Abstract
Patients on mechanical ventilation may receive intravenous fluids via restrictive or liberal fluid management. A clear and objective differentiation between restrictive and liberal fluid management strategies is lacking in the literature. The liberal approach has been described as involving fluid rates ranging from 1.2 to 12 times higher than the restrictive approach. A restrictive fluid management may lead to hypoperfusion and distal organ damage, and a liberal fluid strategy may result in endothelial shear stress and glycocalyx damage, cardiovascular complications, lung edema, and distal organ dysfunction. The association between fluid and mechanical ventilation strategies and how they interact toward ventilator-induced lung injury (VILI) could potentiate the damage. For instance, the combination of a liberal fluids and pressure-support ventilation, but not pressure control ventilation, may lead to further lung damage in experimental models of acute lung injury. Moreover, under liberal fluid management, the application of high positive end-expiratory pressure (PEEP) or an abrupt decrease in PEEP yielded higher endothelial cell damage in the lungs. Nevertheless, the translational aspects of these findings are scarce. The aim of this narrative review is to provide better understanding of the interaction between different fluid and ventilation strategies and how these interactions may affect lung and distal organs. The weaning phase of mechanical ventilation and the deresuscitation phase are not explored in this review.
Take-home message
Ventilatory management may be affected by restrictive and liberal fluid strategies due to physiological interaction between heart–lung, possibly yielding to distal organ damage in critical ill patients. Pre-clinical studies evaluated the effects of different fluid strategies on ventilator-induced lung injury during assisted ventilation, at different PEEP levels, as well as after an abrupt decrease in PEEP.
Similar content being viewed by others
Background
Mechanical ventilation (MV) often results in impaired gas exchange, hemodynamic instability, and injury to endothelial cells. Intravenous (IV) fluid therapy is often required in patients undergoing MV to restore hemodynamics and distal organ perfusion [1, 2]. According to Paracelsus (1493–1541) and previous authors [1]: “Dosis sola facit venenum”, all things are poison and it is the dose that makes something poisonous. Optimizing tissue perfusion and oxygen delivery while preventing fluid overload is a challenge in critically ill patients. Restrictive fluid management [1, 3] can be associated with peripheral hypoperfusion and distal organ damage [4,5,6,7]. However, a more liberal approach could increase mortality because it may lead to endothelial cell damage, lung and peripheral tissue edema, increased intra-abdominal pressure, and gastrointestinal and renal dysfunction [5, 6, 8,9,10,11,12,13,14,15,16,17]. Notwithstanding, the myriad of modes and settings for MV, such as positive end-expiratory pressure (PEEP) and tidal volume (Vt), can have distinct impacts on cardiovascular physiology, as well as volemic status and fluid balance [7, 18, 19]. Variations in pleural (Ppl) and transpulmonary (Ptp) pressures caused by assisted or controlled MV have been shown to affect the preload and afterload as well as capillary transvascular filtration pressures [20,21,22,23]. Some experiments have shown that mismatch between fluid and ventilatory strategies can worsen ventilator-induced lung injury (VILI) as well as reduce cardiac output and tissue perfusion [7, 18, 22]. In specific scenarios, such as acute respiratory distress syndrome (ARDS), more than 60% of patients are dependent on inotropic drugs to achieve an adequate arterial pressure [24] and often require IV fluids as part of hemodynamic support. Although protective MV and a restrictive fluid strategy have been suggested for critically ill patients, this combination may affect distal organs [25, 26]. However, evidence evaluating the interaction between fluid therapies with different modes of MV is scarce. Most clinical studies investigating the impact of restrictive and liberal fluid therapies on organ damage and mortality do not provide detailed information concerning the MV strategy or vice versa.
This narrative review aims to provide better understanding of the crosstalk between fluids and MV strategies and the impact of this interaction on lung and distal organs. The weaning phase of MV and the deresuscitation phase are not explored in this review.
Physiologic rationale: heart–lung interactions and distal organ damage
Because of its location, the heart is inevitably subjected to the mechanical forces of the lungs, namely, Ppl and Ptp [27,28,29]. These forces can have an impact on at least two factors regulating cardiac output: venous return and the heart’s ability to deal with preload during the systolic phase [28, 29]. During spontaneous breathing, Ppl is negative during the expiratory phase and even more negative during inspiration [30], favoring systemic venous return in normo- or hypervolemia. During positive pressure ventilation, the increase in intrathoracic pressure increases right atrial pressure, reducing systemic venous return [27, 29, 31, 32]. The left ventricle, in turn, has its afterload reduced by a lower transmural pressure and a transiently increased preload by a higher alveolar pressure that squeezes blood toward the left ventricle [29]. Left ventricular afterload is reduced due to an increase in pleural pressure during MV, whereas left ventricular transmural pressure tends to decrease because it is the difference between ventricular and pleural pressure. Thus, during MV when Ppl is positive, transmural pressure decreases. However, over time, the transmural pressure may recover due to increased stressed volume or vessel tone, which in turn can increase the mean systemic filling pressure, favoring venous return [33]. Under protective MV, about 70% ± 27% of airway pressure (Paw) is transmitted to juxtacardiac pleura, 37% ± 17% to the pericardium, and 43% ± 11% to the vena cava; these numbers can be even higher when chest wall compliance is reduced [32]. Organ perfusion pressure is determined by the difference between inflow and outflow pressure, therefore a higher intrathoracic pressure during positive pressure ventilation may potentially compromise organ perfusion, ultimately leading to organ damage. Because the right ventricle has less contractile reserve than the left ventricle, intrathoracic pressure and afterload swings during the respiratory cycle have a greater effect on the former than on the latter [34]. This concept becomes especially important in ARDS, where hypoxic vasoconstriction can increase right ventricular afterload, which may lead to right cardiac failure [35].
