Abstract
Purpose
The goal of the study was to compare the effects of different assisted ventilation modes with pressure controlled ventilation (PCV) on lung histology, arterial blood gases, inflammatory and fibrogenic mediators in experimental acute lung injury (ALI).
Methods
Paraquat-induced ALI rats were studied. At 24 h, animals were anaesthetised and further randomized as follows (n = 6/group): (1) pressure controlled ventilation mode (PCV) with tidal volume (V T) = 6 ml/kg and inspiratory to expiratory ratio (I:E) = 1:2; (2) three assisted ventilation modes: (a) assist-pressure controlled ventilation (APCV1:2) with I:E = 1:2, (b) APCV1:1 with I:E = 1:1; and (c) biphasic positive airway pressure and pressure support ventilation (BiVent + PSV), and (3) spontaneous breathing without PEEP in air. PCV, APCV1:1, and APCV1:2 were set with P insp = 10 cmH2O and PEEP = 5 cmH2O. BiVent + PSV was set with two levels of CPAP [inspiratory pressure (P High = 10 cmH2O) and positive end-expiratory pressure (P Low = 5 cmH2O)] and inspiratory/expiratory times: T High = 0.3 s and T Low = 0.3 s. PSV was set as follows: 2 cmH2O above P High and 7 cmH2O above P Low. All rats were mechanically ventilated in air and PEEP = 5 cmH2O for 1 h.
Results
Assisted ventilation modes led to better functional improvement and less lung injury compared to PCV. APCV1:1 and BiVent + PSV presented similar oxygenation levels, which were higher than in APCV1:2. Bivent + PSV led to less alveolar epithelium injury and lower expression of tumour necrosis factor-α, interleukin-6, and type III procollagen.
Conclusions
In this experimental ALI model, assisted ventilation modes presented greater beneficial effects on respiratory function and a reduction in lung injury compared to PCV. Among assisted ventilation modes, Bi-Vent + PSV demonstrated better functional results with less lung damage and expression of inflammatory mediators.
Similar content being viewed by others
Introduction
Assisted mechanical ventilation is frequently used in acute lung injury (ALI) and during the weaning process [1] as it requires less sedation and no paralysis thus preventing muscle atrophy [2, 3] and haemodynamic impairment [4–6], and reducing the time required for invasive ventilatory support as well as the length of stay in the intensive care unit [7]. The activation of the respiratory muscles induces more negative pleural pressures during inspiration diminishing atelectasis, improving oxygenation, and reducing mechanical stress. Conversely, spontaneous breathing during assisted mechanical ventilation may exacerbate lung injury, since it can increase patient-ventilator asynchrony and rapid shallow breathing, inducing further atelectasis and tidal recruitment–derecruitment [8]. Additionally, negative pleural pressures may increase intrathoracic blood volume, worsening pulmonary oedema and lung damage [9].
Pressure limited assisted ventilation modes, such as pressure assist control (A-PCV), pressure support (PSV), or airway pressure release (BiVent/APRV) ventilation have been frequently used [10]. In A-PCV and BiVent/APRV the inspiratory–expiratory trigger is time-cycled while in PSV it is flow cycled. The time-cycled inspiratory–expiratory triggering has been associated with increased patient ventilatory asynchrony due to neuromechanical uncoupling [11]. Extended inspiratory and shortened expiratory time may further improve alveolar recruitment and reduce lung injury [12, 13]. On the other hand, an increased inspiratory time could promote higher lung injury due to increased stress and strain with time [14–16]. During BiVent/APRV, unrestricted spontaneous breathing is possible at any moment of the mechanically supported ventilatory cycle. Since excessive spontaneous breathing is associated with increased respiratory effort, the combination of pressure limited time cycled breaths in BiVent/APRV with PSV has been introduced in new ventilators [17].
So far, to our knowledge, no experimental study in ALI has evaluated the impact of assisted ventilation modes on ventilator associated lung injury (VALI). Therefore, in an experimental model of mild ALI, we hypothesized that: (1) compared to pressure controlled ventilation (PCV), assisted ventilation per se may lead to less atelectasis resulting in better respiratory function, lower alveolar stress and strain, with less VALI; and (2) among pressure assisted ventilation modes, BiVent + PSV may be the optimal protective ventilatory strategy, due to the combination of pressure limited controlled time cycled and spontaneous flow cycled breaths. For this purpose, we compared the effects of different assisted ventilation modes with PCV on lung histology, arterial blood gases, inflammatory and fibrogenic mediators in experimental ALI.
