Abstract
Nanotechnology has the potential to circumvent several drawbacks of conventional therapeutic formulations. In fact, significant strides have been made towards the application of engineered nanomaterials for the treatment of cancer with high specificity, sensitivity and efficacy. Tailor-made nanomaterials functionalized with specific ligands can target cancer cells in a predictable manner and deliver encapsulated payloads effectively. Moreover, nanomaterials can also be designed for increased drug loading, improved half-life in the body, controlled release, and selective distribution by modifying their composition, size, morphology, and surface chemistry. To date, polymeric nanomaterials, metallic nanoparticles, carbon-based materials, liposomes, and dendrimers have been developed as smart drug delivery systems for cancer treatment, demonstrating enhanced pharmacokinetic and pharmacodynamic profiles over conventional formulations due to their nanoscale size and unique physicochemical characteristics. The data present in the literature suggest that nanotechnology will provide next-generation platforms for cancer management and anticancer therapy. Therefore, in this critical review, we summarize a range of nanomaterials which are currently being employed for anticancer therapies and discuss the fundamental role of their physicochemical properties in cancer management. We further elaborate on the topical progress made to date toward nanomaterial engineering for cancer therapy, including current strategies for drug targeting and release for efficient cancer administration. We also discuss issues of nanotoxicity, which is an often-neglected feature of nanotechnology. Finally, we attempt to summarize the current challenges in nanotherapeutics and provide an outlook on the future of this important field.
Similar content being viewed by others
1 Introduction
Cancer is one of the foremost causes of death globally. Despite efforts to mitigate risk factors in recent decades, the prevalence of cancer is continuing to increase [1]. Current standards of care combine precise staging of cancer with chemotherapy, radiotherapy, and/or surgical resection. Radiotherapy and chemotherapy are known for significant adverse effects [2], with most methods targeting non-specifically any rapidly dividing cells irrespective of whether they are tumorous or not. Furthermore, poor pharmacokinetic characteristics of anticancer drugs arising from poor solubility, stability, and metabolism pose different challenges of toxicity, inefficacy and limited bio-distribution. Thus, it is imperative to develop effective formulations that can address the above cited challenges and provide selective targeting of tumor sites without significant damage to the viability of healthy tissues [3,4,5,6,7,8,9].
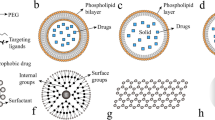
In the paradigm of ‘nanomedicine’, nanotechnology is being embraced to obtain effective drug delivery, establish novel in vitro diagnostics, and develop nano-based implants [7, 10, 11]. There is an exponential growth in the field of nano-based sensing and drug delivery [12,13,14,15,16,17,18,19,20]. Nano-based modalities provide enhanced transport across biological barriers, enable selective targeting of malignant tissues/cells, and offer strategies for sustained release of a drug [21, 22]. As illustrated in Fig. 1, a wide range of nanomaterials have been fabricated using organic, inorganic, lipid and protein compounds typically in the range of 1–100 nm and deliver various antitumor drugs by fine-tuning the chemical composition, size, and shape (morphology) that can control the functionality of the nanomaterials. Specifically, the use of nanocarriers for drug delivery offers many advantages; (i) circumvent the problems of solubility and stability of anticancer drugs; (ii) prevents the drug from degradation from proteases and other enzymes and increase the half-life of the drug in the systemic circulation; (iii) improves drug distribution and targeting; (iv) helps in the sustained release of drug by targeting the cancer sites and (v) helps in delivery of multiple drugs and, therefore helps in reducing drug resistance [23]. Thus, nanotechnology is creating new opportunities for designing materials that can revolutionize the approaches to drug delivery and transform the landscape of the pharmacological treatment of cancer [7, 24,25,26].
In this review, we discuss the development of ‘smart’ nanomaterials for treating cancer, with emphasis on the strategies of drug targeting and triggering sustained release of drug from the nanocarriers. Later, we elaborate upon the design and fabrication of nanomaterials, along with different types of nanomaterials used in cancer therapeutics including liposomes, dendrimers, inorganic nanomaterials and polymeric nanomaterials. We also discuss the current challenges and perspectives of nanomaterials in effective cancer management.
2 Approaches for drug vehicles, targeting, and release
It is well-known that the activity of the anticancer drugs is greatly attenuated by the time drug reaches the target, which can render the treatment to be ineffective and increase off-target effects. The effectiveness of anticancer drug treatment can be achieved only when the administered drug is of proper dosage and display maximal activity in the cancer cells. Thus, the nanomaterials used for targeting tumor cells should have the capability of increasing local concentration of the drugs in and around tumor cells, thereby reducing the potential toxicity toward healthy cells [27]. The efficient delivery of nanomaterials to the target tissues can be classified as passive and active targeting, as discussed below.
2.1 Passive targeting
The most common route of administration of nanomaterial-based anticancer drugs is intravenous injection. This approach bypasses the absorption step across the intestinal epithelium required after oral administration [28]. At tumor sites, the vascular barrier is disrupted, and this enables nanocarriers to accumulate in the tumor tissue as depicted in Fig. 2 [29]. The gaps between the endothelial cells in the tumor vasculature can range from 200 to 2000 nm depending on the tumor type, localization, and environment. Moreover, due to the poor lymphatic function, the nanoparticles are not rapidly cleared and accumulate in the tumor interstitium [30]. This is known as enhanced permeability and retention (EPR) effect, which is the basis of passive targeting [31]. This accumulation of the drug at the tumor sites is a passive process, and it requires prolonged circulation of the drug for appropriate drug delivery. The accumulation of the nanocarriers is essentially depends on physicochemical properties such as size, shape (morphology), surface charge and surface chemistry [32]. The extent and kinetics of nanomaterial accumulation at the tumor site are influenced by their size. The nanocarriers need to be smaller than the cut-off of the proportions in the neovasculature, with the extravasation to the tumor acutely affected by the size of the vehicle. Further, the biodistribution of the nanomaterial–drug formulation is influenced by blood perfusion, passive interactions with biomolecules along the route, and immunological clearance processes such as phagocytosis or renal clearance [33].
Graphical illustration of passive and active drug targeting strategies. In passive targeting, the nanocarriers pass through the leaky walls and accumulate at the tumor site by the enhanced permeability and retention (EPR) effect. Active targeting can be achieved using specific ligands that bind to the receptors on the tumor cells
There are several studies reporting on successful applications of passive targeting of tumor cells and a successful translation into clinical therapeutics. The first FDA (the Food and Drug Administration, national agency of the United States Department of Health and Human Services) approved nano-drug is one consisting of PEGylated liposome entrapped doxorubicin (DOX) targeted against HIV-related Kaposi sarcoma tumor, and ovarian cancer. Entrapping doxorubicin inside the lipid material resulted in substantial reduction in the cellular and systemic toxicity of the drug, and resulted in improved pharmacokinetics for the drug, controlled biodistribution, and release [34]. A recent FDA-approved nano-formulation comprising of liposomal entrapped cytarabine–daunorubicin combination (CPX-351 Vyxeos™) has shown 9.6 months of overall survival compared with 6.0 months of survival for the free form of the drug in patients with newly diagnosed high-risk acute myeloid leukemia [35]. Many such formulations have been approved [34], opening new avenues toward cancer therapeutics.
An additional layer of targeting functionalities can be applied to these nano-formulations to improve their biodistribution and minimize immune clearance. The concept has been discussed in the active targeting section of this review. Therapeutic efficacy of passive targeted approaches is limited by the heterogeneity of the EPR effects seen within and between different tumors. Due to variable endothelial gaps resulting from vigorous tumorous cell growth, it can result in non-uniform extravasation of nanoparticles into the target area [36]. Additionally, while it is evident that nanoparticles permeability should normally be at higher rates in hypoxic core of tumor area rather than the periphery, few studies contrast this observation [37]. This heterogeneity adds another layer of complexity to passive targeting. Similarly, extravasation has been shown to not only depend on permeability, but also on the blood flow rate around the tumor site. This vascularization displays spatial and temporal heterogeneity within and between tumor cells adding another level of challenges to passive targeting [38]. Moreover, for nanomaterials that do extravasate by crossing the vasculature, deeper penetration to tumor site is impeded by the interstitial tumor matrix. Here, the size and size-dependent properties of the material will be the key to improving penetration into the matrix. Wong et al. have proposed a multi-factorial nanosystem that changes size upon reaching different locations of the tumor sites. They have developed gelatin particles, 100 nm in diameter, which upon extravasation into tumor tissue reduce in size to 10 nm, through degradation by tumor-associated matrix metalloproteinases [39].