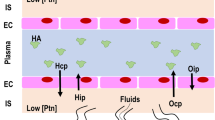
Pulmonary transvascular filtration pressure is defined as the difference between vascular hydrostatic pressure (Ph) and Ppl. In spontaneous breathing and assisted ventilation (such as pressure-support ventilation [PSV]), a more negative inspiratory Ppl may increase transvascular filtration pressure. In the presence of extremely negative Ppl, due to intense inspiratory effort against obstructed airways, a sudden increase in pulmonary transvascular filtration pressure and lung edema may occur [36]. The association between negative Ppl, resulting from spontaneous breathing (or assisted ventilation), and hypervolemia (that may be caused by liberal fluids) increases Ph, thus increasing the risk of edema, which can be even worse in the presence of increased vascular permeability [37]. These mechanisms are presented in Fig. 1.

Adapted from Vieillard-Baron et al. [20]
Hemodynamic changes in controlled and assisted mechanical ventilation. In pressure-support ventilation, pleural pressure (Ppl) is lowered by inspiratory efforts, leading to higher venous return and lower right ventricular (RV) afterload. Increased transmural pressure (caused by the decrease in Ppl from inspiratory effort) increases hydrostatic pressure (Ph) in the microvasculature, worsening edema. Increased flow in lung vessels may also lead to shear stress, causing further endothelial damage and protein and fluid leak into alveolar space. Transvascular filtration pressure (PTvF) is higher in pressure-support ventilation than in pressure control ventilation (even at the same transpulmonary pressure (Ptp) given by the difference between alveolar pressure (Palv) and Ppl).
The MV mode, whether spontaneous or controlled, can change intrathoracic pressures and may lead to changes in hemodynamics [35]. Given the significant hemodynamic impact of the heart–lung interactions in critically ill patients, the use of hemodynamic tests and indices have been widely endorsed to better predict volume responsiveness [38].
Liberal and restrictive fluid management: search for an objective definition
Restrictive versus liberal fluid management have been compared in various settings, albeit not clearly defined [5, 7] due to the different terminologies adopted in clinical studies. “Conservative” [7, 39,40,41,42,43,44] and “restrictive” [34, 45,46,47,48,49,50] are used interchangeably without any clearly defined pattern regarding fluid rates. There is an overall lack of consensus on this; for example, a “restrictive” approach (6 ml/kg/h) has been compared with a “conservative” approach (12 ml/kg/h) [51]. Higher fluid rates are frequently named “liberal”, and older clinical studies use labels such as “standard”, “high volume”, and even “aggressive” [11, 52,53,54]. Experimental and clinical studies have so far used the term “liberal” over a remarkably wide range from 1.2 to 12 times the fluid rates referred to as restrictive (Additional file 1: Table S1) [10, 45, 46, 48, 55,56,57].
Impact of restrictive versus liberal fluid management on lung and distal organ damage
Recent surgical and intensive care guidelines—such as Enhanced Recovery from Anesthesia and Surgery (ERAS) and UK guidelines for the management of ARDS—support restrictive fluid therapies [6, 58]. Evidence points to a significant association between liberal fluids, hypervolemia, and glycocalyx damage (shown by increased plasma syndecan-1 [59,60,61,62], hyaluronic acid [60], and heparan sulfate [17, 61]). Also, increased central venous and capillary hydrostatic pressures may reduce organ perfusion pressure and facilitate lung interstitial edema [2]. In murine models of acute lung injury, increased capillary hydrostatic pressure caused by liberal fluids was shown to promote perivascular lung edema than a restrictive approach [7, 18]. In addition, higher fluid rates could even increase the risk of developing ARDS after surgery [63]. Thus, the main consequences of liberal fluids may be lung edema, reduced oxygen delivery, and distal organ damage.
Organ damage may also be caused by insufficient fluid therapy. For example, an excessively restrictive approach can lead to renal hypoperfusion and further functional impairment [6, 34, 64]. The Surviving Sepsis Campaign indicates that there is not enough evidence to recommend restrictive fluids in the first 24 h of resuscitation in patients with signs of hypoperfusion and volume depletion [4]. The BaSICS study showed no difference in 90-day mortality in patients in the intensive care unit (ICU) when comparing slower versus faster crystalloid infusion rates [65]. In major abdominal surgery, restrictive fluids resulted in higher acute kidney injury, need for renal replacement therapy, and surgical-site infection rates than a liberal approach (8.6% versus 5.0%, 16.5% versus 13.6%, and 0.3% versus 0.9%, respectively; all p < 0.05) [34].
Even in specific syndromes, such as ARDS, it seems that distinct phenotypes (hyper- or hypoinflammatory) may respond differently to restrictive or liberal approaches, as demonstrated in the cohort in the Fluids and Catheters Treatment Trial [39]. In this study, subphenotype I (mainly trauma, aspiration, or pneumonia) had lower 90-day mortality under restrictive fluid management (26% versus 18%), whereas patients with subphenotype II (sepsis as a primary risk factor and a lower central venous pressure) had lower mortality under a liberal fluid management (40 versus 50%). Thus, fluid therapy should be individualized according to the patient’s specific needs. ERAS guidelines strongly recommend avoiding excessively restrictive or liberal fluid regimes during lung surgery [66]. ERAS also supports goal-directed fluid therapy with dynamic monitoring over a liberal fluid management for renal transplantation [67]. There is no mention of the relationship between fluid and MV strategies.