Materials and methods
Detailed methods are described in the Electronic Supplementary Material (ESM) accompanying this article, and briefly summarized here.
Animal preparation and experimental protocol
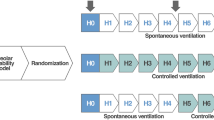
This study was approved by the Ethics Committee of the Carlos Chagas Filho Institute of Biophysics, Health Sciences Centre, Federal University of Rio de Janeiro. A total of 36 Wistar rats (250–300 g) were initially assigned into two groups. In Control (C, n = 6), sterile saline solution (0.9% NaCl, 1.0 ml) was intraperitoneally (i. p.) injected and in acute lung injury (ALI, n = 30) groups, paraquat (15 mg/kg, i. p.) was administered [18]. Twenty-four hours after saline or paraquat administration, animals were sedated (diazepam 1 mg, i. p.), anaesthetised (thiopental sodium 20 mg/kg, i. p.), and tracheotomised. A polyethylene catheter (PE-10) was introduced into the femoral artery to collect blood sampling (300 μl) and measure mean arterial pressure (SCIREQ, Montreal, Canada) (Baseline). The fraction of inspired oxygen (FiO2) was adjusted to 1.0 and, after 5 min, arterial oxygen partial pressure (PaO2), arterial carbon dioxide partial pressure (PaCO2) and pH were measured (i-STAT, Abbott Laboratories, Illinois, USA). FiO2 was then reduced to 0.21 to avoid absorption atelectasis and, after 5 min, the Control group was kept in spontaneous ventilation (room air) without positive-end expiratory pressure (PEEP), while ALI groups were further randomized as follows (n = 6/group): (1) pressure controlled ventilation mode (PCV) with inspiratory pressure (Pins) sufficient to achieve a tidal volume (V T) = 6 ml/kg (Pins = 10 cmH2O), and inspiratory time (T I) = 0.2 s, respiratory rate (RR) = 100 bpm/min, inspiratory to expiratory ratio (I:E) = 1:2, and PEEP = 5 cmH2O (ALI-PCV); (2) three assisted ventilation modes: (a) assist-pressure controlled ventilation (ALI-APCV1:2) with Pins = 10 cmH2O, PEEP = 5 cmH2O, T I = 0.2 s, RR = 100 bpm, and I:E = 1:2; (b) assist-pressure controlled ventilation (ALI-APCV1:1) with Pins = 10 cmH2O, PEEP = 5 cmH2O, T I = 0.3 s, RR = 100 bpm, and I:E = 1:1; and (c) biphasic positive airway pressure and pressure support ventilation (BiVent + PSV) with two levels of CPAP [inspiratory pressure (P High = 10 cmH2O) and positive end-expiratory pressure (P Low = 5 cmH2O)] and inspiratory/expiratory times: T High = 0.3 s and T Low = 0.3 s. PSV was set as follows: 2 cmH2O above P High and 7 cmH2O above P Low; and (3) spontaneous breathing without PEEP (ALI-NV) in air. Animals were mechanically ventilated in Servo i (MAQUET, Solna, Sweden). After a 1 h ventilation period, FiO2 was set at 1.0 and, after 5 min, arterial blood gases were analysed (End). Following this step, FiO2 was reduced to 0.21 and, after 5 min, ventilatory and mechanical parameters were measured. After the ventilation period, a laparotomy was done immediately after the determination of lung mechanics (End), and heparin (1,000 IU) was intravenously injected. The trachea was clamped at 5 cmH2O PEEP in all groups to standardize the pressure condition. The abdominal aorta and vena cava were sectioned, yielding a massive haemorrhage that quickly killed the animals. Lungs were removed en bloc and prepared for histology (light and electron microscopy) and mRNA expression of tumour necrosis factor (TNF)-α, interleukin (IL)-6, interferon (IFN)-γ, transforming growth factor (TGF)-β, and type III procollagen (PCIII) in lung tissue were measured. A schematic flow chart of study design and the timeline representation of the procedure are shown in the electronic supplementary material.
Data acquisition
Airflow, V T, airway (Paw) and oesophageal (Pes) pressures were measured [18]. The durations of inspiration and expiration and the duration of the respiratory cycle were measured on the flow signal. Using these variables, respiratory frequency (f) and minute ventilation (V′E) were computed. In addition, mean airway pressure and P 0.1 was measured throughout acquisition periods.
