78(3):240–254. https://doi.org/10.1007/bf00398919
Halbach P, Nakamura K-i, Wahsner M, Lange J, Sakai H, Käselitz L, Hansen RD, Yamano M, Post J, Prause B, Seifert R, Michaelis W, Teichmann F, Kinoshita M, Märten A, Ishibashi J, Czerwinski S, Blum N (1989) Probable modern analogue of Kuroko-type massive sulphide deposits in the Okinawa Trough back-arc basin. Nature 338(6215):496–499. https://doi.org/10.1038/338496a0
Ishibashi J, Sano Y, Wakita H, Gamo T, Tsutsumi M, Sakai H (1995) Helium and carbon geochemistry of hydrothermal fluids from the Mid-Okinawa Trough back arc basin, southwest of Japan. Chem Geol 123(1–4):1–15. https://doi.org/10.1016/0009-2541(95)00051-M
Ishibashi J, Noguchi T, Toki T, Miyabe S, Yamagami S, Onishi Y, Yamanaka T, Yokoyama Y, Omori E, Takahashi Y, Hatada K, Nakaguchi Y, Yoshizaki M, Konno UTA, Shibuya T, Takai KEN, Inagaki F, Kawagucci S (2014) Diversity of fluid geochemistry affected by processes during fluid upwelling in active hydrothermal fields in the Izena Hole, the middle Okinawa Trough back-arc basin. Geochem J 48(4):357–369. https://doi.org/10.2343/geochemj.2.0311
Ishibashi J, Ikegami F, Tsuji T, Urabe T (2015) Hydrothermal activity in the Okinawa Trough back-arc basin: Geological background and hydrothermal mineralization. In: Ishibashi J, Okino K, Sunamura M (eds) Subseafloor biosphere linked to hydrothermal systems: TAIGA concept. Springer Japan, Tokyo, pp 337–359. https://doi.org/10.1007/978-4-431-54865-2_27
James RH, Allen DE, Seyfried WE Jr (2003) An experimental study of alteration of oceanic crust and terrigenous sediments at moderate temperatures (51 to 350°C): insights as to chemical processes in near-shore ridge-flank hydrothermal systems. Geochim Cosmochim Acta 67(4):681–691. https://doi.org/10.1016/S0016-7037(02)01113-4
Jamieson JW, Hannington MD, Tivey MK, Hansteen T, Williamson NMB, Stewart M, Fietzke J, Butterfield D, Frische M, Allen L, Cousens B, Langer J (2016) Precipitation and growth of barite within hydrothermal vent deposits from the Endeavour Segment, Juan de Fuca Ridge. Geochim Cosmochim Acta 173:64–85. https://doi.org/10.1016/j.gca.2015.10.021
Kasaya T, Machiyama H, Kitada K, Nakamura K (2015) Trial exploration for hydrothermal activity using acoustic measurements at the North Iheya Knoll. Geochem J 49(6):597–602. https://doi.org/10.2343/geochemj.2.0389
Kawagucci S (2015) Fluid geochemistry of high-temperature hydrothermal fields in the Okinawa Trough: how and where TAIGA of Methane is generated. In: Okino K, Ishibashi J, Sunamura M (eds) Subseafloor biosphere linked to global hydrothermal systems; TAIGA concept. Springer, Tokyo Heidelberg New York Dordrecht London, pp 387–403. https://doi.org/10.1007/978-4-431-54865-2_30
Kawagucci S, Shirai K, Lan TF, Takahata N, Tsunogai U, Sano Y, Gamo T (2010) Gas geochemical characteristics of hydrothermal plumes at the HAKUREI and JADE vent sites, the Izena Cauldron. Okinawa Trough Geochem J 44(6):507–518. https://doi.org/10.2343/geochemj.1.0100
Kawagucci S, Chiba H, Ishibashi J, Yamanaka T, Toki T, Muramatsu Y, Ueno Y, Makabe A, Inoue K, Yoshida N, Nakagawa S, Nunoura T, Takai K, Takahata N, Sano Y, Narita T, Teranishi G, Obata H, Gamo T (2011) Hydrothermal fluid geochemistry at the Iheya North field in the mid-Okinawa Trough: implication for origin of methane in subseafloor fluid circulation systems. Geochem J 45(2):109–124. https://doi.org/10.2343/geochemj.1.0105
Kawagucci S, Ueno Y, Takai K, Toki T, Ito M, Inoue K, Makabe A, Yoshida N, Muramatsu Y, Takahata N, Sano Y, Narita T, Teranishi G, Obata H, Nakagawa S, Nunoura T, Gamo T (2013) Geochemical origin of hydrothermal fluid methane in sediment-associated fields and its relevance to the geographical distribution of whole hydrothermal circulation. Chem Geol 339:213–225. https://doi.org/10.1016/j.chemgeo.2012.05.003