Over the last decades, attempts to improve outcomes by fluid balance have ranged from dehydration and negative fluid balance to normovolemia and even moderate hypervolemia as primary therapeutic goals [68]. The 2018 European Society of Intensive Care Medicine consensus statement on fluid therapy in neurointensive care [69] suggests targeting normovolemia during fluid replacement in patients with a brain injury. It also suggests fluid balance, arterial blood pressure, and variables such as cardiac output and blood lactate as primary and safety endpoints to titrate fluids.
Impact of assisted versus controlled mechanical ventilation on lung and distal organ damage
Some studies have suggested that assisted spontaneous breathing modes such as PSV could be associated with a reduction in VILI and length of stay in the ICU, and an increase in ventilator-free days in experimental and clinical studies [70,71,72,73,74,75]. Although assisted ventilation can prevent the harmful effects of controlled MV, intense inspiratory efforts during assisted ventilation can also dramatically change the intrathoracic pressures. This can lead to increased lung perfusion and transvascular filtration pressures and facilitate alveolar edema. Increased inspiratory efforts may lead to patient self-inflicted lung injury (P-SILI) and negative pressure edema [76]. This situation could be even worse in lungs with endothelial injury. A recent study [77] hypothesized that intrapulmonary dyssynchrony (i.e., pendelluft, defined in this study as the percentage of the Vt that moves during inspiration from the non-dependent to the dependent lung region) could be a leading mechanism for VILI and P-SILI. The authors showed that regional pendelluft during BiPAP may reflect local swings in Ppl during spontaneous breathing and be associated with an increase in specific inflammatory biomarkers in patients with ARDS. On the other hand, muscle paralysis and controlled ventilation were shown to be safer than spontaneous breathing in severe acute lung injury in an animal model [78].
Protective controlled ventilation (low Vt and moderate-to-high PEEP after recruitment maneuver) is associated with a lower incidence of acute kidney injury [79, 80] and reduced pulmonary complications and mortality [6, 66]. However, depending on airway pressures, it affects hemodynamics [22]. Higher PEEP and peak inspiratory pressures may be associated with distal organ damage as long as hemodynamics are altered and vasopressin secretion is increased [18, 81, 82]. Also, a high PEEP may produce a masking effect on the PaO2/FiO2 ratio due to changes in hemodynamics—namely a reduction in cardiac output and a proportional reduction in venous admixture.
Interaction between mechanical ventilation and fluid management
In 1947, researchers first showed a reduction in renal blood flow, glomerular filtration rate, and urine output during positive airway pressure [83]. Since then, only a few studies have assessed the interaction between fluid and ventilatory strategies. Here, we discuss the evidence comparing lung and organ damage under assisted or controlled ventilation in restrictive and liberal fluid management.
PSV is a frequently used mode of assisted ventilation in patients who are breathing spontaneously. Intense inspiratory efforts during assisted ventilation could lead to hemodynamic impairment [7, 20, 21, 70], higher transpulmonary pressures [78], increased lung perfusion, and likely P-SILI. Judicious adjustment of delta pressure during PSV [84] or assisted modes and higher PEEP levels [85, 86] can help prevent P-SILI and possibly protect patients during assisted ventilation, mainly with liberal fluid management. The increased transvascular pressure (caused by increased inspiratory efforts and liberal fluids) might cause vascular shear stress, ongoing endothelial damage, and alveolar edema in patients with high capillary permeability, as observed in sepsis and ARDS [17, 24]. The combination of liberal fluids and PSV increased alveolar diffuse damage and MMP-9 gene expression and decreased specific biomarkers associated with epithelial integrity (occludin, zona occludens-1, and claudin-4) [7]. Although no differences in kidney morphology were observed, NGAL (neutrophil gelatinase associated lipocalin) expression during PSV was lower with a liberal fluids approach compared with a restrictive fluids approach.
The effects of controlled ventilation on cardiac output and tissue perfusion partially depend on Vt. In this context, both pressure control ventilation (PCV) and volume control ventilation (VCV) with the same tidal volume resulted in comparable cardiac output during MV. However, PCV may result in higher cardiac outputs when lower Vt are used [35, 87, 88]. The reduction in cardiac output observed in VCV partially explains the negative impact of positive pressure ventilation on renal function. However, other mechanisms may play a role in the development of kidney injury, including redistribution of intrarenal blood flow, hyperactivation of the sympathetic nervous system, and the action of inflammatory mediators [89]. In an attempt to improve cardiac output, liberal fluids strategy may be advised. First, to improve cardiac output, the patient must be fluid responsive (if cardiac output response is negligible, fluid should be stopped) [38, 90]; second, stretched alveolar epithelial cells can have disrupted tight junctions [23]; in this case, a high hydrostatic pressure could worsen lung edema. It has been demonstrated that in VCV, Vt is positively and linearly correlated with Ppl. Vascular filtration pressure for an intrathoracic vessel is the difference between hydrostatic vascular pressure and Ppl, and researchers have shown that superior vena cava transmural pressure decreased during inspiration in VCV, whereas right atrium transmural pressure did not [32]. This reduction of transmural pressure in intrathoracic vessels could be protective with a liberal fluid management, because it would reduce transvascular filtration and formation of edema. Although no clinical studies have investigated this interaction, chloride-rich fluids can promote renal vasoconstriction, which could be even worse in the presence of positive pressure ventilation [90].