Unfortunately, the understanding of EPR effects is limited by the unavailability of accurately recapitulated solid tumor models in humans. In fact, most of our current knowledge is based on a few subcutaneous tumor xenograft models that divide vigorously resulting in very high EPR effects. Therefore, the knowledge from experimentation using these models could provide a false impression about the efficacy of passive targeted nanomaterials [40]. Moreover, it is imperative to state that there is also a lack of patient-based experimental data on the EPR phenomenon. Therefore, further advances in understanding tumor biology, understanding EPR effects in varieties of the tumor is essential. Such thoughtful knowledge will be useful in the rational tailoring of nanomaterials, which can be used for personalized tumor medicine for even higher therapeutic benefits.
2.2 Active targeting
Active targeting, also known as the ligand-mediated targeted approach, involves affinity based recognition, retention and facilitated uptake by the targeted cells (Fig. 2) [32]. Chemical affinity for active targeting is based on different specific molecular interactions such as receptor–ligand-based interactions, charge-based interactions and facilitated motif-based interactions with substrate molecules [41, 42]. Diverse biomolecules can constitute a ligand, including antibodies, proteins, nucleic acids, peptides, carbohydrates and small organic molecules such as vitamins [43,44,45]. Target substrates can be surface molecules expressed in diseased cells, proteins, sugars or lipids present in the organs, molecules present (or secreted by tumor cells) in the microenvironment of the diseased cells or even the physicochemical environment in the vicinity [46]. Nanomaterial-based smart, targeted systems exploit the multivalent nature of interactions of ligands with the target antigens. When multiple ligand molecules are accumulated onto the nanosystems, there is an overall increase in the avidity of the nanoparticles for its cognate target [45]. Also, binding of one ligand molecule generally facilitates binding of consequent molecules through cooperativity effects, collectively enhancing the binding efficiency and subsequent actions.
Active targeting approach has been exploited to increase internalization of nanoparticles by the target cells and improve the drug delivery efficacy. In one study, anti-HER2 targeting ligand moieties functionalized on the surface of liposome increased the cellular uptake of the nanoparticles in HER2-expressing cancer cells. In contrast, non-HER2 targeting moieties or non-targeted liposome nanoparticles resulted in the accumulation of particles in the perivascular and stromal space of the tumor site in higher proportion. These accumulated nanoparticles were captured and quickly cleared by macrophages resulting in suboptimal tumor cell internalization [47]. In another study, Zhou et al. [48] synthesized insulin-like growth factor 1 (IGF1) and conjugated it to magnetic iron oxide nanoparticles (IONPs) having the anthracycline doxorubicin as therapeutic payload. Upon intravenous administration of nanoparticles into patient-derived xenograft (PDX) prototype of pancreatic cancer, exceptional tumor targeting and penetration was obtained. Targeted therapy using theranostic IGF1-iron oxide nanoparticles-doxorubicin significantly inhibited the growth of pancreatic PDX tumors showing potential for improved therapeutic outcomes as shown in Fig. 3. Further, they measured the localization and internalization of these nanoparticles using magnetic resonance imaging (MRI) exploiting IONPs properties as contrast agents. These targeted magnetic nanosystems could also be used in photothermal therapy, wherein, their specific localization in tumor sites can be used to induce a local thermal ablation of the tumor sites upon passing alternating magnetic field (AMF). The heat generated due to Neel and Brownian relaxation as well as hysteresis loss can be used to kill tumor cells in the vicinity of IONPs [49].

(adapted with permission from [48])
In vitro and in vivo effects of IGF1-IONPs (insulin-like growth factor 1-iron oxide nanoparticles) and IGF1-IONPs-doxorubicin on cell proliferation and viability. a The effect of IGF1R in MIAPaCa-2 cells was assessed by immunofluorescence labeling employing an anti-IGF1R antibody (shown in red color). b Prussian blue staining of cells incubated for 4 h with different treatments at 20 μg/mL of iron equivalent dose. The cells are also counterstained with nuclear fast red. c The in vitro influence of IGF1 and IGF1-IONPs on cell proliferation. The % of viable cells after 96 h incubation with IGF1 or IGF1-IONPs, and for 4 h at equivalent IGF1 concentrations was estimated by cell proliferation assay, wherein *P < 0.05; **P < 0.001. d The in vivo effect on tumor cell proliferation of IGF1-IONPs in human pancreatic PDX-tumor xenografts. By using immunofluorescence labeling of an anti-Ki67 antibody, the Ki67-positive cells in tumor sections after two tail vein injections of 20 mg/kg iron dose of IGF1-IONPs are measured. e In vitro cytotoxicity of unconjugated and conjugated doxorubicin in MIA PaCa-2 cells. The scale bars are 100 μm
Usually, targeting based approaches exploit the subtle differences in the expression of substrate molecules between cancer and normal cells. For example, epidermal growth factor receptor (EGFR, responsible for epithelial tissue development and homeostasis) is overexpressed in cancerous cells relative to normal cells, as cancer cells grow and divide vigorously [50]. This concentration difference on the cell surface is the basis for studies targeting cancer cells overexpressing EGFR [51, 52]. Normally, given the complexity of nanoparticles administration routes and undesirable interactions with non-specific molecules within the organisms, the difference in the nanoparticle’s affinity towards cancerous and normal cells would not be sufficient for high specificity and efficient delivery to the target site required for wide utility for biomedical applications. Recent approaches have explored concomitantly targeting multiple surface receptors with single nanoparticle systems conjugated with multiple ligands [53]. Bhattacharyya et al. have fabricated and characterized such dual ligand–receptor nanosystems using gold (Au) nanoparticles. They employed EGFR and folate receptor (FR) overexpressed in ovarian cancer as target surface molecules, and used monoclonal antibodies against these receptors as dual ligands for Au nanoparticle targeting. They observed that this dual targeting system is more efficient in delivering Au nanoparticles to cancer cells than their corresponding single ligand system [54].
However, there are multiple factors that need to be optimized for effective use of active-targeted cancer therapeutics. Ligand density on the nanoparticles dictates the strength of avidity towards the substrate, so approaches used to conjugate ligands on the surface of nanoparticles are critical aspects of the targeted systems. Generally, covalent conjugation methods have been utilised, but systems with physical absorption using affinity complexes can also be used effectively [55]. The critical aspect to this conjugation is to maintain the stability of the conjugated ligands during the adverse environment presented by the physiological environment, and various approaches have been undertaken to achieve it [32]. Interestingly, in contrast to the more-ligand-more-targeting notion, there have been a few observations wherein increasing ligand density to increase total affinity did not always have a linear relationship with ligand density. This phenomenon has been explained based on molecular saturation, improper orientation of ligands, bond constraints, and steric constraints from neighboring molecules on the nanoparticles [56]. Similarly accumulating a high degree of hydrophobicity on the nanoparticles led to increased susceptibility towards macrophage uptake, without offering a significant advantage for rapid target cell internalization [57]. These studies do raise concerns about how an appropriate optimization of targeting moieties, conjugation approaches and densities play an essential role in the desired outcomes of the therapeutic nanosystems.
Targeting specificity and payload delivery capacity are two critical parameters required to optimize the efficiency and viability of a nanoparticle-based active targeted systems in in vivo settings. Specificity is defined as how effective the interaction is between the ligand-conjugated nanoparticles with their target molecules weighted against off-target effects incurred before reaching the target molecules. This specificity is dictated mostly by the interactions presented during the biodistribution process. Since there are a multitude of smaller interactions presented by diverse complex biomolecules based on simple van der Waals interactions, the cumulative effects of these smaller interactions can hinder nanoparticles approach to their target sites. Similarly, in an in vivo environment, many smaller proteins and intrinsic biomolecules bind non-specifically on the surface of nanoparticles, commonly known as Vroman’s effect [58], leading to changed ‘identity’ of the whole nanosystem. This alteration could cause nanoparticles to lose their specificity leading to sub-optimal localization in desired sites or at cellular targets. Since actively-targeted nanosystem rely on being in the vicinity of the target sites to home in and execute their functions, biodistribution profile is very critical to its proper functioning. The biodistribution profile is also strongly influenced by active clearance processes posed by various immune cells, and blood flow/renal filtration rate. Since tumor blood flow is low compared to observed in other organs and bodily tissues, the increased affinity based on the ligands cannot compensate for the clearance processes [32]. Therefore, actively-targeted nanosystems need to be developed with extended blood circulation times and biocompatible profiles, along with neutral coating to prevent extensive non-specific binding of blood molecules.