Kawagucci S, Miyazaki J, Noguchi T, Okamura K, Shibuya T, Watsuji T, Nishizawa M, Watanabe H, Okino K, Takahata N, Sano Y, Nakamura K, Shuto A, Abe M, Takaki Y, Nunoura T, Koonjul M, Singh M, Beedessee G, Khishma M, Bhoyroo V, Bissessur D, Kumar LS, Marie D, Tamaki K, Takai K (2016) Fluid chemistry in the Solitaire and Dodo hydrothermal fields of the Central Indian Ridge. Geofluids 16(5):988–1005. https://doi.org/10.1111/gfl.12201
Kumagai H, Nozaki T, Ishibashi J, Maeda L, CK16-01 on-board member (2017) Cruise report SIP-HOT II “Explorer” (SIP–Hydrothermal deposit in Okinawa Trough) CK16-01 (Exp. 908). JAMSTEC, Yokosuka, Japan
Kusakabe M, Mayeda S, Nakamura E (1990) S, O and Sr isotope systematics of active vent materials from the Mariana backarc basin spreading axis at 18°N. Earth Planet Sci Lett 100(1):275–282. https://doi.org/10.1016/0012-821X(90)90190-9
Lee C-S, Shor GG Jr, Bibee LD, Lu RS, Hilde TWC (1980) Okinawa Trough: origin of a back-arc basin. Mar Geol 35(1–3):219–241. https://doi.org/10.1016/0025-3227(80)90032-8
Letouzey J, Kimura M (1986) The Okinawa Trough: genesis of a back-arc basin developing along a continental margin. Tectonophysics 125(1–3):209–230. https://doi.org/10.1016/0040-1951(86)90015-6
Masaki Y, Kinoshita M, Inagaki F, Nakagawa S, Takai K (2011) Possible kilometer-scale hydrothermal circulation within the Iheya-North field, mid-Okinawa Trough, as inferred from heat flow data. JAMSTEC Rep Res Dev 12:1–12. https://doi.org/10.5918/jamstecr.12.1
McArthur JM, Howarth RJ, Bailey TR (2001) Strontium isotope stratigraphy: LOWESS Version 3: best fit to the marine sr-isotope curve for 0–509 Ma and accompanying look-up table for deriving numerical age. J Geol 109(2):155–170. https://doi.org/10.1086/319243
Michard G, Albarède F, Michard A, Minster JF, Charlou JL, Tan N (1984) Chemistry of solutions from the 13°N East Pacific Rise hydrothermal site. Earth Planet Sci Lett 67(3):297–307. https://doi.org/10.1016/0012-821X(84)90169-9
Miyazaki J, Makabe A, Matsui Y, Ebina N, Tsutsumi S, Ishibashi J, Chen C, Kaneko S, Takai K, Kawagucci S (2017) WHATS-3: An improved flow-through multi-bottle fluid sampler for deep-sea geofluid research. Frontiers Earth Sci. https://doi.org/10.3389/feart.2017.00045
Miyoshi Y, Ishibashi J, Shimada K, Inoue H, Uehara S, Tsukimura K (2015) Clay minerals in an active hydrothermal field at Iheya-North-Knoll. Okinawa Trough Resour Geol 65(4):346–360. https://doi.org/10.1111/rge.12078
Mokadem F, Parkinson I, Hathorne E, Anand P, Allen J, Burton K (2015) High-precision radiogenic strontium isotope measurements of the modern and glacial ocean: limits on glacial–interglacial variations in continental weathering. Earth Planet Sci Lett 415:111–120. https://doi.org/10.1016/j.epsl.2015.01.036
Monma H, Iwase R, Mitsuzawa K, Kaiho Y, Fujiwara Y, Amitani Y, Aoki M (1996) Deep tow survey in Nanseishoto Region (K95–07-NSS) (In Japanese with English abstract). JAMSTEC J Deep Sea Res 12:195–210
Mottl MJ, Holland HD (1978) Chemical exchange during hydrothermal alteration of basalt by seawater—I. Experimental results for major and minor components of seawater. Geochim Et Cosmochim Acta 42(8):1103–1115. https://doi.org/10.1016/0016-7037(78)90107-2
Mottl MJ, Seewald JS, Wheat CG, Tivey MK, Michael PJ, Proskurowski G, McCollom TM, Reeves E, Sharkey J, You CF, Chan LH, Pichler T (2011) Chemistry of hot springs along the Eastern Lau Spreading Center. Geochim Cosmochim Acta 75(4):1013–1038. https://doi.org/10.1016/j.gca.2010.12.008