It has been shown that PSV in combination with a restrictive fluid strategy resulted in less lung epithelial damage in a model of acute lung injury. One likely explanation is that damage to tight junctions, which was identified by a decrease in occludin expression, was observed in animals during PSV combined only with a liberal fluid strategy but not with a restrictive fluid approach. The interaction between the mode of MV and the fluid strategy may have a mechanistic relationship [7]. In addition, edema may increase further if tight junction connections, which are constitutive in epithelial and endothelial structural cells, are lost during the stretch movements produced by tensile stress in PSV.
The choice of PEEP levels should also take into account the volume status. It has been shown in clinical studies that a high PEEP level can decrease kidney function despite the fluid strategy because it can increase peak inspiratory pressures [18]. The combination of high PEEP and liberal fluids worsened lung injury in a murine model of ARDS [18]. In addition, an abrupt decrease in PEEP has been shown to increase club cell-16 protein, a marker of alveolar epithelial cell damage marker, in an experimental model of ARDS. When combined with a liberal fluid management, it worsened diffuse alveolar damage and increased the levels of inflammatory and endothelial cell damage biomarkers [19]. Table 1 summarizes the main findings from pre-clinical studies investigating interactions between MV and fluid management.
Clinical implications
The clinical evidence is scarce and mainly limited to a few experimental studies, therefore the effects of interaction between MV and fluid management on organ damage are still poorly understood. Thus, it would be reckless to address clinical recommendations based on it. Nevertheless, some possible clinical implications from these experimental studies should be pointed out. First, whenever restrictive or liberal fluids are strongly recommended, caution should be taken when choosing the ventilatory strategy. Especially in the early phase of resuscitation, when large amounts of fluids are warranted, assisted ventilation and intense inspiratory efforts may cause higher transvascular filtration pressures, vascular edema, and epithelial cell damage, especially with concurrent lung and endothelial damage. Experimental data show that despite protective ventilation, high and rapid intravenous fluid boluses can be associated with worsened lung injury and respiratory function [91]. In this setting, careful titration of fluid therapy or opting for controlled ventilation is probably beneficial. In contrast, whenever a patient receives a combined strategy with restrictive fluids and controlled MV, distal organ damage should be closely monitored, especially when high PEEP is used or when decreased lung compliance leads to high peak inspiratory pressures. Ventilatory settings should also be carefully titrated because the association between higher PEEPs and liberal fluids may worsen lung injury, and the association between higher PEEPs and restrictive fluids may aggravate distal organ damage.
Conclusions
The understanding of physiological heart–lung interaction is fundamental to optimize fluid strategies and mechanical ventilation setting. Accepted definitions of restrictive or liberal fluid strategies do not exist. Both restrictive and liberal fluid strategies may lead to hypoperfusion and edema of distal organs, respectively. Assisted ventilation may cause self-inflicted lung injury associated with liberal fluid strategies, while controlled ventilation may impair hemodynamics, and thus distal organ damage with restrictive fluid strategies, especially when high PEEP levels are used. Gradual transitioning of ventilatory patterns is suggested to promote lung protection due to the impact on vascular compartment. Optimization of the type and mechanical ventilation setting should consider careful titration of fluid strategies in critically ill patients.
Availability of data and materials
Not applicable.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- BiPAP:
-
Biphasic positive airway pressure
- CPAP:
-
Continuous positive airway pressure
- ERAS:
-
Enhanced recovery from anesthesia and surgery
- ICU:
-
Intensive care unit
- MV:
-
Mechanical ventilation
- NGAL:
-
Neutrophil gelatinase associated lipocalin
- PCV:
-
Pressure control ventilation
- PEEP:
-
Positive end-expiratory pressure
- P-SILI:
-
Patient self-inflicted lung injury
- PSV:
-
Pressure support ventilation
- VCV:
-
Volume-controlled ventilation
- VILI:
-
Ventilator-induced lung injury
References
Malbrain MLNG, Langer T, Annane D, Gattinoni L, Elbers P, Hahn RG et al (2020) Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the International Fluid Academy (IFA). Ann Intensive Care 10(1):64. https://doi.org/10.1186/s13613-020-00679-3
De Backer D, Aissaoui N, Cecconi M, Chew MS, Denault A, Hajjar L et al (2022) How can assessing hemodynamics help to assess volume status? Intensive Care Med 48:1482–1494. https://doi.org/10.1007/s00134-022-06808-9
Malbrain MLNG, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal P-J, Joannes-Boyau O et al (2018) Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care 8(1):66. https://doi.org/10.1186/s13613-018-0402-x
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C et al (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 7(11):1181–1247. https://doi.org/10.1007/s00134-021-06506-y