Histology
Light microscopy
Right lungs were immersed in 3% buffered formaldehyde, paraffin embedded, and stained with haematoxylin-eosin. Volume fraction of the lung occupied by hyperinflated structures (alveolar ducts, alveolar sacs or alveoli wider than 120 μm), collapsed alveoli or normal pulmonary areas were determined by the point-counting technique [19] at a magnification of ×200 across ten random, non-coincident microscopic fields [20–22].
Transmission electron microscopy
Three slices 2 × 2 × 2 mm were cut from three different segments of the lung and fixed [2.5% glutaraldehyde and phosphate buffer 0.1 M (pH 7.4)] for electron microscopy analysis (JEOL 1010 Transmission Electron Microscope, Tokyo, Japan). For each electron microscopy image (15/animal), the following structural damages were analysed: (a) alveolar capillary membrane, (b) type II epithelial cells, and (c) endothelial cells. The pathologic findings were graded according to a 5-point semi-quantitative severity-based scoring system as: 0 = normal lung parenchyma, 1 = changes in 1–25%, 2 = changes in 26–50%, 3 = changes in 51–75%, and 4 = changes in 76–100% of examined tissue [21].
Cytokines mRNA expression using ribonuclease protection assay
Four animals of each group were submitted to the aforementioned protocols to analyse mRNA expression for cytokines by using ribonuclease protection assay (RPA). The in vitro transcription kit and a customized template set [containing lymphotoxin (LT) α, LTβ, TNF-α, IL-6, IFN-γ, IFN-β, TGF-β1, TGF-β2, TGF-β3, macrophage inflammatory factor (MIF), and two housekeeping genes glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) and L32 (ribosomal RNA)] were used to synthesize a radiolabeled probe set using [α-32P] UTP. Density of each cytokine mRNA is expressed relative to that of the housekeeping gene GAPDH. These values were then related to C-NV group [23].
Expression of type III procollagen mRNA
Lung parenchyma strips (3 × 3 × 10 mm) were longitudinally cut from left lungs. Quantitative real-time reverse transcription (RT) polymerase chain reaction (PCR) was performed to measure the relative levels of type III procollagen (PCIII) expression. The relative amount of expression of PCIII was calculated as a ratio of PCIII and control gene (GAPDH) and expressed as fold change relative to C.
Statistical analysis
The normality of the data (Kolmogorov–Smirnov test with Lilliefors’ correction) and the homogeneity of variances (Levene median test) were tested. If both conditions were satisfied, the effects of different ventilatory strategies in C and ALI groups were analysed by using one-way ANOVA followed by Tukey’s test. Otherwise, One-way ANOVA on ranks followed by Dunn’s post hoc test was selected. The significance level was always set at 5%. The parametric data were expressed as mean ± SD, while the non-parametric data were expressed as median (interquartile range). All tests were performed using SigmaStat 3.1 (Jandel Corporation, San Raphael, CA, USA).
Results
Mean arterial pressure was maintained stable (70–90 mmHg) throughout the experiments.
Minute ventilation and tidal volume were higher while mean airway pressure was lower during assisted breathing than PCV (Table 1). Among the assisted ventilation modes, APCV1:2 induced the highest minute ventilation. Inspiratory pressures were similar in all assisted modes (P insp = 10 cmH2O) as well as the mean airway pressure (Table 1). P 0.1 was lower in BiVent + PSV compared to other groups.
ALI groups presented worse oxygenation than C. Baseline PaO2, PaCO2, and pHa did not differ among ALI groups (Table 2). After 1 h ventilation, APCV1:1 and BiVent + PSV presented similar PaO2 which was higher than APCV1:2. All assisted ventilation groups showed better oxygenation, lower PaCO2 and higher pHa compared to ALI-NV and ALI-PCV (Table 2).
ALI animals demonstrated a higher amount of alveolar collapse than C. Assisted ventilation groups showed a reduction in alveolar collapse compared to PCV. However, APCV1:1 led to hyperinflation (Table 3).
All ALI animals presented swelling and injury of cytoplasmic organelles of type II pneumocyte (PII) with aberrant lamellar bodies and endothelial injury (Table 4; Fig. 1). However, in BiVent + PSV alveolar capillary damage was less pronounced with no detachment of alveolar epithelium or denudation of epithelial basement membrane (Fig. 1).