Most cognate substrates for nanoparticles bound ligands are present in the extravascular space of tumor outside of the blood vessels epithelial lining. Active targeting, therefore, relies extensively on endoplasmic retention effects to reach the targets. Therefore, it is essential to consider how we can exploit the endoplasmic retention effects to achieve active targeting. Endoplasmic retention effects vary with tumor types such that some cancers have wide epithelial fenestrations so that nanoparticles with broader size range can be effectively used. However, in some tumor cases the size of nanoparticles should be tuned according to the vasculature lining gap size [59]. Endoplasmic retention is only one of the mechanisms describing tumor biology. There is a multitude of other factors that can present potential challenges for nanotherapeutics such as low blood circulation rate in tumor vessels, tumor site macrophages, and extracellular matrices environment around tumor cells. These factors play significant roles in how targeted nanoparticles find their substrate and effectively deliver drugs payload. A clear understanding of these factors will provide important synthesis strategies for targeted nanoparticles therapy—active or passive targeting alike.
2.3 Drug release strategy
Payload delivery capacity depends on how effectively drugs have been packaged, and how drug release mechanisms are programmed into the nanosystems. Drug ‘packaging’ efficacy depends on encapsulation or drug conjugation efficiency. Different nanoparticles provide different means of entrapping drug molecules, as described later in the section. Modulating rate of drug release in response to an activation signal constitutes an essential strategy to achieve controlled release purposes as well as maintaining effective therapeutic dosage over a stretch of time. There are two categories of nanosystems, open-loop control systems and closed-loop control systems, grouped according to what activation factors stimulate drug release as schematically shown in Fig. 4. In open-loop control systems, external factors such as magnetic pulses, thermal, acoustic pulses or electric fields control drug release. In contrast, in closed-loop systems the drug release rate is controlled by the presence and intensity of internal stimuli in the vicinity of the target sites [60, 61]. A few current strategies are based on the ‘chemistry’ programmed into the nanosystems that are responsive towards pH or temperature, erosion due to the local chemical environment, redox reaction-based release, and enzyme-mediated release as discussed below [62].
2.3.1 Redox-activated drug release
In redox-activated drug release mechanism, a redox-responsive nanocarrier containing functional groups that reacts upon contact with oxidizing and/or reducing environment in and around cancer cells (peroxides, GSH, and free radicals), undergoing to chemical bond cleavage [63]. The chemical changes can also introduce changes in the hydrophobicity of the polymer, changing the integrity of nanoparticles and thereby leading to release of drug cargo. For example, in poly(propylene sulfide) polymer nanoparticles, disulfide bonds act as a redox-responsive motif, and upon reacting with H2O2 leads to a change of hydrophobicity of the polymers causing a collapse of nanoparticles and thus drug release [64]. Redox-response moieties can also respond to the stimuli in a non-linear fashion. This complexity allows a prompt reaction to the high concentration of stimuli, but not to low concentrations, achieving controlled specificity [65].
2.3.2 pH-mediated drug release
The extracellular microenvironment of tumor tissues is acidic, due to secreted lactic acid caused by glycolysis in anorexia. Studies show that the pH value drops to around 6.5 from physiological pH of 7.4 during the tumoral metastasis or development [66]. This gradient in the pH profile between pathological cells and normal cells can be exploited for controlled drug release. Multiple types of chemical bonds have already been investigated to meet the drug development requirement that can ease the drug release process. pH-labile covalent bonds with benzoic-imine bond, 1,3,5-triazaadamantane (TAA) group, the hydrazone bond are developed as a proof of principle systems for pH-mediated response systems [67]. Gao et al. have used benzoic-imine bonds to attach α-cyclodextrin directly to mesoporous silica nanoparticles (MSNs) which were partially hydrolyzed in the extracellular tumor space and completely hydrolysed inside endosomes with low pH ~ 5. They have measured the cumulative release of loaded doxorubicin drug in different pH concentrations to confirm the functionality of the system [68].
2.3.3 Other stimuli-response systems
Other stimuli have been investigated for controlled release, including heat generated under a magnetic field [49], photo-inducible systems [69], ultrasound inducible systems [70] and electrochemically triggered [71] controlled release of drugs. With current advances in molecular biology and enzyme engineering, there is no limitation to using chemistry methods for surface modification or functionalization of nanoparticles for specificity. Biomolecule incorporation and conjugation methods will assist equally in development of well-controlled drug delivery systems, filling in shortcomings one system presents. Table 1 presents different nanocarriers loaded with drugs that are released to tumor sites based on specific stimuli.
3 Influence of physicochemical properties on nanocarriers
The design of highly efficient nanocarriers that meet the requirements for a drug delivery vehicle is an intricate process. A wide range of materials have been used to develop nanocarriers. The primary requirements in precisely engineering these nanomaterials as drug-delivery platforms for sustained release based on their size, shape, composition, surface charge, and biocompatibility, as illustrated in Fig. 1. The physicochemical properties of nanomaterials affect the adhesion to cells, their interaction, and accumulation which leads to therapeutic or toxic effects [23, 100, 101]. Thus, it is fundamental to engineer the nanomaterials to maximize their utility in biomedical applications. The ensuing section discusses major physicochemical properties of nanomaterials and their design considerations for therapeutic and diagnostic applications.
3.1 Size and shape of the nanoparticles
The size and shape of nanomaterials determine the extent of their tumor accumulation and in vivo distribution. The size of the nanomaterials also influences the uptake of the drug by the cells and interactions with specific tissues for therapeutic purposes. Additionally, the size and shape of the nanomaterials impact the drug loading and release, along with the stability [102]. Recent studies have demonstrated that size and shape of the gold (Au) nanoparticles influence the transfection efficiency of small interfering RNA (siRNA). To ascertain this dependence, three different sizes and two different shapes (13 nm sphere, 50 nm sphere and 40 nm star) of siRNA-conjugated gold nanoconstructs were developed to check the in vitro response of U87 glioblastoma cells targeting the expression of isocitrate dehydrogenase 1. Cellular uptake of larger particles (50 nm spheres and 40 nm stars) was higher when compared to 13 nm spheres, establishing that the size and shape of the nanoconstructs not only influenced the kinetics of cellular uptake but also affected intracellular distribution as depicted in Fig. 5 [103]. In another study, ultrasmall Au nanoparticles of sizes ranging from 2 to 15 nm coated with tiopronin were evaluated for their localization and penetration into breast cancer cells, and it was found that accumulation of smaller nanoparticles was higher in tumor tissues in mice [104]. Similarly, mesoporous silica nanoparticles of different sizes (280, 170, 110, 50 and 30 nm) were examined for the uptake by HeLa cells, revealing the maximum uptake by cells of 50 nm sized mesoporous silica nanoparticles, showing the suitability to be used as carrier vehicles for drug delivery [105].

(reproduced with permission from [103])
Cellular uptake of gold nanoconstructs by U87 glioblastoma cells. A Transmission electron micrographs of Au nanoparticles displaying 13 nm spheres, 50 nm spheres and 40 nm stars; B cellular uptake kinetics of Au nanoparticles-siRNA constructs by cells showing size and shape dependent uptake; C transmission electron images illustrating the process of cellular uptake after treatment with 0.5 nM of Au nanoparticles-siRNA constructs for 24 h. The vesicle membranes disrupted by the treatment with 50 nm spheres is signified by orange arrows, and the nanoconstructs distributed outside the vesicles is represented by yellow arrows
In addition to the size of the nanomaterials, the shape of the nanomaterials is equally important in drug delivery. Chithrani et al. [106] have studied the effect of the shape of Au nanoparticles (rod and spherical) on cellular uptake and established that the nanoparticles uptake is shape and size dependent, with uptake of spherical nanoparticles efficient compared to their rod-shaped counterparts. Furthermore, silicon-based nanoparticles with quasi-hemispherical, discoidal and cylindrical shapes were used to study the effect of shape-dependent distribution, with discoidal particles distributed to most of the organs tested as compared to other shapes that had less diverse biodistributions [107]. Likewise, Huang et al. have developed different shaped mesoporous silica nanoparticles (sphere, rod, and long rod) functionalized with fluorescein isothiocyanate (FITC) and rhodamine B isothiocyanate (RITC) for imaging and quantification of mesoporous silica nanoparticle uptake. The results designated that long rods are more easily internalized by A375 human melanoma cells, when compared to short rods and spheres shapes [108]. From the above discussion, it can be concluded that nanomaterials for therapeutic applications need to be engineered carefully with respect to their size and shape, because both of them have noteworthy impact on the uptake process of cells, and can potentially induce cellular responses.