Rahbari NN, Zimmermann JB, Schmidt T, Koch M, Weigand MA, Weitz J (2009) Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg 96(4):331–341. https://doi.org/10.1002/bjs.6552
Nelson G, Bakkum-Gamez J, Kalogera E, Glaser G, Altman A, Meyer LA et al (2019) Guidelines for perioperative care in gynecologic/oncology: enhanced recovery after surgery (ERAS) society recommendations—2019 update. Int J Gynecol Cancer 29(4):651–668. https://doi.org/10.1136/ijgc-2019-000356
De Carvalho EB, Fonseca ACF, Magalhães RF, Pinto EF, dos Samary CS, Antunes MA et al (2022) Effects of different fluid management on lung and kidney during pressure-controlled and pressure-support ventilation in experimental acute lung injury. Physiol Rep 10(17):e15429. https://doi.org/10.14814/phy2.15429
Boyd JH, Forbes J, Nakada T, Walley KR, Russell JA (2011) Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 39(2):259–265. https://doi.org/10.1097/CCM.0b013e3181feeb15
Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H et al (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34(2):344–353
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, DeBoisblanc B et al (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354(24):2564–2575. https://doi.org/10.1056/NEJMoa062200
Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K et al (2003) Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 238(5):641. https://doi.org/10.1097/01.sla.0000094387.50865.23
Malbrain MLNG, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ et al (2014) Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther 46(5):361–380. https://doi.org/10.5603/AIT.2014.0060
Chowdhury AH, Cox EF, Francis ST, Lobo DN (2012) A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 256(1):18–24. https://doi.org/10.1097/SLA.0b013e318256be72
Salahuddin N, Sammani M, Hamdan A, Joseph M, Al-Nemary Y, Alquaiz R et al (2017) Fluid overload is an independent risk factor for acute kidney injury in critically Ill patients: results of a cohort study. BMC Nephrol 18(1):45. https://doi.org/10.1186/s12882-017-0460-6
de Mendes RS, Pelosi P, Schultz MJ, Silva PL (2020) Fluids in ARDS: more pros than cons. Intensive Care Med Exp 8(Suppl 1):32. https://doi.org/10.1186/s40635-020-00319-x
Ingelse SA, Geukers VG, Dijsselhof ME, Lemson J, Bem RA, Van Woensel JB (2019) Less is more?—a feasibility study of fluid strategy in critically ill children with acute respiratory tract infection. Front Pediatr 7:496. https://doi.org/10.3389/fped.2019.00496
Hippensteel JA, Uchimido R, Tyler PD, Burke RC, Han X, Zhang F et al (2019) Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care 23(1):259. https://doi.org/10.1186/s13054-019-2534-2
Felix NS, Maia LA, Rocha NN, Rodrigues GC, Medeiros M, da Silva LA et al (2022) Biological impact of restrictive and liberal fluid strategies at low and high PEEP levels on lung and distal organs in experimental acute respiratory distress syndrome. Front Physiol. https://doi.org/10.3389/fphys.2022.992401
Rocha NN, Samary CS, Antunes MA, Oliveira MV, Hemerly MR, Santos PS et al (2021) The impact of fluid status and decremental PEEP strategy on cardiac function and lung and kidney damage in mild-moderate experimental acute respiratory distress syndrome. Respir Res 22(1):1–12. https://doi.org/10.1186/s12931-021-01811-y
Vieillard-Baron A, Matthay M, Teboul JL, Bein T, Schultz M, Magder S et al (2016) Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 42:739–749. https://doi.org/10.1007/s00134-016-4326-3
Yoshida T, Fujino Y, Amato MBP, Kavanagh BP (2017) Fifty years of research in ARDS spontaneous breathing during mechanical ventilation risks, mechanisms, and management. Am J Respir Crit Care Med 195:985–992. https://doi.org/10.1164/rccm.201604-0748CP
Roosens CD, Ama R, Leather HA, Segers P, Sorbara C, Wouters PF et al (2006) Hemodynamic effects of different lung-protective ventilation strategies in closed-chest pigs with normal lungs. Crit Care 34(12):2990–2886. https://doi.org/10.1097/01.CCM.0000242758.37427.16
Cavanaugh KJ Jr, Oswari J, Margulies SS (2001) Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol 25(5):584–591. https://doi.org/10.1165/ajrcmb.25.5.4486
Mekontso Dessap A, Boissier F, Charron C, Bégot E, Repessé X, Legras A et al (2016) Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 42(5):862–870. https://doi.org/10.1007/s00134-015-4141-2
Upadhyaya VD, Shariff MZ, Mathew RO, Hossain MA, Asif A, Vachharajani TJ (2020) Management of acute kidney injury in the setting of acute respiratory distress syndrome: review focusing on ventilation and fluid management strategies. J Clin Med Res 12(1):1. https://doi.org/10.14740/jocmr3938
Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V et al (2003) Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 289(16):2104–2112. https://doi.org/10.1001/jama.289.16.2104
Pinsky MR (2018) Cardiopulmonary interactions: physiologic basis and clinical applications. Ann Am Thorac Soc 15(Suppl 1):S45–S48. https://doi.org/10.1513/AnnalsATS.201704-339FR
Magder S (2018) Heart–lung interaction in spontaneous breathing subjects: the basics. Ann Transl Med 6(18):348. https://doi.org/10.21037/atm.2018.06.19
Vistisen ST, Enevoldsen JN, Greisen J, Juhl-Olsen P (2019) What the anaesthesiologist needs to know about heart–lung interactions. Best Pract Res Clin Anaesthesiol 33(2):165–177. https://doi.org/10.1016/j.bpa.2019.05.003
Neupane K, Jamil RT (2020) Physiology, transpulmonary pressure. StatPearls Publishing, Treasure Island