Electron microscopy of lung parenchyma in the Control (C) and Acute Lung Injury (ALI) groups. NV spontaneously breathing rats, PCV pressure controlled mode, APCV1:2 and APCV1:1: assist-pressure controlled ventilation with I:E = 1:2 and I:E = 1:1, respectively, BiVent + PSV biphasic positive airway pressure and pressure support ventilation. Note the cytoplasmatic degeneration of type II cell (PII) with aberrant lamellar bodies as well as alveolar capillary membrane damage in the ALI group (arrows). The ellipse indicates that the detachment of the alveolar capillary membrane is reduced in APCV1:1. In the BiVent + PSV group the damage in alveolar capillary membrane is less intense. IE interstitial edema. Photomicrographs are representative of data obtained from lung sections derived from five animals
LT-α, LT-β, IFN-β, TGF-β2, TGF-β3, and MIF mRNA were not expressed in any groups. TNF-α, IL-6, IFN-γ (Fig. 2a), and PCIII (Fig. 2b) expressions were lower in assisted ventilation modes. IL-6, IFN-γ, TGF-β1 and PCIII expressions were more reduced in BiVent + PSV. Among assisted ventilation groups PCIII expression was higher in APCV1:1 (Fig. 2b).
Cytokine mRNA expression investigated by RNase Protection Assay (Panel A) and type III procollagen (PCIII) mRNA expression analysed using real-time polymerase chain reaction (Panel B) in the Control (C) and Acute Lung Injury (ALI) groups. Data are normalized to GAPDH expression. In panel B, the y axis represents fold increase compared with C [non-ventilated (NV) animals]. NV spontaneously breathing rats, PCV pressure controlled mode, APCV1:2 and APCV1:1: assist-pressure controlled ventilation with I:E = 1:2 and I:E = 1:1, respectively, BiVent + PSV biphasic positive airway pressure and pressure support ventilation. Values are means (± SD) four animals per group. §Significantly different from ALI-NV (P < 0.05). #Significantly different from ALI-PCV (p < 0.05). †Significantly different from ALI-APCV1:2 (P < 0.05). ‡Significantly different from ALI-APCV1:1 (P < 0.05). *All ALI data were significantly different from C-NV (P < 0.05)
Discussion
In the present experimental model of mild ALI, the assisted ventilation modes employed improved gas-exchange, reduced atelectasis, and inflammatory and fibrogenic mediators in lung tissue compared to PCV. Furthermore, BiVent + PSV led to a lower inspiratory effort, alveolar capillary membrane injury, and inflammatory and fibrogenic mediators compared to A-PCV.
Acute lung injury was induced by paraquat, an herbicide that accumulates predominantly in the lung and induces alveolar epithelial damage due to its action on type II pneumocytes. This model leads to a well reproducible lung injury characterized by alveolar collapse, interstitial oedema, and hyaline membranes, but no alveolar oedema [22], and presents an amount of atelectasis similar to that observed in human ALI/ARDS [24]. In our study, the controlled mechanical ventilation group was set with low V T (6 ml/kg) in line with current recommendations [25]. The level of PEEP was 5 cmH2O, based on previous observations from our group showing that higher PEEP levels led to deterioration in gas-exchange respiratory mechanics in a similar ALI model in rats [22, 26]. Moreover, the same driving pressure, i.e. difference between inspiratory plateau and end-expiratory pressure, was used during controlled and assisted ventilation modes, suggesting that the morphofunctional and molecular differences were probably related to the type of ventilation, allowing a direct comparison among the different ventilatory techniques. Blood gas-analysis was performed with FiO2 = 1.0 to avoid possible confounding effects of ventilation/perfusion mismatch in the interpretation of the gas-exchange data [27]. However, this study was conducted with FiO2 = 0.21 in order to avoid possible iatrogenic effects induced by high concentration of oxygen on lung parenchyma [21, 28]. Mean airway pressure was lower during PCV compared to other forms of assisted ventilation, which presented no significant differences among A-PCV1:2, A-PCV1:1, and BiVent + PSV. The I:E time during PCV was 1:2 comparable to that set during A-PCV1:2, although I:E may increase during A-PCV since inspiratory breath may begin before the set expiratory time [29]. Pulmonary histology was evaluated at comparable airway pressure, thus, the morphometrical changes reflect only the effects of different modes of mechanical ventilation. To our knowledge, no prospective randomized controlled experimental or clinical study has compared the effects of different assisted ventilation modes with controlled mechanical ventilation at the same driving pressure on lung mechanics and histology, and inflammatory and fibrogenic responses. Moreover, no study has evaluated the impact of different I:E ratio during A-PCV and BiVent + PSV on lung injury. In fact, previous reports have compared assisted with controlled mechanical ventilation focusing on respiratory muscle changes and prevention of muscle atrophy [30–32].