3.2 Surface charge of nanoparticles
Like other physicochemical properties, the surface charge of nanomaterials governs their biomedical potency and applicability. The surface charge of the nanoparticles is one of the leading factors to direct the interaction at the nano-bio interface [23]. The cellular entry of nanomaterials depends on surface charge [109]. Additionally, the in vivo biodistribution of nanoparticles suggest that the negatively charged particles accumulate in tumor sites more efficiently [110]. Similarly, the cellular uptake and in vivo fate of micellar nanoparticles have been explored, wherein negatively charged micellar nanoparticles were taken up by tumor cells, and the mechanism of internalization was determined to occur through multiple distinct endocytic pathways including clathrin-mediated endocytosis, caveolae-mediated endocytosis, and macropinocytosis. Likewise, the in vivo distribution of nanoparticles indicated that highly charged nanoparticles were taken up by liver cells. Uptake was less effective with the negatively charged particles, however, indicating the role of negative surface charge on the nanoparticles, which can reduce the undesirable clearance by liver cells [111]. Finally, the surface charge significantly affects the internalization process and the cellular endocytosis mechanism as discussed above [112].
In general, positively charged nanomaterials may internalize efficiently at cell membranes, because of the negative charge on the cell surface [113]. This phenomenon can be further exploited for potential therapeutic purposes, employing nanoparticles as drug or gene delivery carriers. Insightful results have been obtained in the recent past, when cationic liposomes were developed to target the tumors that accumulated in tumor tissues [114, 115]. Moreover, the studies of Villanueva et al. have demonstrated that the internalization of magnetic nanoparticles inside HeLa cells is dependent on the nanoparticle surface charge and incubation time. Specifically, cationic magnetic nanoparticles are retained by the cells for extended period, inducing no cytotoxicity [116]. Additionally, charge switchable nanoparticles have also been developed, and such nanoparticles are reported to change their surface charge in response to external stimuli, with such charge switchable nanoparticles having positive impact toward enhanced cellular uptake [117, 118]. From the discussion above, it is evident that the surface charge of the nanomaterials affects their cellular uptake, and these particles can be efficiently used in cancer treatment based on the cell type and mechanism of endocytosis. Also, it is apparent that the nanomaterials distribution within the cell is strongly governed by their surface charge, which needs to be engineered to avoid undesirable uptake from the normal cells to achieve target specific action without adverse impact on normal cells.
3.3 Surface chemistry of nanoparticles
To develop nanomaterials for specific biomedical applications, surface chemistry design is indispensable. In addition to tailoring the surface corona, engineered nanomaterials reduce their toxicity and enhance their stability [23, 44, 119]. In this context, many studies have demonstrated that cellular interactions of polymer-based nanomaterials are highly influenced by their surface chemistry [120,121,122]. Recently, PLGA [poly(lactic-co-glycolic acid)] based nanomaterials have been developed, demonstrating that suitable surface coating of the nanomaterials provides extended circulation time. This increased circulation time can also lead to higher potency and specific antitumor activity. As an example, drug-coated nanoparticles completely inhibited lung tumor in mice, leading to enhanced survival rate and reduced adverse effect when compared to the free drug [123]. Similarly, PLGA nanoparticles were coated with polyvinyl alcohol (PVA) or vitamin E TPGS to evaluate cellular uptake by Caco-2 cells. The cellular uptake of surface modified PLGA nanoparticles were in the order of vitamin E TPGS-coated PLGA > PVA-coated PLGA > naked PLGA nanoparticles [124]. This pronounced variance from different surface coating suggests that chemical modification of nanoparticles is one of the most effectual means to control and restrain cellular interactions of nanomaterials, and hence their biological consequences.
The surface chemistry of Au nanoparticles and their use in cancer treatment have been extensively studied [125, 126]. The influence of surface coating on the toxicity and cellular uptake of Au nanorods were studied revealing the surface chemistry dependent cellular uptake of Au nanorods covered with poly(diallyldimethylammonium chloride) [PDDAC] [127]. Likewise, PEG capped Au nanoparticles coated with [Pt(1R,2R-diaminocyclohexane) (H2O)2]2NO3 were taken up, and localized in the lung epithelial and colon cancer cell lines showing more significant effects than the drug alone [128]. Similarly, mesoporous silica nanoparticles coated with different functional groups resulted in different mechanisms of endocytosis by HeLa cells, providing evidence of surface functional group-dependent uptake [129]. Likewise, functionalized carbon nanotubes are extensively used as drug delivery vehicles for delivering small interfering RNA (siRNA), paclitaxel and doxorubicin (DOX) [130,131,132,133]. The surface chemistry of the nanomaterials can provide control of the therapeutic effects by reducing the potential undesired side effects. However, surface functionalization needs to be systematically studied before clinical translation. In addition to the above discussion, there are tools that are currently available to shield nanomaterials for targeting cancer cells. Further, antibodies, small proteins, peptides, nucleic acid-based ligands, aptamers, small molecules, and oligosaccharides are used as targeting ligands [134,135,136,137,138,139]. Nevertheless, it is essential to choose the right type of ligand for improved and efficient targeting of the tumor cells. Since the fate of nanoparticles may be altered due to the surface conjugation of ligands, the nanomaterials further need to be carefully investigated, following their surface decoration to reduce unwanted toxicity effects, and to evaluate their increased specificity and sensitivity post-modification.
In summary, the physicochemical properties such as size, shape, surface charge, and surface chemistry influence the mechanisms of cellular uptake, distribution and therapeutic nature of material. In addition, many other factors have a profound consequence on nanomaterials uptake and distribution in cells. The purity of the nanoparticles, surface to volume ratio, chemical composition, aggregation states, crystal planes, stability, nanoparticle–protein interactions, incubation conditions, cell types, cell treatment, and other factors may also contribute to the cellular uptake and distribution.
4 Nanomaterial as drug delivery agents
A wide range of nanotherapeutics, composed of organic and inorganic nanomaterials have been developed with multiple types of drugs or molecules for cancer imaging, detection and treatment. In this section, multiple nanocarriers have been discussed including liposomes, dendrimers, polymeric nanoparticles, and metal nanoparticles.
4.1 Inorganic nanoparticles
This category of nanomaterials forms a significant fraction of current drug delivery systems due to their precise control of size and shape, tuneable physicochemical properties, controlled surface chemistry and diverse multifunctionality. A range of inorganic nanomaterials have been developed in recent past with meticulous properties and employed in biomedical applications especially in cancer treatment and management. Among the inorganic nanomaterials, metal nanoparticles and metal oxides have gained noteworthy consideration due to their exceptional properties and recent progress in the fundamental understanding through the development of innovative techniques. Other major nanomaterials that have noticeable contribution in drug delivery are carbon-based nanostructures and mesoporous silica nanoparticles. Table 2 highlights various inorganic nanocarriers for delivery of anticancer therapeutics. The table illustrates the type of inorganic nanomaterial used as nanocarrier, the explicit drug loaded on the carrier and the cancer cells.