Berger D, Bloechlinger S, Takala J, Sinderby C, Brander L (2014) Heart–lung interactions during neurally adjusted ventilatory assist. Crit Care 18(5):1–11. https://doi.org/10.1186/s13054-014-0499-8
Lansdorp B, Hofhuizen C, van Lavieren M, van Swieten H, Lemson J, van Putten MJAM et al (2014) Mechanical ventilation-induced intrathoracic pressure distribution and heart–lung interactions. Crit Care Med 42(9):1983–1990. https://doi.org/10.1097/CCM.0000000000000345
Magder SA, Lichtenstein S, Adelman AG (1983) Effects of negative pleural pressure on left ventricular hemodynamics. Am J Cardiol 52(5):588–593. https://doi.org/10.1016/0002-9149(83)90032-2
Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D et al (2018) Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med 378(24):2263–2274. https://doi.org/10.1056/NEJMoa1801601
Mahmood SS, Pinsky MR (2018) Heart–lung interactions during mechanical ventilation: the basics. Ann Transl Med 6(18):349. https://doi.org/10.21037/atm.2018.04.29
Bhattacharya M, Kallet RH, Ware LB, Matthay MA (2016) Negative-pressure pulmonary edema. Chest 150:927–933. https://doi.org/10.1016/j.chest.2016.03.043
Brochard L, Slutsky A, Pesenti A (2017) Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med 195:438–442. https://doi.org/10.1164/rccm.201605-1081CP
Pinsky MR, Cecconi M, Chew MS, De Backer D, Douglas I, Edwards M et al (2022) Effective hemodynamic monitoring. Crit Care 26(1):294. https://doi.org/10.1186/s13054-022-04173-z
Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT et al (2017) Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 195(3):331–338. https://doi.org/10.1164/rccm.201603-0645OC
Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC et al (2017) Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med 43(2):155–170. https://doi.org/10.1007/s00134-016-4573-3
Evans RG, Naidu B (2012) Does a conservative fluid management strategy in the perioperative management of lung resection patients reduce the risk of acute lung injury? Interact Cardiovasc Thorac Surg 15(3):498–504. https://doi.org/10.1093/icvts/ivs175
Ingelse SA, IJland MM, van Loon LM, Bem RA, van Woensel JBM, Lemson J (2021) Early restrictive fluid resuscitation has no clinical advantage in experimental severe pediatric acute respiratory distress syndrome. Am J Physiol Cell Mol Physiol 320(6):L1126–L1136. https://doi.org/10.1152/ajplung.00613.2020
Herbert JA, Valentine MS, Saravanan N, Schneck MB, Pidaparti R, Fowler AA et al (2016) Conservative fluid management prevents age-associated ventilator induced mortality. Exp Gerontol 81:101–109. https://doi.org/10.1016/j.exger.2016.05.005
Griffiths MJD, McAuley DF, Perkins GD, Barrett N, Blackwood B, Boyle A et al (2019) Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res 6(1):e000420. https://doi.org/10.1136/bmjresp-2019-000420
Holte K, Hahn RG, Ravn L, Bertelsen KG, Hansen S, Kehlet H (2007) Influence of “liberal” versus “restrictive” intraoperative fluid administration on elimination of a postoperative fluid load. Anesthesiology 106(1):75–79. https://doi.org/10.1097/00000542-200701000-00014
Holte K, Kristensen BB, Valentiner L, Foss NB, Husted H, Kehlet H (2007) Liberal versus restrictive fluid management in knee arthroplasty: a randomized, double-blind study. Anesth Analg 105(2):465–474. https://doi.org/10.1213/01.ane.0000263268.08222.19
Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I (2005) Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 103(1):25–32. https://doi.org/10.1097/00000542-200507000-00008
Lobo SM, Ronchi LS, Oliveira NE, Brandão PG, Froes A, Cunrath GS et al (2011) Restrictive strategy of intraoperative fluid maintenance during optimization of oxygen delivery decreases major complications after high-risk surgery. Crit Care 15(5):R226. https://doi.org/10.1186/cc10466
Palomba H, Treml RE, Caldonazo T, Katayama HT, Gomes BC, Malbouisson LMS et al (2022) Intraoperative fluid balance and cardiac surgery-associated acute kidney injury: a multicenter prospective study. Brazilian J Anesthesiol (English Ed) 72(6):688–694. https://doi.org/10.1016/j.bjane.2022.07.006
Harbell MW, Kraus MB, Bucker-Petty SA, Harbell JW (2021) Intraoperative fluid management and kidney transplantation outcomes: a retrospective cohort study. Clin Transplant 35(12):e14489. https://doi.org/10.1111/ctr.14489
Futier E, Constantin J-M, Petit A, Chanques G, Kwiatkowski F, Flamein R et al (2010) Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 145(12):1193–1200. https://doi.org/10.1001/archsurg.2010.275
Bennett J, McDonald T, Lieblich S, Piecuch J (1999) Perioperative rehydration in ambulatory anesthesia for dentoalveolar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 88(3):279–284. https://doi.org/10.1016/S1079-2104(99)70028-4
Ogendran S, Asokumar B, Cheng DCH, Chung F (1995) A prospective randomized double-blinded study of the effect of intravenous fluid therapy on adverse outcomes on outpatient surgery. Anesth Analg 80(4):682–686. https://doi.org/10.1097/00000539-199504000-00006
Rajdev K, Leifer L, Sandhu G, Mann B, Pervaiz S, Lahan S et al (2021) Aggressive versus conservative fluid resuscitation in septic hemodialysis patients. Am J Emerg Med 46:416–419. https://doi.org/10.1016/j.ajem.2020.10.037
Marjanovic G, Villain C, Juettner E, Hausen A, Hoeppner J, Hopt UT et al (2009) Impact of different crystalloid volume regimes on intestinal anastomotic stability. Ann Surg 249(2):181–185. https://doi.org/10.1097/SLA.0b013e31818b73dc