Assisted mechanical ventilation was associated with better aeration, less atelectasis, and higher V T compared to PCV leading to an overall increase in total minute ventilation, oxygenation improvement, and a reduction in PaCO2. The main determinant of alveolar recruitment is the transpulmonary pressure achieved at end-inspiration and end-expiration [33]. During controlled mechanical ventilation inspiratory airway pressure reflects the transpulmonary pressure. Conversely, during assisted ventilation, inspiratory airway pressures may not reflect the real transpulmonary pressure, being affected by the reduction in pleural pressure generated by the activation of the inspiratory respiratory muscles. Since in assisted ventilation V T was higher and the inspiratory and expiratory airway pressures were comparable to PCV, the negative pleural pressure generated by inspiratory efforts could better recruit the atelectatic lung units, in accordance with previous experimental studies [34, 35]. In a clinical study, Putensen et al. [6] found that oxygenation and shunt did not differ between PSV and controlled mechanical ventilation. During PSV a progressive derecruitment may occur due to a decrease in I:E ratio and mean airway pressure [36], limiting the positive effects on alveolar recruitment determined by the inspiratory effort. Our data suggest that time cycled inspiratory–expiratory triggering may be efficient to avoid alveolar derecruitment observed during the entire flow cycled modes such as PSV, minimizing differences among different assisted ventilation modes.
We measured the P 0.1 as an indicator of the degree of inspiratory effort [37]. P 0.1 was lower in BiVent + PSV compared to A-PCV in spite of equal levels of anaesthesia, which may indicate reduced inspiratory effort, a better adaptation to the ventilator, and a reduction in inspiratory transpulmonary pressure. In this line, Sassoon et al. [3] showed that partial respiratory muscle activation can reduce muscle dysfunction. Henzler et al. [29] found that A-PCV1:1 led to a decreased respiratory effort compared to BiVent + spontaneous breathing in a surfactant depletion ALI model in pigs. Conversely, in the present study, since BiVent was associated with PSV, spontaneous breaths were partially supported, thus reducing the inspiratory effort. Therefore, different assisted ventilation modes, set with similar parameters, may affect the inspiratory drive differently.
Lung hyperinflation was increased in assisted ventilation, mainly in A-PCV1:1, suggesting the role of inspiratory to expiratory time in an inhomogeneous lung parenchyma with different time constant. The increase in hyperinflation during assisted breathing can be explained by the higher transpulmonary pressure achieved during inspiration in the presence of active breathing efforts. However, our data also suggest that modalities of assisted ventilation which favoured lower inspiratory to expiratory ratio may lead to less hyperinflation. Interestingly, BiVent + PSV was associated with lower hyperinflation compared to A-PCV 1:1, which could be due to a better animal-ventilator interaction reduced respiratory drive, with a consequent decrease in the inspiratory transpulmonary pressure.
We tested the presence of several inflammatory and fibrogenic mediators in the lung tissue, but we found measurable amounts only of IL-6, TNF-α, TGF-β1, and IFN-γ mRNA expressions [38]. Furthermore, lung tissue mRNA expression of PCIII was evaluated as it is the first collagen to be remodelled in the evolution of lung fibrogenesis [23] and has been used as an early marker of lung parenchyma remodelling [26]. Although tidal volume was higher during assisted ventilation, IL-6, TNF-α, TGF-β1, and IFN-γ expressions were lower than PCV. A-PCV1:1 yielded alveolar hyperinflation resulting in a raise in PCIII mRNA expression in the lung compared to APCV1:2 and BiVent + PSV. IL-6 and TNF-α mRNA expressions were lower during A-PCV1:1 compared to A-PCV 1:2. Our data are in line with previous studies which showed association between hyperinflation and increased PCIII expression [21, 22, 39]. Different factors could have promoted reduced lung injury during assisted ventilation: (a) recruitment of dependent atelectatic lung regions, reducing opening and closing during tidal breath, thus limiting shear stress forces [17]; (b) more homogeneous distribution of regional transpulmonary pressures [40]; (c) variability of breathing pattern [41]; (d) redistribution of perfusion towards non-atelectatic injured areas [42]; and (e) improved lymphatic drainage [43]. Among assisted ventilation modes, IL-6, IFN-γ, TGF-β, and PCIII were lower in BiVent + PSV associated with reduced P 0.1, suggesting that assisted ventilation with high inspiratory effort may increase the risk of VALI. Therefore, P 0.1 could be used to optimize assisted ventilation setting achieving optimal recruitment with minimal stress and strain.