4.1.1 Metal nanoparticle and metal oxides
Metal and metal oxide nanoparticles are one of the most useful materials as drug delivery vehicles due to their controllable size and shape, biocompatibility and easy surface functionalization. The noble metal nanostructures, particularly Au nanoparticles, are widely used for delivering drugs [140,141,142]. Recently, Wan et al. have reported the in vitro anticancer effects of docetaxel conjugated Au doped apatite. Wherein, the material display higher cytotoxicity against human liver cancer cells HepG2, and revealed to have improved bioavailability at the site [140]. Furthermore, Au nanoparticles coated with Pc4, a fluorescent photodynamic therapy (PDT) drug have been developed by functionalizing prostate-specific membrane antigen (PSMA-1) ligand to actively target the disease biomarkers to increase tumor residence time, and internalization by receptor-mediated endocytosis. The efficacy of a theranostics for prostate cancer has also been evaluated through in vitro and in vivo studies [141]. The in vitro studies discovered that the nanoparticle–drug conjugate was more efficient in killing PMSA-expressing cells. This observation provides insights on the active targeting and delivery of Pc4 drug due to the conjugation of Au nanoparticles with PMSA-1 ligand and internalization via clathrin-mediated endocytosis. The in vivo studies have further established that tumor volume reduces post-PDT as demonstrated by the decrease in fluorescence intensity. In one of the recent reports the drug release and stability of pH-sensitive Au nanoparticles loaded with 5-fluorouracil capped with cetyltrimethylammonium bromide (CTAB) was achieved by incorporating into gel and cream bases [142]. The ex vivo permeability of these formulations tested on mice dorsal skin and in vivo anticancer activity were evaluated in A431 tumor-bearing mice. The study has shown the sustained and pH-dependent release, in which the volume of the tumor reduced compared to the untreated control. The outcomes of the study are promising and recommend a topical nanoformulation to enhance drug efficacy against skin cancer. It has been demonstrated that Au nanoparticles decorated with two different anticancer drugs not only prolong the drug circulation time but also enhanced drug targeting and reduced the risk of drug resistance [143].
Similar to Au nanoparticles, silver (Ag) nanoparticles have also been demonstrated to be used as anticancer agents for the treatment of multiple types of cancer [144,145,146,147]. Ag nanoparticles have been used to deliver drugs that can elevate the therapeutic indices of the drug [148]. Ag nanoparticles conjugated with phytopharmaceuticals can serve as non-toxic delivery vehicles, contrast agents and photothermal agents for cancer therapy. Biogenic Ag nanoparticles can be employed against prostate and colon cancer. For instance, Ag nanoparticles synthesized using Indigofera hirsuta leaf extract and pollen extract of Phoenix dactylifera showed dose-dependent cytotoxicity against different cancers [149, 150]. A unique drug delivery system in which Ag nanoparticles coated with a camptothecin-based polymer prodrug was developed for the sustained release of the drug based on pH sensitivity [151]. Another potential strategy for inhibiting tumor metastasis and overcoming drug resistance was developed by co-delivering the drugs with particles featuring different physicochemical properties [152].
Iron oxide nanoparticles (IONPs) have emerged as theranostic nanoparticles providing a means to address the unmet clinical challenges in the treatment of cancer by imaging drug delivery and tumor response [153,154,155,156,157]. Contemporarily, double receptor targeted iron oxide nanoparticles loaded with paclitaxel drug delivery systems have been developed against prostate cancer [158]. The results demonstrated that these iron oxide nanoparticles are effectively internalized by human prostate cancer cell line PC-3. The in vitro magnetic resonance imaging confirmed the enhanced binding and accumulation of iron oxide nanoparticles in PC-3 cells, when compared with normal prostate epithelial cells. Recently, a theranostic nanoparticle to enhance intra-tumoral drug delivery by overcoming drug resistance and providing image-guided drug delivery by reducing the systemic toxicity was developed using iron oxide nanoparticles. In the study, three different targeted nanoparticles and one non-targeted nanoparticle were used to study the uptake and distribution of iron oxide nanoparticles in the PANC02 mouse pancreatic cancer cell line. The study also demonstrated the detection of residual tumors following intraperitoneal therapy signifying the possibility of image-guided surgery to remove drug-resistant tumors [159].
In another study, iron oxide nanoparticles were used to deliver OVA, an anticancer vaccine. OVA formulated with iron oxide nanoparticles significantly promoted the activation of immune cells and cytokine production, inducing potent humoral and cellular immune responses. The data indicated that OVA-iron oxide nanoparticles inhibited tumor growth effectively in mice and had good tissue compatibility with organs after intra-tumoral injection as depicted in Fig. 6 [188].

(reproduced with permission from [188])
Effect of OVA-iron oxide nanoparticles: macrophages activation with different concentrations of OVA, and production of a TNF-α, b IL-6, c IFN-γ. Saline and LPS served as negative and positive control; d size of the tumor measured after 22nd day of mice immunization; e histological sections of different organs on 23rd day after immunization of mice with different treatments (1) control, (2) soluble OVA, (3) iron oxide nanoparticles and (4) OVA-iron oxide nanoparticles
Apart from iron oxide nanoparticles several other metal oxide nanoparticles have been constructed and used for imaging, and drug delivery applications [165, 189,190,191]. However, their use is often limited due to the accumulation of metal in the body after drug administration causing toxicity. It is recommended that additional studies must be carried out to address the toxicity concerns, since the metal-based nanoparticles are easy to tune with the required properties for efficient loading of drugs and their potential may be excessively high in the field of biology and medicine.
4.1.2 Carbon-based materials
Carbon-based nanomaterials have also been extensively studied in imaging, delivery and diagnosis of cancer, due to their attractive characteristics such as high surface area, high drug loading capacity, and easily modifiable surfaces [7, 192,193,194,195,196,197]. Among the carbon nanomaterials, carbon nanotubes (CNTs) and graphene have been most commonly investigated in cancer therapeutic applications. Carbon nanotubes can assist as drug delivery systems for effective targeting to cancer cells. Recent investigations on multi-walled carbon nanotubes (MWCNTs) for the co-delivery of drugs have revealed that the release of drug at the cancer site, and the uptake by the cells showed the potential for treating multi-drug resistant cancer [198]. Wang et al., developed a multi-walled carbon nanotube platform with improved circulation half-life, and active targeting ability with high drug loading ratio. A pH sensitive nanoplatform can generate heat, following light absorption upon irradiation with near-IR (NIR) light and due to the toxicity of DOX, offering a potential multimodal nanomedicine for efficient cancer treatment [199]. In another report, multi-walled carbon nanotube were decorated with TiO2–Au nanocomposite, and the system was observed to be efficient in inducing toxicity to A549 and MCF7 cancer cell lines [200]. Likewise, intracellular drug delivery can be enhanced by utilizing carbon nanotube-based phototherapies. In this context, Levi-Polyachenko et al. have demonstrated increased cell membrane permeability by hyperthermia from multi-walled carbon nanotube, thereby enhancing drug delivery to tumor targets [201]. Similarly, complete tumor eradication has been achieved employing cut-single walled carbon nanotubes coated with BSA-reduced Au nanoparticles, enhancing doxorubicin drug release when combined with phototherapy with an 808 nm laser in a nude mouse model [202]. Alginate and chitosan coated single walled carbon nanotubes loaded with curcumin could target human lung adenocarcinoma (A549) cells, as shown in one recent report [203].
The delivery of PEGylated multi-walled carbon nanotubes conjugated with doxorubicin efficiently released 57% of the drug at lower pH within 24 h, and could inhibit HepG2 cells when compared to free doxorubicin [204]. A recent investigation reported that single walled carbon nanotubes were toxic, and induced death of the organs at higher dosages, whereas multi-walled carbon nanotubes in lower dosages could effectively deliver drug for targeted therapy of abnormal cells in breast cancer [205]. A novel drug delivery system based on carbon nanospheres for delivery of cancer therapeutics has been evaluated for internalization, and possible mechanism of endocytosis and biodistribution in mice [206]. The carbon spheres provided high drug loading capacity along with sustained release of drug under acidic pH, which is the normal tumor microenvironment. In vivo fluorescence imaging revealed the distribution of the drug in organs and these carbon nanospheres exercised antitumor effect in SCID mice bearing oesophageal tumors.
Graphene and its derivatives comprise an important class of materials that is widely used in drug and gene delivery, cell imaging, photothermal cancer therapy and biosensing [207,208,209,210]. Recently, a hybrid material based on graphene oxide (GO) coated with β-cyclodextrin (CD) and poly(amido amine) dendrimer (DEN) was used to deliver doxorubicin, camptothecin (CPT) and a photosensitizer (protoporphyrin IX (PpIX)). The drug loading capacity of hybrid material was in the order of camptothecin > protoporphyrin IX > doxorubicin, and displayed enhanced cytotoxicity [211]. A multi-functional graphene oxide based drug delivery system could target cancerous tissues, and exhibit antitumor effect with no systemic toxicity in B16 tumor-bearing mice [212]. To overcome the hypoxia-mediated chemoresistance of oral squamous cell carcinoma (OSCC), platinum loaded, polyethylene glycol-modified graphene quantum dots (GPt) have been utilized. The tested glycol-modified graphene quantum dots exhibited strong inhibitory effect on the tumor growth with minimal systemic drug toxicity in an oral squamous cell carcinoma xenograft mouse tumor model [209].