Nakamura K, Kawagucci S, Kitada K, Kumagai H, Takai K, Okino K (2015) Water column imaging with multibeam echo-sounding in the mid-Okinawa Trough: implications for distribution of deep-sea hydrothermal vent sites and the cause of acoustic water column anomaly. Geochem J 49(6):579–596. https://doi.org/10.2343/geochemj.2.0387
Nishizawa A, Kaneda K, Oikawa M, Horiuchi D, Fujioka Y, Okada C (2019) Seismic structure of rifting in the Okinawa Trough, an active backarc basin of the Ryukyu (Nansei-Shoto) island arc–trench system. Earth Planets Space 71(1):21. https://doi.org/10.1186/s40623-019-0998-6
Noguchi T, Shinjo R, Ito M, Takada J, Oomori T (2011) Barite geochemistry from hydrothermal chimneys of the Okinawa Trough: insight into chimney formation and fluid/sediment interaction. J Mineral Petrol Sci 106(1):26–35. https://doi.org/10.2465/jmps.090825
Nozaki T, Nagase T, Takaya Y, Yamasaki T, Otake T, Yonezu K, Ikehata K, Totsuka S, Kitada K, Sanada Y, Yamada Y, Ishibashi J, Kumagai H, Maeda L, the D. V. Chikyu Expedition Scientists (2021) Subseafloor sulphide deposit formed by pumice replacement mineralisation. Sci Rep 11(1):8809. https://doi.org/10.1038/s41598-021-87050-z
Pezzopane SK, Wesnousky SG (1989) Large earthquakes and crustal deformation near Taiwan. J Geophys Res Solid Earth 94(B6):7250–7264. https://doi.org/10.1029/JB094iB06p07250
Piepgras DJ, Wasserburg GJ (1985) Strontium and neodymium isotopes in hot springs on the East Pacific Rise and Guaymas Basin. Earth Planet Sci Lett 72(4):341–356. https://doi.org/10.1016/0012-821X(85)90057-3
Pin C, Briot D, Bassin C, Poitrasson F (1994) Concomitant separation of strontium and samarium-neodymium for isotopic analysis in silicate samples, based on specific extraction chromatography. Anal Chim Acta 298(2):209–217. https://doi.org/10.1016/0003-2670(94)00274-6
Redfield AC (1934) On the proportions of organic derivatives in sea water and their relation to the composition of plankton. In: Daniel RJ (ed) James Johnston Memorial Volume. University Press of Liverpool, Liverpool, pp 176–192
Reeves EP, Seewald JS, Saccocia P, Bach W, Craddock PR, Shanks WC, Sylva SP, Walsh E, Pichler T, Rosner M (2011) Geochemistry of hydrothermal fluids from the PACMANUS, Northeast Pual and Vienna Woods hydrothermal fields, Manus Basin, Papua New Guinea. Geochim Cosmochim Acta 75(4):1088–1123. https://doi.org/10.1016/j.gca.2010.11.008
Rosenbauer RJ, Bischoff JL, Zierenberg RA (1988) The laboratory albitization of mid-ocean ridge basalt. J Geol 96(2):237–244
Saegusa S, Tsunogai U, Nakagawa F, Kaneko S (2006) Development of a multibottle gas-tight fluid sampler WHATS II for Japanese submersibles/ROVs. Geofluids 6(3):234–240. https://doi.org/10.1111/j.1468-8123.2006.00143.x
Sakai H, Gamo T, Kim E-S, Shitashima K, Yanagisawa F, Tsutsumi M, Ishibashi J, Sano Y, Wakita H, Tanaka T, Matsumoto T, Naganuma T, Mitsuzawa K (1990) Unique chemistry of the hydrothermal solution in the mid-Okinawa Trough Backarc Basin. Geophys Res Lett 17(12):2133–2136. https://doi.org/10.1029/GL017i012p02133
Sedwick PN, McMurtry GM, Hilton DR, Goff F (1994) Carbon dioxide and helium in hydrothermal fluids from Loihi Seamount, Hawaii, USA: temporal variability and implications for the release of mantle volatiles. Geochim Cosmochim Acta 58(3):1219–1227. https://doi.org/10.1016/0016-7037(94)90587-8
Seyfried WE Jr, Bischoff JL (1981) Experimental seawater-basalt interaction at 300°C, 500 bars, chemical exchange, secondary mineral formation and implications for the transport of heavy metals. Geochim Cosmochim Acta 45(2):135–147. https://doi.org/10.1016/0016-7037(81)90157-5