Limitations
Our study has several limitations: (1) we used a specific mild ALI model induced by paraquat, thus, we cannot extend our data to other models of more severe ALI; (2) the study period was short (1 h), therefore our results cannot be directly shifted to longer periods of ventilation. These experimental data should be confirmed in large animals, with greater duration of analysis, and in clinical studies, in order to better understand the potentially relevant consequences in the weaning of critically ill patients; (3) our results cannot be generalized for other types of assisted ventilation modes and higher levels of inspiratory support; (4) we found a higher variability of tidal volume in the A-PCV1:1 group which suggests a worse animal-ventilator interaction. Although respiratory drive and functional parameters were not different compared to A-PCV1:2, VALI was lower in A-PCV1:1 than to A:PCV1:2; (5) the respiratory effort was estimated by measuring the respiratory drive, even though the direct transpulmonary pressure was not evaluated. In this line, several studies have shown that the respiratory drive is well correlated with the inspiratory effort [37, 44]; and (6) we did not measure inflammatory mediators in the blood or analyse distal organ injury.
Conclusions
In the present study, evidence shows that the assisted ventilation modes employed presented greater beneficial effects on respiratory function and a reduction in lung injury in mild experimental ALI. Among different assisted modes, BiVent + PSV further improved oxygenation, reduced inspiratory effort, and decreased lung injury.
References
Esteban A, Alía I, Tobin MJ, Gil A, Gordo F, Vallverdú I, Blanch L, Bonet A, Vázquez A, de Pablo R, Torres A, de La Cal MA, Macías S, Spanish Lung Failure Collaborative Group (1999) Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Am J Respir Crit Care Med 159:512–518
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB (2008) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358:1327–1335
Sassoon CS, Zhu E, Caiozzo VJ (2004) Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med 170:626–632. doi:10.1164/rccm.200401-042OC
Kaplan LJ, Bailey H, Formosa V (2001) Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care 5:221–226. doi:10.1186/cc1027
Staudinger T, Kordova H, Röggla M, Tesinsky P, Locker GJ, Laczika K, Knapp S, Frass M (1998) Comparison of oxygen cost of breathing with pressure-support ventilation and biphasic intermittent positive airway pressure ventilation. Crit Care Med 26:1518–1522
Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J (1999) Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 159:1241–1248
Putensen C, Zech S, Wrigge H, Zinserling J, Stüber F, Von Spiegel T, Mutz N (2001) Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 164:43–49
Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L (2006) Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 32:1515–1522. doi:10.1007/s00134-006-0301-8
Kallet RH, Daniel BM, Gropper M, Matthay MA (1998) Acute pulmonary edema following upper airway obstruction: case reports and brief review. Respir Care 43:476–480
Rose L, Hawkins M (2008) Airway pressure release ventilation, biphasic positive airway pressure: a systematic review of definitional criteria. Intensive Care Med 34:1766–1773. doi:10.1007/s00134-008-1216-3
Kondili E, Xirouchaki N, Georgopoulos D (2007) Modulation and treatment of patient-ventilator dyssynchrony. Curr Opin Crit Care 13:84–89. doi:10.1097/MCC.0b013e328011278d
Fujita Y, Maeda Y, Fujino Y, Uchiyama A, Mashimo T, Nishimura M (2006) Effect of peak inspiratory flow on gas exchange, pulmonary mechanics, and lung histology in rabbits with injured lungs. J Anesth 20:96–101. doi:10.1007/s00540-005-0374-5
D’Angelo E, Pecchiari M, Saetta M, Balestro E, Milic-Emili J (2004) Dependence of lung injury on inflation rate during low-volume ventilation in normal open-chest rabbits. J Appl Physiol 97:260–268. doi:10.1152/japplphysiol.01175.2003