Multifunctional graphene smart nanomaterials have been developed for drug delivery and cellular imaging in cancer treatment [210, 213]. Recently, nanographene oxide complexed with upconverting nanoparticles were used for tumor imaging and photothermal therapy, signifying the potential of multifunctional graphene for clinical antitumor treatments [213]. The combination of chemotherapy with photothermal therapy has proved to be efficient when magnetic graphene oxide modified with PEG and cetuximab was used against CT-26 murine colorectal cells [214]. The active targeting was achieved using cetuximab, an epidermal growth factor receptor (EGFR) monoclonal antibody, since epidermal growth factor receptor is highly expressed on the tumor surface of colorectal cancer cells. The pH dependent release studies indicated the drug release was greater at pH 5.5 than pH 7.4 and could effectively target epidermal growth factor receptor-expressing CT-26 murine colorectal cells. The in vivo antitumor studies suggested that the tumor volume drastically reduced in mice in the presence of magnetic nanocarrier, magnet and laser. There was 29-fold increase in therapeutic efficacy of the nanocarrier during the combination therapy when compared to control.
Liu et al., developed graphene oxide modified with chitosan followed by conjugation with hyaluronic acid and an anti-cancer drug SNX-2112. The graphene oxide based carrier was found to be effective in inhibiting and killing A549 cells, and displayed lesser toxicity against normal human bronchial epithelial cells [215]. Similarly, graphene oxide with galactosylated chitosan with doxorubicin have been developed for the treatment of cancer. These nanocarriers were stable and their release was reported to be pH responsive. The in vivo antitumor effect of galactosylated graphene oxide was better than the chitosan graphene oxide, which was demonstrated by tumor weight and volume [216]. Clearly, carbon-based nanomaterials have led to the improvement in cancer therapy due to their unique properties. All these observations are motivating and may change the face of cancer treatment and management.
4.1.3 Mesoporous silica nanomaterials
Mesoporous silica nanomaterials (MSNs) have emerged as another class of drug delivery carriers, due to their surface properties such as large surface area, uniform porosity, stability, low toxicity and narrow size distribution [217]. The designing of multifunctional delivery platforms using mesoporous silica nanomaterials with different characteristics is possible because of facile modification of their surface. These features have led many researchers to load cargos on to mesoporous silica nanomaterials for transporting them to the tumor tissues [218,219,220]. Many anticancer drug applications are limited due to its solubility, stability, and bioavailability of the drug. In this context, Li et al., have developed novel nanocarrier systems for tumor targeting and precise release of curcumin. These surface modifiable mesoporous silica nanomaterials have been exploited to deliver curcumin to breast cancer cell lines that were loaded with hyaluronan or polyethyleneimine-folic acid and were tested on mouse xenograft model [221]. The folic acid modified mesoporous silica nanomaterials showed an enhanced cellular uptake than hyaluronan mesoporous silica nanomaterials and both nanoformulations had better cellular uptake when compared with that of a non-targeted nanocarrier. These nanoformulations showed better biocompatibility with low toxicity and inhibited tumor growth to a greater extent than curcumin alone. In a related study, to treat the multidrug resistant cancer cells with elevated Bcl-2 levels, Xu et al. [222] have developed macroporous silica nanoparticles with a peptide loading efficiency of 40%, which upon administration induced apoptosis. Often in the breast cancer cells, Mucin 1 (MUC1), a cell surface protein, will be overexpressed. In vivo studies of MUC1 aptamer-capped mesoporous silica nanomaterials on MDA-MB-231 tumor-bearing Balb/c mice were found to effectively target breast cancer cells and induce a dramatic reduction in cell viability [223].
Additionally, mesoporous silica nanomaterials can release cargo in response to stimuli. These smart nanosystems trigger the release of the drug trapped in the pores to the target sites in the presence of either endogenous or exogenous stimuli, with control on the administered dose. The pH responsive release of the drug is widely employed, since the tumor microenvironment will be slightly more acidic than the normal tissues. Liu et al. [224], utilized hollow mesoporous silica nanomaterials to release doxorubicin to HeLa cells in an acidic environment exhibiting anticancer effect with good biocompatibility. Additionally, mesoporous silica nanomaterials for the CD44-targeting pH responsive smart drug delivery system were developed by hyaluronic acid end-capping and loaded with doxorubicin. The fabricated nanoparticles enhanced cellular uptake via CD44 receptor-mediated endocytosis by HeLa cells. The cytotoxicity of doxorubicin-loaded mesoporous silica nanomaterials toward cancer cells overexpressing CD44 receptor was enhanced with IC50 of 0.56 μg/mL whereas; the normal cells showed lower cytotoxicity with the IC50 of 1.03 μg/mL [225]. Likewise, external stimuli mediated treatment of cancer with mesoporous silica nanomaterials is seen as suitable drug delivery candidates since the external stimuli will be independent of complicated tumor physiological microenvironment. Recently, core–shell nanoparticles were also developed with a magnetic core and mesoporous silica nanomaterials shell to effectively deliver epirubicin. There was a 27% increase in the cellular uptake of cells treated with magnetic mesoporous silica nanomaterials with epirubicin in the presence of external magnetic field when compared to free epirubicin [226].
Multifunctional mesoporous silica nanomaterials have been employed to provide a synergistic blend of different assemblies into nanoplatforms with enhanced antitumor activity and less cytotoxicity to normal cells. These particles can selectively target human osteosarcoma cells and are capable of pH-responsive antitumor drug delivery. The nanosystems exhibited higher internalization degree into human osteosarcoma cells and induced almost 100% osteosarcoma cell death with a low doxorubicin loading of 2.5 µg/mL. The cytotoxicity of nanoplatform was eightfold higher than that of the free drug [227]. It is evident that mesoporous silica nanomaterials are one of the promising nanocarriers for efficient delivery of cancer therapeutics due to their useful properties. The possibility of using mesoporous silica nanomaterials as potential nanocarriers has driven interest in many biomedical applications. However, more in-depth studies are required to understand the pharmacokinetic and pharmacodynamic properties of these systems before clinical translation of mesoporous silica-based nanomaterials.
4.2 Organic nanomaterials
Organic nanomaterials are promising candidates for the development of drug delivery systems. Significant properties of any nanomaterial used in biomedical delivery are its biocompatibility and biodegradability [228], with the discharged carrier degraded into nontoxic components and cleared through the circulation. These attractive properties along with low toxicity have enabled the nanomedicine research community to use organic nanomaterials as drug delivery vehicles to target specific tissues and controlled release of the drug molecules. To date, many types of organic nanocarriers have been developed such as liposomes, polymeric nanoparticles, dendrimers and micelles. A summary of different organic nanomaterials used as drug delivery carrier for anticancer drugs and the targets is shown in Table 3.