Shinjo R, Kato Y (2000) Geochemical constraints on the origin of bimodal magmatism at the Okinawa Trough, an incipient back-arc basin. Lithos 54(3–4):117–137. https://doi.org/10.1016/S0024-4937(00)00034-7
Shinjo R, Chung S-L, Kato Y, Kimura M (1999) Geochemical and Sr-Nd isotopic characteristics of volcanic rocks from the Okinawa Trough and Ryukyu Arc: implications for the evolution of a young, intracontinental back arc basin. J Geophys Res Solid Earth 104(B5):10591–10608. https://doi.org/10.1029/1999JB900040
Shiraki R, Sakai H, Endoh M, Kishima N (1987) Experimental studies on rhyolite- and andesite-seawater interactions at 300°C and 1000 bars. Geochem J 21(4):139–148. https://doi.org/10.2343/geochemj.21.139
Sibuet J-C, Letouzey J, Barbier F, Charvet J, Foucher J-P, Hilde TWC, Kimura M, Chiao L-Y, Marsset B, Muller C, Stéphan J-F (1987) Back arc extension in the Okinawa Trough. J Geophys Res Solid Earth 92(B13):14041–14063. https://doi.org/10.1029/JB092iB13p14041
Spivack AJ, Palmer MR, Edmond JM (1987) The sedimentary cycle of the boron isotopes. Geochim Cosmochim Acta 51(7):1939–1949. https://doi.org/10.1016/0016-7037(87)90183-9
Stevenson E, Burton K, Rickaby R, Parkinson I, Anand P, Hathorne E (2009) Strontium stable isotope behavior in foraminiferal calcite and the retrieval of marine records. EOS Trans Am Geophys Union 90
Suzuki R, Ishibashi J, Nakaseama M, Konno U, Tsunogai U, Gena K, Chiba H (2008) Diverse range of mineralization induced by phase separation of hydrothermal fluid: case study of the Yonaguni Knoll IV hydrothermal field in the Okinawa Trough back-arc basin. Resour Geol 58(3):267–288. https://doi.org/10.1111/j.1751-3928.2008.00061.x
Takai K, Mottl MJ, Nielsen SH, the Expedition 331 Scientists (eds) (2011) Proceedings of the Integrated Ocean Drilling Program, vol 331. Integrated Ocean Drilling Program Management International, Inc., Tokyo
Takai K, Kumagai H, Kubo Y, CK14-04 on-board member (2015) Cruise report SIP-HOT I “Pathfinder” (SIP–Hydrothermal deposit in Okinawa Trough) CK14-04 (Exp. 907). JAMSTEC, Yokosuka, Japan
Toki T, Itoh M, Iwata D, Ohshima S, Shinjo R, Ishibashi J, Tsunogai U, Takahata N, Sano Y, Yamanaka T, Ijiri A, Okabe N, Gamo T, Muramatsu Y, Ueno Y, Kawagucci S, Takai K (2016) Geochemical characteristics of hydrothermal fluids at Hatoma Knoll in the southern Okinawa Trough. Geochem J 50(6):493–525. https://doi.org/10.2343/geochemj.2.0449
Totsuka S, Shimada K, Nozaki T, Kimura J, Chang Q, Ishibashi J (2019) Pb isotope compositions of galena in hydrothermal deposits obtained by drillings from active hydrothermal fields in the middle Okinawa Trough determined by LA-MC-ICP-MS. Chem Geol 514:90–104. https://doi.org/10.1016/j.chemgeo.2019.03.024
Tsunogai U, Toki T, Nakayama N, Gamo T, Kato H, Kaneko S (2003) WHATS: a new multi-bottle gas-tight sampler for sea-floor vent fluids (in Japanese with English abstract). Chikyukagaku (geochemistry) 37(3):101–109. https://doi.org/10.14934/chikyukagaku.37.101
Von Damm KL (1995) Controls on the chemistry and temporal variability of seafloor hydrothermal fluids. In: Humphris SE, Zierenberg RA, Mullineaux LS, Thomson RE (eds) Seafloor hydrothermal systems: physical, chemical, biological, andgeological interactions, vol 91. AGU Monograph. American Geophysical Union, Washington DC, pp 222–247
Von Damm KL, Bischoff JL (1987) Chemistry of hydrothermal solutions from the southern Juan de Fuca Ridge. J Geophys Res 92(11):11334–11346. https://doi.org/10.1029/JB092iB11p11334
Von Damm KL, Edmond JM, Grant B, Measures CI, Walden B, Weiss RF (1985a) Chemistry of submarine hydrothermal solutions at 21°N, East Pacific Rise. Geochim Cosmochim Acta 49(11):2197–2220. https://doi.org/10.1016/0016-7037(85)90222-4