Kotlińska-Hasiec E, Rutyna RR, Rzecki Z, Czarko-Wicha K, Gagała J, Pawlik P et al (2017) The effect of crystalloid infusion on body water content and intra-abdominal pressure in patients undergoing orthopedic surgery under spinal anesthesia. Adv Clin Exp Med 26(8):1189–1196. https://doi.org/10.17219/acem/63140
Shin CH, Long DR, McLean D, Grabitz SD, Ladha K, Timm FP et al (2018) Effects of intraoperative fluid management on postoperative outcomes: a hospital registry study. Ann Surg 267(6):1084–1092. https://doi.org/10.1097/SLA.0000000000002220
Griffiths M, Fan E, Baudouin SV (2019) New UK guidelines for the management of adult patients with ARDS. Thorax 74:931–933. https://doi.org/10.1136/thoraxjnl-2018-212885
Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M et al (2014) Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care 18(5):538. https://doi.org/10.1186/s13054-014-0538-5
Belavić M, Sotošek Tokmadžić V, Fišić E, Brozović Krijan A, Strikić N, Lončarić Katušin M et al (2018) The effect of various doses of infusion solutions on the endothelial glycocalyx layer in laparoscopic cholecystectomy patients. Minerva Anestesiol 84(9):1032–1043. https://doi.org/10.23736/S0375-9393.18.12150-X
Powell M, Mathru M, Brandon A, Patel R, Frölich M (2014) Assessment of endothelial glycocalyx disruption in term parturients receiving a fluid bolus before spinal anesthesia: a prospective observational study. Int J Obstet Anesth 23(4):330–334. https://doi.org/10.1016/j.ijoa.2014.06.001
Li X, Sun S, Wu G, Che X, Zhang J (2020) Effect of hydroxyethyl starch loading on glycocalyx shedding and cerebral metabolism during surgery. J Surg Res 246:274–283. https://doi.org/10.1016/j.jss.2019.09.030
Blum JM, Stentz MJ, Dechert R, Jewell E, Engoren M, Rosenberg AL et al (2013) Preoperative and intraoperative predictors of postoperative acute respiratory distress syndrome in a general surgical population. J Am Soc Anesthesiol 118(1):19–29. https://doi.org/10.1097/ALN.0b013e3182794975
Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R (2010) Fluid balance and acute kidney injury. Nat Rev Nephrol 6(2):107–115. https://doi.org/10.1038/nrneph.2009.213
Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC et al (2021) Effect of slower vs faster intravenous fluid bolus rates on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA 326(9):830–838. https://doi.org/10.1001/jama.2021.11444
Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M et al (2019) Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothoracic Surg 55(1):91–115. https://doi.org/10.1093/ejcts/ezy301
Tan JHS, Bhatia K, Sharma V, Swamy M, van Dellen D, Dhanda R et al (2022) Enhanced recovery after surgery recommendations for renal transplantation: guidelines. Br J Surg 110(1):57–59. https://doi.org/10.1093/bjs/znac325
Wiegers EJA, Lingsma HF, Huijben JA, Cooper DJ, Citerio G, Frisvold S et al (2021) Fluid balance and outcome in critically ill patients with traumatic brain injury (CENTER-TBI and OzENTER-TBI): a prospective, multicentre, comparative effectiveness study. Lancet Neurol 20(8):627–638. https://doi.org/10.1016/S1474-4422(21)00162-9
Oddo M, Poole D, Helbok R, Meyfroidt G, Stocchetti N, Bouzat P et al (2018) Fluid therapy in neurointensive care patients: ESICM consensus and clinical practice recommendations. Intensive Care Med 44(4):449–463. https://doi.org/10.1007/s00134-018-5086-z
Magalhães PAF, De Padilha GA, Moraes L, Santos CL, De Maia LA, Braga CL et al (2020) Effects of pressure support ventilation on ventilator-induced lung injury in mild acute respiratory distress syndrome depend on level of positive end-expiratory pressure. Eur J Anaesthesiol 35(4):298–306. https://doi.org/10.1097/eja.0000000000000763
Pinto EF, Santos RS, Antunes MA, Maia LA, Padilha GA, de A. Machado J, et al (2020) Static and dynamic transpulmonary driving pressures affect lung and diaphragm injury during pressure-controlled versus pressure-support ventilation in experimental mild lung injury in rats. Anesthesiology 132(2):307–320. https://doi.org/10.1097/ALN.0000000000003060
Saddy F, Oliveira GP, Garcia CSNB, Nardelli LM, Rzezinski AF, Ornellas DS et al (2010) Assisted ventilation modes reduce the expression of lung inflammatory and fibrogenic mediators in a model of mild acute lung injury. Intensive Care Med 36(8):1417–1426. https://doi.org/10.1007/s00134-010-1808-6
van Haren F, Pham T, Brochard L, Bellani G, Laffey J, Dres M et al (2019) Spontaneous breathing in early acute respiratory distress syndrome: insights from the Large Observational Study to UNderstand the Global Impact of Severe Acute Respiratory FailurE Study. Crit Care Med 47(2):229–238. https://doi.org/10.1097/CCM.0000000000003519
Santos CL, Santos RS, Moraes L, Samary CS, Felix NS, Silva JD et al (2017) Effects of pressure support and pressure controlled ventilation on lung damage in a model of mild extrapulmonary acute lung injury with intra-abdominal hypertension. PLoS ONE 12(5):e0178207. https://doi.org/10.1371/journal.pone.0178207
Saddy F, Moraes L, Santos CL, Oliveira GP, Cruz FF, Morales MM et al (2013) Biphasic positive airway pressure minimizes biological impact on lung tissue in mild acute lung injury independent of etiology. Crit Care 17(5):R228. https://doi.org/10.1186/cc13051
Yoshida T, Grieco DL, Brochard L, Fujino Y (2020) Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Curr Opin Crit Care 26(1):59–65. https://doi.org/10.1097/MCC.0000000000000691