4.2.1 Liposomes
The use of a nanoparticles for medicine was first described in 1965, with liposomes as the first ones to be used [229]. Liposomes are spherical vesicles composed of a lipid bilayer of either synthetic or natural phospholipids surrounding an internal aqueous phase. The structure of liposomes can be engineered to encapsulate the hydrophobic or hydrophilic drugs, or other small molecules, in the lipid bilayer or aqueous core, respectively [230]. Liposome-based drug carrier systems have been developed to prolong the circulation time of the drugs and reduce toxicity to healthy tissues around. Correspondingly, these vehicles offer several other advantages including biocompatibility, self-assembly, and high drug cargo loading [231]. Due to the morphological similarity with cellular membranes and ability to integrate with various substances, liposomes serve as an ideal drug-carrier systems. Over the past 20 years, commendable progress has been made in biomedical applications of liposomes improving the therapeutic index of the encapsulated drugs. There are different classes of liposomes used as drug delivery platforms for enhancing the efficacy of cancer therapeutics [232]. Liposomes can be conjugated with poly(ethylene glycol) (PEG), targeting ligands and/or antibodies, polysaccharides on the external surface to enhance solubility, to increase the hydrophilicity and to provide passive and active targeting functions, in due course attaining high drug efficiency with low toxicity [233]. By exploiting the extended circulation property of PEGylated liposomes and biocompatibility, biodegradability and hydrophilicity of polysialic acid, a negatively charged polysaccharides drug delivery systems developed that has been used to prolong the circulation time of the liposomes, increasing the ability of epirubicin to reach the tumor sites. These antitumor studies revealed that modified liposomes had lower systemic toxicity and prolonged the survival time of the treated mice by suppressing the tumor growth more strongly [234]. Likewise, doxorubicin-loaded modified PEGylated liposomes were developed for targeted delivery of drug to hepatocellular carcinoma. Soybean phosphatidylcholine/cholesterol was used in the molar ratio of 3:2 to prepare liposomes by thin film hydration method, and doxorubicin was remotely loaded into the liposomes via the ammonium gradient method. Later liposomes were PEGylated (PLS) by a PEG-lipid post-insertion technique followed by covalent coupling with lactoferrin (Lf) to the surface of liposomes as illustrated in Fig. 7a. These liposomal formulations exhibited negative zeta potential values and an in vitro release study demonstrated that the liposomal formulations displayed good stability, and an extended circulation time required to avoid drug clearance before arrival at the target cells. The in vitro cytotoxicity studies revealed that doxorubicin formulations had increased antiproliferative effect and was time and dose-dependent as depicted in Fig. 7b. Doxorubicin-loaded lactoferrin-PLS displayed stronger inhibitory effects in ASGPR-positive HCC cells than with unmodified PEGylated liposomes. The in vivo antitumor studies were conducted on male BALB/C nude mice bearing a HepG2 tumor model. Interestingly, there was a significant tumor growth inhibition in the treatment group with doxorubicin-loaded lactoferrin-PLS of HepG2 tumors when compared to only doxorubicin-loaded PLS and free doxorubicin, with no significant change in the body weight observed as shown in Fig. 7c–g. This important study signposts the strategy of modifying the surface of liposomes for effective delivery of anticancer drugs to treat hepatocellular carcinoma [235]. Similarly, the PEGylated liposomes have been used in delivering celastrol, irinotecan, resveratrol in the treatment of breast cancer and glioblastoma [236, 237].

(reproduced with permission from [235])
Scheme representing the formulation of doxorubicin loaded PEGylated liposome, and doxorubicin loaded lactoferrin modified PEGylated liposome (a); effect of cell viability of free DOX and the liposomal formulations evaluated by MTT assay in HepG2, BEL7402, and SMMC7721 cells at different time intervals (b); relative tumor volume of various liposomal formulations injected to tumor-bearing mice through tail veins every 7 days at a dose of 5 mg/kg DOX (c); change in the body weight of tumor-bearing mice after each treatment (d); image of tumors excised on 21st day from each treatment group (e); relative tumor volume at the time of sacrifice from each treatment group (f); tumor weight at the time of sacrifice from each treatment group (g)
Approaches for co-delivery of different chemotherapeutics have been developed as a useful method for the treatment of cancer. Combination therapy has been demonstrated to be effective and has substantial evidence showing that synergistic effects that are superior to the totality of the therapeutic consequences of the individual drug [238,239,240]. Several strategies have also been developed to accomplish liposomal codelivery of chemotherapeutic agents. These liposome-based combinational formulations have significant popularity due to augmented anticancer effects, antiproliferative activity, apoptosis, and cytotoxicity while diminishing the systemic toxicity. Besides, liposomal co-delivery of chemotherapeutic agents can minimize cancer cell drug resistance and make them more sensitive to individual drugs. In a recent study, co-delivery of two chemotherapeutic agents (tamoxifen and imatinib mesylate) using a liposome carrier system was developed to treat breast cancer. Tamoxifen and imatinib mesylate were released in controlled manner from the temperature sensitive liposomes prepared using a combination of phospholipids with a transition temperature near to 39 °C. The dual drug-loaded thermo-sensitive liposomes exhibited significantly larger release rate of both the drugs at 40 °C and displayed synergistic inhibition of breast cancer cell proliferation. The findings highlighted the development of a thermo-responsive liposomal drug delivery system for combinational breast cancer treatment [97]. Similarly, pH sensitive liposomes have also proved to be effective in increasing the drug accumulation in resistant tumor cells and are potent drug carriers that can overcome multidrug resistance. Xia et al., constructed a pH sensitive liposome formulation by loading tariquidar (TQR) and doxorubicin to overcome multidrug resistance of drug-resistant ovarian cancer cells. The tariquidar and doxorubicin-loaded pH sensitive liposome formulation exhibited outstanding tumour inhibition against the tested cells by increasing the accumulation of doxorubicin in cells, allowing them to enter specifically into the nuclei [241]. Additionally, a newer generation of liposomes are emerging, focusing on redox sensitive liposomes, magnetic liposomes, enzyme sensitive liposomes and multifunctional smart liposomes [242,243,244,245].
The above discussion signifies the importance of liposomes in drug delivery systems for the treatment of cancer. These nanocarriers help overcome the unwanted side effects in normal tissues and increase circulation time, bioavailability, and accumulation of drug at target-site by reducing toxicity and protect the chemotherapeutic agents from the surrounding environment. In spite of widespread research and the preclinical development of liposomal formulations from several decades, only a few liposomal drug formulations have been approved by the FDA for clinical use [246]. Formulations have been approved for the treatment of Kaposi’s sarcoma, acute lymphoblastic leukemia, pancreatic cancer, ovarian cancer, multiple myeloma and metastatic breast cancer including Doxil®, Myocet®, DaunoXome®, DepoCyte®, Lipoplatin®. This major setback has led to the development of ligand-directed liposomes for active targeting and treatment of different types of cancer.
4.2.2 Polymeric nanoparticles
Polymeric nanoparticles are colloidal nanoparticles wherein therapeutic molecules will be encapsulated or adsorbed or conjugated in the polymer matrix. These nanoparticles can be synthesized using synthetic and natural polymers, and have been extensively used in drug delivery applications [265, 266]. These nanoparticles can be customized for various biomedical applications due to their unique characteristics such as drug solubility, stability, and preferential accumulation [267]. Polymeric nanoparticles serve as a versatile platform to deliver drugs due to their different chemical composition, charge and physical structure. Moreover, they have gained commercial importance because of their tunable drug release kinetics. Drugs can be efficiently delivered using polymeric nanoparticles by active or passive targeting the cancer cells. Tumor-specific targeting at the surface of the cancer cells has also been explored to eradicate tumor cells. Various ligands such as antibodies, proteins, peptides, aptamers and small molecules have been used to target specific cells [268]. The targeting of cells by nanoparticles results in highly specific delivery of cargos, resulting in high concentrations of the therapeutic within the cell. Several studies have demonstrated enhanced antitumor activity with targeting moieties. Surface modified polylactic acid (PLA) nanoparticles have been reported and employed for delivery of docetaxel (DTX) as a targeted drug delivery system for the treatment of liver cancer. Docetaxel-loaded galactosamine combined with polydopamine-modified nanoparticles synthesized from d-a-tocopherol polyethylene glycol 1000 succinate-poly(lactide) (Gal-pD-TPGS-PLA/NPs) were found to inhibit the growth of HepG2 cells more effectively than TPGS-PLA/NPs, pD-TPGS-PLA/NPs, and a clinically available docetaxel formulation (Taxotere®). The in vivo transplantable liver tumor bearing BALB/c nude mice treated with docetaxel loaded Gal-pD-TPGS-PLA/NPs exhibited noticeable tumor growth inhibition when compared to other nanoformulations and free Taxotere®. The authors have suggested that the antitumor effect of the surface modified docetaxel loaded polylactic acid nanoparticles resulted from the targeted delivery to HepG2 cells [269]. In another study, resveratrol encapsulated PLGA [poly(lactic-co-glycolic acid)] nanoparticles have been constructed for prostate cancer therapy. These nanoparticles exhibited a significant decrease in cell viability and greater cytotoxicity toward LNCaP cells when compared to free resveratrol. The cytotoxicity assay demonstrated that resveratrol conjugated poly(lactic-co-glycolic acid) nanoparticles had two-fold lower IC50 and IC90 values in comparison to only resveratrol [253].