Von Damm KL, Edmond JM, Measures CI, Grant B (1985b) Chemistry of submarine hydrothermal solutions at Guaymas Basin, Gulf of California. Geochim Cosmochim Acta 49(11):2221–2237. https://doi.org/10.1016/0016-7037(85)90223-6
Wei W (2007) Fluid origins, paths, and fluid-rock reactions at convergent margins, using halogens, Cl stable isotopes, and alkali metals as geochemical tracers. University of California, San Diego
White WM, Hofmann AW, Puchelt H (1987) Isotope geochemistry of Pacific Mid-Ocean Ridge Basalt. J Geophys Res Solid Earth 92(B6):4881–4893. https://doi.org/10.1029/JB092iB06p04881
Wilckens FK, Reeves EP, Bach W, Seewald JS, Kasemann SA (2019) Application of B, Mg, Li, and Sr isotopes in acid-sulfate vent fluids and volcanic rocks as tracers for fluid-rock interaction in back-arc hydrothermal systems. Geochem Geophys Geosyst 20(12):5849–5866. https://doi.org/10.1029/2019GC008694
Williams LB, Hervig RL, Holloway JR, Hutcheon I (2001) Boron isotope geochemistry during diagenesis. Part I. Experimental determination of fractionation during illitization of smectite. Geochim Et Cosmochim Acta 65(11):1769–1782. https://doi.org/10.1016/S0016-7037(01)00557-9
Yamaoka K, Hong E, Ishikawa T, Gamo T, Kawahata H (2015) Boron isotope geochemistry of vent fluids from arc/back-arc seafloor hydrothermal systems in the western Pacific. Chem Geol 392:9–18. https://doi.org/10.1016/j.chemgeo.2014.11.009
Yeats CJ, Hollis SP, Halfpenny A, Corona J-C, LaFlamme C, Southam G, Fiorentini M, Herrington RJ, Spratt J (2017) Actively forming Kuroko-type volcanic-hosted massive sulfide (VHMS) mineralization at Iheya North, Okinawa Trough, Japan. Ore Geol Rev 84:20–41. https://doi.org/10.1016/j.oregeorev.2016.12.014
Yokoo Y, Nakano T, Nishikawa M, Quan H (2004) Mineralogical variation of Sr–Nd isotopic and elemental compositions in loess and desert sand from the central Loess Plateau in China as a provenance tracer of wet and dry deposition in the northwestern Pacific. Chem Geol 204(1):45–62. https://doi.org/10.1016/j.chemgeo.2003.11.004
Yoshimura T, Wakaki S, Ishikawa T, Gamo T, Araoka D, Ohkouchi N, Kawahata H (2020) A systematic assessment of stable Sr isotopic compositions of vent fluids in arc/back-arc hydrothermal systems: effects of host rock type, phase separation, and overlying sediment. Frontiers Earth Sci. https://doi.org/10.3389/feart.2020.591711
You CF, Gieskes JM (2001) Hydrothermal alteration of hemi-pelagic sediments: experimental evaluation of geochemical processes in shallow subduction zones. Appl Geochem 16:1055–1066. https://doi.org/10.1016/S0883-2927(01)00024-5
Yuan D, Zhu J, Li C, Hu D (2008) Cross-shelf circulation in the Yellow and East China Seas indicated by MODIS satellite observations. J Mar Syst 70(1):134–149. https://doi.org/10.1016/j.jmarsys.2007.04.002
Zhang X, Zhai S, Yu Z (2020) Strontium isotope compositions of hydrothermal barite from the Yonaguni IV: insight into fluid/sediment interaction and barite crystallization condition. J Ocean Univ China 19(2):377–385. https://doi.org/10.1007/s11802-020-4021-4
Zhang X, Zhai S, Sun Z, Yu Z, Guo K, Xu J (2022) Rare earth elements and Sr, S isotope compositions of hydrothermal deposits from the Okinawa Trough: insight into mineralization condition and metal sources. Mar Geol 443:106683. https://doi.org/10.1016/j.margeo.2021.106683
Acknowledgements