Broccard AF, Hotchkiss JR, Suzuki S, Olson D, Marini JJ (1999) Effects of mean airway pressure and tidal excursion on lung injury induced by mechanical ventilation in an isolated perfused rabbit lung model. Crit Care Med 27:1533–1541
Marini JJ, Ravenscraft SA (1992) Mean airway pressure: physiologic determinants and clinical importance-Part 1: Physiologic determinants and measurements. Crit Care Med 20:1461–1472
Marini JJ, Ravenscraft SA (1992) Mean airway pressure: physiologic determinants and clinical importance-part 2: clinical implications. Crit Care Med 20:1604–1616
Putensen C, Wrigge H (2004) Clinical review: biphasic positive airway pressure and airway pressure release ventilation. Crit Care 8:492–497. doi:10.1186/cc2919
Rocco PR, Negri EM, Kurtz PM, Vasconcellos FP, Silva GH, Capelozzi VL, Romero PV, Zin WA (2001) Lung tissue mechanics and extracellular matrix remodeling in acute lung injury. Am J Respir Crit Care Med 164:1067–1071
Weibel ER (1990) Morphometry: stereological theory and practical methods. In: Gil J (ed) Models of lung disease-microscopy and structural methods. Marcel Dekker, New York, pp 199–247
Riva DR, Oliveira MBZ, Rzezinski AF, Rangel G, Capelozzi VL, Zin WA, Morales MM, Pelosi P, Rocco PRM (2008) Recruitment maneuver in pulmonary and extrapulmonary experimental acute lung injury. Crit Care Med 36:1900–1908. doi:10.1097/CCM.0b013e3181760e5d
Pássaro CP, Silva PL, Rzezinski AF, Abrantes S, Santiago VR, Nardelli L, Santos RS, Barbosa CM, Morales MM, Zin WA, Amato MB, Capelozzi VL, Pelosi P, Rocco PR (2009) Pulmonary lesion induced by low and high positive end-expiratory pressure levels during protective ventilation in experimental acute lung injury. Crit Care Med 37:1011–1017. doi:10.1097/CCM.0b013e3181962d85
Steimback PW, Oliveira GP, Rzezinski AF, Silva PL, Garcia CS, Rangel G, Morales MM, Lapa E, Silva JR, Capelozzi VL, Pelosi P, Rocco PR (2009) Effects of frequency and inspiratory plateau pressure during recruitment manoeuvres on lung and distal organs in acute lung injury. Intensive Care Med 35:1120–1128. doi:10.1007/s00134-009-1439-y
Leite-Junior JH, Garcia CS, Souza-Fernandes AB, Silva PL, Ornellas DS, Larangeira AP, Castro-Faria-Neto HC, Morales MM, Negri EM, Capelozzi VL, Zin WA, Pelosi P, Bozza PT, Rocco PR (2008) Methylprednisolone improves lung mechanics and reduces the inflammatory response in pulmonary but not in extrapulmonary mild acute lung injury in mice. Crit Care Med 36:2621–2628. doi:10.1097/CCM.0b013e3181847b43
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354:1775–1786
[No authors listed] (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342:1301–1308
Farias LL, Faffe DS, Xisto DG, Santana MC, Lassance R, Prota LF, Amato MB, Morales MM, Zin WA, Rocco PR (2005) Positive end-expiratory pressure prevents lung mechanical stress caused by recruitment/derecruitment. J Appl Physiol 98:53–61. doi:10.1152/japplphysiol.00118.2004
Kulkarni AC, Kuppusamy P, Parinandi N (2007) Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid Redox Signal 9:1717–1730
dos Santos CC, Slutsky AS (2006) The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol 68:585–618. doi:10.1146/annurev.physiol.68.072304.113443
Henzler D, Pelosi P, Bensberg R, Dembinski R, Quintel M, Pielen V, Rossaint R, Kuhlen R (2006) Effects of partial ventilatory support modalities on respiratory function in severe hypoxemic lung injury. Crit Care Med 34:1738–1745. doi:10.1097/01.CCM.0000218809.49883.54
Powers SK, DeCramer M, Gayan-Ramirez G, Levine S (2008) Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm. Crit Care 12:191. doi:10.1186/cc7095
Futier E, Constantin JM, Combaret L, Mosoni L, Roszyk L, Sapin V, Attaix D, Jung B, Jaber S, Bazin JE (2008) Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm. Crit Care 12:R116. doi:10.1186/cc3908
Shanely RA, Van Gammeren D, Deruisseau KC, Zergeroglu AM, McKenzie MJ, Yarasheski KE, Powers SK (2004) Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am J Respir Crit Care Med 170:994–999. doi:10.1164/rccm.200304-575OC
Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ (2001) Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 164:122–130
Wrigge H, Zinserling J, Neumann P, Muders T, Magnusson A, Putensen C, Hedenstierna G (2005) Spontaneous breathing with airway pressure release ventilation favors ventilation in dependent lung regions and counters cyclic alveolar collapse in oleic-acid-induced lung injury: a randomized controlled computed tomography trial. Crit Care 9:R780–R789. doi:10.1186/cc3908
Wrigge H, Zinserling J, Neumann P, Defosse J, Magnusson A, Putensen C, Hedenstierna G (2003) Spontaneous breathing improves lung aeration in oleic acid-induced lung injury. Anesthesiology 99:376–384
Dembinski R, Max M, Bensberg R, Rossaint R, Kuhlen R (2002) Pressure support compared with controlled mechanical ventilation in experimental lung injury. Anesth Analg 94:1570–1576
Alberti A, Gallo F, Fongaro A, Valenti S, Rossi A (1995) P0.1 is a useful parameter in setting the level of pressure support ventilation. Intensive Care Med 21:547–553
Fanelli V, Mascia L, Puntorieri V, Assenzio B, Elia V, Fornaro G, Martin EL, Bosco M, Delsedime L, Fiore T, Grasso S, Ranieri VM (2009) Pulmonary atelectasis during low stretch ventilation: “open lung” versus “lung rest” strategy. Crit Care Med 37:1046–1053. doi:10.1097/CCM.0b013e3181968e7e
Garcia CS, Abreu SC, Soares RM, Prota LF, Figueira RC, Morales MM, Capelozzi VL, Zin WA, Rocco PR (2008) Pulmonary morphofunctional effects of mechanical ventilation with high inspiratory air flow. Crit Care Med 36:232–239. doi:10.1097/01.CCM.0000295309.69123.AE
D’Angelo E (1984) Factors affecting the distribution of transpulmonary pressure in animals and in man. Bull Eur Physiopathol Respir 20(5):415–422
Spieth PM, Carvalho AR, Pelosi P, Hoehn C, Meissner C, Kasper M, Hübler M, von Neindorff M, Dassow C, Barrenschee M, Uhlig S, Koch T, de Abreu MG (2009) Variable tidal volumes improve lung protective ventilation strategies in experimental lung injury. Am J Respir Crit Care Med 179:684–693. doi:10.1164/rccm.200806-975OC
Gama de Abreu M, Spieth PM, Pelosi P, Carvalho AR, Walter C, Schreiber-Ferstl A, Aikele P, Neykova B, Hübler M, Koch T (2008) Noisy pressure support ventilation: a pilot study on a new assisted ventilation mode in experimental lung injury. Crit Care Med 36:818–827. doi:10.1097/01.CCM.0000299736.55039.3A
Moriondo A, Mukenge S, Negrini D (2005) Transmural pressure in rat initial subpleural lymphatics during spontaneous or mechanical ventilation. Am J Physiol Heart Circ Physiol 289:H263–H269. doi:10.1152/ajpheart.00060.2005
Pelosi P, Chiumello D, Calvi E, Taccone P, Bottino N, Panigada M, Cadringher P, Gattinoni L (2001) Effects of different continuous positive airway pressure devices and periodic hyperinflations on respiratory function. Crit Care Med 29:1683–1689
Acknowledgments
We would like to express our gratitude to Mr. Andre Benedito da Silva for animal care, Mrs. Miriam Regina Taborda Simone and Ana Lucia Neves da Silva for their help with microscopy, Ms. Jaqueline Lima do Nascimento for her skillful technical assistance during the experiments, Mrs. Moira Elizabeth Schöttler for assistance in editing the manuscript, and Maquet for borrowing us Servo-I ventilator. Supported by Centres of Excellence Program (PRONEX-FAPERJ), Brazilian Council for Scientific and Technological Development (CNPq), Carlos Chagas Filho, Rio de Janeiro State Research Supporting Foundation (FAPERJ), São Paulo State Research Supporting Foundation (FAPESP).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saddy, F., Oliveira, G.P., Garcia, C.S.N.B. et al. Assisted ventilation modes reduce the expression of lung inflammatory and fibrogenic mediators in a model of mild acute lung injury. Intensive Care Med 36, 1417–1426 (2010). https://doi.org/10.1007/s00134-010-1808-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1808-6