Cornejo RA, Arellano DH, Ruiz-Rudolph P, Guiñez DV, Morais CCA, Gajardo AIJ et al (2022) Inflammatory biomarkers and pendelluft magnitude in ARDS patients transitioning from controlled to partial support ventilation. Sci Rep 12(1):1–9. https://doi.org/10.1038/s41598-022-24412-1
Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y (2013) The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit Care Med 41(2):536–545. https://doi.org/10.1097/CCM.0b013e3182711972
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342(18):1301–1308. https://doi.org/10.1056/NEJM200005043421801
Fontanarosa PB, Ranieri VM, Giunta F, Suter PM, Slutsky AS (2000) Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA 284(1):43–44. https://doi.org/10.1001/jama.284.1.43
Panitchote A, Mehkri O, Hastings A, Hanane T, Demirjian S, Torbic H et al (2019) Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care 9(1):1–10. https://doi.org/10.1186/s13613-019-0552-5
Jacob LP, Chazalet JJ, Payen DM, Villiers SM, Boudaoud S, Teillac P et al (1995) Renal hemodynamic and functional effect of PEEP ventilation in human renal transplantations. Am J Respir Crit Care Med 152(1):103–107. https://doi.org/10.1164/ajrccm.152.1.7599806
Drury DR, Henry JP, Goodman J (1947) The effects of continuous pressure breathing on kidney function. J Clin Invest 26(5):945–951. https://doi.org/10.1172/JCI101889
Bertoni M, Spadaro S, Goligher EC (2020) Monitoring patient respiratory effort during mechanical ventilation: lung and diaphragm-protective ventilation. In: Vincent JL (ed) Annual update in intensive care and emergency medicine 2020. Springer, Cham, pp 21–35. https://doi.org/10.1007/978-3-030-37323-8_2
Kiss T, Bluth T, Braune A, Huhle R, Denz A, Herzog M et al (2019) Effects of positive end-expiratory pressure and spontaneous breathing activity on regional lung inflammation in experimental acute respiratory distress syndrome. Crit Care Med 47(4):e358. https://doi.org/10.1097/CCM.0000000000003649
Morais CCA, Koyama Y, Yoshida T, Plens GM, Gomes S, Lima CAS et al (2018) High positive end-expiratory pressure renders spontaneous effort noninjurious. Am J Respir Crit Care Med 197(10):1285–1296. https://doi.org/10.1164/rccm.201706-1244OC
Poelaert JI, Visser CA, Everaert JA, Koolen JJ, Colardyn FA (1993) Acute hemodynamic changes of pressure-controlled inverse ratio ventilation in the adult respiratory distress syndrome: a transesophageal echocardiographic and Doppler study. Chest 104(1):214–219. https://doi.org/10.1378/chest.104.1.214
Singer M, Vermaat J, Hall G, Latter G, Patel M (1994) Hemodynamic effects of manual hyperinflation in critically ill mechanically ventilated patients. Chest 106(4):1182–1187. https://doi.org/10.1378/chest.106.4.1182
Pannu N, Mehta RL (2004) Effect of mechanical ventilation on the kidney. Best Pract Res Clin Anaesthesiol 18(1):189–203. https://doi.org/10.1016/j.bpa.2003.08.002
Monnet X, Malbrain MLNG, Pinsky MR (2023) The prediction of fluid responsiveness. Intensive Care Med 49(1):83–86. https://doi.org/10.1007/s00134-022-06900-0
Bihari S, Dixon D-L, Lawrence MD, De Bellis D, Bonder CS, Dimasi DP et al (2017) Fluid-induced lung injury—role of TRPV4 channels. Pflügers Arch J Physiol 469(9):1121–1134. https://doi.org/10.1007/s00424-017-1983-1
Acknowledgements
We would like to thank Moira Elizabeth Shottler, mBA, Rio de Janeiro, Brazil, and Lorna O’Brien (authorserv.com) for editing assistance.
Funding
The Brazilian Council for Scientific and Technological Development (CNPq) funded research projects and scholarships for students, Rio de Janeiro State Research Foundation (FAPERJ) funded research projects and scholarships for students, Coordination for the Improvement of Higher Education Personnel (CAPES) funded publication costs and scholarships for students, and the National Institute of Science and Technology for Regenerative Medicine/CNPq funded research projects.
Author information
Authors and Affiliations
Contributions
EBC, DB, CR, MM, PP, PRMR, and PLS wrote the manuscript and revised the final version. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MLNGM is co-founder, past-President and current Treasurer of WSACS (The Abdominal Compartment Society, http://www.wsacs.org). He is member of the medical advisory Board of Pulsion Medical Systems (part of Getinge group), Serenno Medical, Potrero Medical, Sentinel Medical and Baxter. He consults for BBraun, Becton Dickinson, ConvaTec, Spiegelberg, and Holtech Medical, and received speaker's fees from PeerVoice. He holds stock options for Serenno and Potrero Medical. He is co-founder and President of the International Fluid Academy (IFA). The IFA (http://www.fluidacademy.org) is integrated within the not-for-profit charitable organization iMERiT, International Medical Education and Research Initiative, under Belgian law. The other authors have no potential conflicts of interest with regard to the contents of this review paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Restrictive and liberal fluid strategies in experimental and clinical studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Carvalho, E.B., Battaglini, D., Robba, C. et al. Fluid management strategies and their interaction with mechanical ventilation: from experimental studies to clinical practice. ICMx 11, 44 (2023). https://doi.org/10.1186/s40635-023-00526-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-023-00526-2