The authors express their heartfelt gratitude to the professors and staff of the University of the Ryukyus for their examination of this work, which has enhanced the quality of the manuscript. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study was funded by the Council for Science, Technology, and Innovation (CSTI) cross-ministerial Strategic Innovation Promotion Program (SIP), Okinawa Research Core for Highly Innovative Discipline Science for Marine Science (ORCHIDS), Japan Science and Technology Agency (JST) Research Institute of Science and Technology for Society (RISTEX) grant (Grant Number: JPMJRX19IA), Japan Society for the Promotion of Science (JSPS) KAKENHI grants (Grant Numbers: JP18H03733 and JP20H04315), and The Research Institute for Humanity and Nature (RIHN) LINKAGE project (No. 14200145).
Author information
Authors and Affiliations
Contributions
TT conceived the study, obtained funding, and conducted the sampling, analysis, data processing, and manuscript writing and revision. TN conducted the analysis and data processing and wrote the first draft of the manuscript. YU conducted the analysis and data processing and wrote the first draft of the manuscript. RS obtained funding, conducted the analysis and data processing, and wrote and revised the manuscript. SH-W analyzed and processed the data and wrote the first draft of the manuscript. JI conceived the study, obtained funding, conducted the sampling and analysis, and revised the manuscript. SK conceived the study, conducted sampling, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Data from volcanic rocks.
For our analyses, we used previously reported data (Shinjo et al. 1999; Shinjo and Kato 2000) and rock samples collected from dredges and dives (using remotely operated vehicles and submersibles) of the SOT during several cruises. Table
3 lists the locations and identities of the sampling points, which are plotted as solid yellow circles in Fig. 1b. Analytical procedures were based on previously described methods (Pin et al. 1994). Before collecting measurements, we rinsed the rocks in an ultrasonic bath and repeatedly washed them in hot ultrapure water for 1–3 weeks to eliminate the influence of seawater. The rock samples were crushed to powder using an agate mill before leaching in hot 6 M HCl (EL grade). After separation, the measurements were collected using thermal ionization mass spectrometry (MAT262; Finnigan). The dried eluent was dissolved in 1 M H3PO4 (2 µL), loaded onto a tantalum filament, and next, the solution was dried with a current. The 87Sr/86Sr values were normalized using the average value (0.71023 ± 0.00060) of the NBS987 standard material (National Bureau of Standards).
Table 3 lists the analytical results for the rock samples. The values ranged from 0.704026 to 0.706077, indicating that they were more enriched in 87Sr than MORB (0.7023–0.7030; White et al. 1987). These rock samples were more radiogenic than basalts collected from the middle to southern regions of the Okinawa Trough (0.703686–0.704620; n = 15; Shinjo et al. 1999). Volcanic rocks collected west of 123°E had a higher 87Sr/86Sr ratio than those collected from the MOT (0.704018–0.704884; n = 14; Shinjo and Kato 2000). However, volcanic rocks east of 123°E had values comparable to those of volcanic rocks from the MOT (Table 3). Based on these integrated data, the 87Sr/86Sr ratio of the volcanic rocks in the Okinawa Trough was found to be 0.7030–0.7049 (bold red bar in Fig. 3).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toki, T., Nohara, T., Urata, Y. et al. Sr isotopic ratios of hydrothermal fluids from the Okinawa Trough and the implications of variation in fluid–sediment interactions. Prog Earth Planet Sci 9, 59 (2022). https://doi.org/10.1186/s40645-022-00519-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40645-022-00519-x