The combination of multiple drugs has been established to be more effective than single drug treatment. It is anticipated that multiple drugs when delivered simultaneously to a cancer cell will exhibit a synergistic effect, when administered in an optimized ratio. Due to the advancements in nanomedicine, several nanoparticle formulations have been developed for co-delivery of cancer chemotherapeutics [270, 271]. Zhang et al., designed pH sensitive TPGS-PAE nanoparticles, polymeric nanoparticles, wherein doxorubicin and curcumin were co-loaded by self-assembly. The nanoformulation exhibited a high rate of apoptosis against human liver cells and stronger anti-angiogenic effects together with inhibition of proliferation, migration, invasion, and tube formation [272].
Another polymeric nanoparticle platform that is gaining significant attention as drug delivery systems is polymer micelle nanoparticles. Recently, Peng et al. prepared a polymeric micelle by incorporating temozolomide (TMZ) and anti-BCL-2 siRNA based on tri-block copolymer conjugated with folic acid as outlined in Fig. 8a for delivering temozolomide and siRNA to overcome the drawbacks of acquired resistance of glioma cells and restriction of blood–brain-barrier (BBB) for drug delivery. The nanocomplexes were spherical in shape, which was confirmed by transmission electron microscopy analysis as shown in Fig. 8b. Further, as illustrated in Fig. 8c, the cell viability of various formulations was investigated on a rat C6 glioma cell line at different temozolomide concentrations. Temozolomide-FaPEC@siRNA exhibited higher cytotoxicity than both temozolomide-FaPEC and temozolomide-PEC, whereas C6 cells incubated with FaPEC@SCR and PEC@SCR exhibited viabilities over 90% even at a very high 100 µg/mL polymer concentration, indicating low cytotoxicity of carrier, a vital characteristic for in vivo application. In vivo, pharmacokinetic studies have also been conducted in the study to reveal the variation in the glioma growth in rat brain for different complexes after 25 days of the first injection. The tumor volume as depicted in Fig. 8d, e was determined by magnetic resonance imaging and showed that temozolomide and siRNA conjugated nanocomplex had a volume of 82 ± 11 mm3 which is much less than the volume resulting with the other treatments. However, the siRNA or temozolomide treatment mediated by the folate-targeted nanocarrier was able to prevent glioma growth, the combination therapy was more effective than the individual treatment [273]. These in vitro and in vivo studies confirmed the effectiveness of combination therapy using temozolomide and siRNA for treatment of glioma and provided understanding on the folate targeted co-delivery of cancer therapeutics. Apart from folate-mediated targeting, aptamer-functionalized PEG-PLGA nanoparticles have also been constructed for anti-glioma drug delivery by active targeting the tumor. PEG-PLGA nanoparticles were conjugated with AS1411, a DNA aptamer, that binds to a protein highly expressed in the plasma membrane of cancer and the endothelial cells of angiogenic blood vessels. The designed nanoformulation was spherical in shape with 156 ± 54 nm size and a negative zeta potential exhibiting increased cytotoxicity in C6 glioma cells. The targeted nanosystem established higher tumor inhibition and prolonged the survival time of rats bearing intracranial C6 glioma, when compared to paclitaxel conjugated nanoparticles and a commercial drug Taxol® [274].

(reproduced with permission from [273])
Illustration of TMZ (temozolomide) and siRNA conjugated preparation of folic acid decorated Fa-PEG-PEI-PCL and release of antitumor therapeutics inside the cancer cells (a); TEM images showing TMZ-conjugated, folic acid-decorated PEC micelle (left) and TMZ and siRNA-conjugated, folic acid-decorated PEC micelle (right) at pH 7.4. All samples were stained with 0.5% uranyl acetate for 1 min. Scale bar: 200 nm (b); in vitro cytotoxicity effect of different nanocomplexes on C6 cells evaluated by CCK8 assay at various TMZ concentrations. Cells were incubated for 48 h and BCL-2 siRNA concentration used is 20 nM (c); Mean tumor volume determined using magnetic resonance imaging measured after 25 days of the first injection. *P < 0.05 vs TMZ-FaPEC@siRNA; #P < 0.05 vs TMZ-PEC@siRNA; ΔP < 0.05 vs TMZ-FaPEC@SCR (d); visualization of tumor growth inhibition in male Sprague–Dawley rats implanted with C6 cells after treatment with different formulations (red arrow indicates the tumor) (e). Fa, folate; PCL, poly(ε-caprolactone); PEG, poly(ethylene glycol); PEI, poly(ethylenimine); TMZ, temozolomide
Therefore, polymeric nanoparticles can be effectively used to deliver cancer therapeutics by active and passive targeting. Also, these platforms can provide competent drug delivery systems responsive to various stimuli to enhance the therapeutic efficacy and reduce the side effects of loaded drugs. Polymeric nanoparticles are efficient in enhancing therapeutic and diagnostic effects over conventional medicines. Several polymer-based therapeutics are currently in the market or undergoing a clinical evaluation to treat cancer. Such clinical trials are projected to intensify the use of polymeric drug delivery systems in the near future.
4.2.3 Dendrimers
Dendrimers are multi-branched molecules with functional groups on the surface with an inner core. These structures can be produced by using macromolecules such as polyamide amine (PAMAM), polypropyleneimine and poly(aryl ether). The most striking properties of dendrimers such as branches, distinct molecular weight and globular assembly with meticulous surface functionality, and multivalency, can be exploited to be used as carriers for drug delivery [275]. In addition to functional groups on their branches, they are suitable for loading and binding diverse hydrophilic and hydrophobic drugs. These dendritic systems have been used to deliver anticancer drugs wherein the drugs are encapsulated/conjugated with dendrimers. These nanocarriers have demonstrated to decrease non-specific toxicities, improve drug delivery profiles, enhance drug stability and bioavailability, targeted drug delivery.
The solubility, biodistribution and resistance of anticancer drugs together form a significant hurdle in improving the pharmacodynamic profile for the treatment of cancer. To overcome these drawbacks, a polyamide amine dendrimer conjugated with paclitaxel and docosahexaenoic acid (DHA) was developed to enhance the anticancer activity by increasing its efficacy and reducing toxicity. The in vitro studies indicated that the nanocarrier developed with docosahexaenoic acid, polyamide amine and conjugated with PTX had a better anticancer activity toward upper gastrointestinal cancer cells when compared to polyamide amine conjugated with PTX [276]. Likewise, half-generation polyamide amine dendrimers reduce cytotoxicity due to the presence of negatively charged carboxylic or cyano groups on their surface. Several researchers have demonstrated that half-generation dendrimers exhibit lower toxicity than the full generation of polyamide amine [277,278,279]. Likewise, Thanh et al., generated Heparin-functionalized monomethoxy PEG-polyamide amine dendrimer (HEP-mPEG) with effective encapsulation of DOX. G4.0-polyamide amine-HEP-mPEG revealed precise release of doxorubicin and had prolonged retention compared to pristine doxorubicin in both Hela and fibroblast NIH3T3 cancer cells. The results demonstrated that the high drug loading capacity and less systemic toxicity of G4.0 polyamide amine-HEP-mPEG/DOX could serve as a suitable drug delivery system [280].
The most effective approach of delivering anticancer drugs is by conjugation of ligands that specifically recognize and binds to the receptors on the tumor cells. In this context, Chittasupho et al., have developed CXCR4 targeted dendrimer for breast cancer therapy. Linear type of FC131 (LFC131) ligand conjugated, doxorubicin encapsulated polyamide amine dendrimer was developed using polyamide amine dendrimer generation 4.0 (D4). The cytotoxicity of the dendrimer encapsulated doxorubicin and LFC131-DOX-D4 to BT-549-Luc cells was evaluated and the IC50 value of LFC131-DOXD4 was 2.8 fold of DOX-D4 against BT-549-Luc cells and it was 6.8 fold of DOX-D4 against T47D cells after 24 h of incubation, indicating that the ligand conjugated doxorubicin encapsulated dendrimer can enhance the cytotoxicity of the drug against the cancer cell lines [281]. Likewise, Öztürk et al., developed a PEF modified dendrimer-based drug delivery system targeting Flt-1 (a receptor for vascular endothelial growth factors (VEGF)) receptor to improve the therapeutic efficacy of gemcitabine in pancreatic cancer. The CFPAC-1 pancreatic adenocarcinoma cell viability decreased, indicating a PEGc polyamide amine-PEG dendrimers anti-cancer effect. Conjugation with anti-Flt-1 antibody improved the accumulation of PEGc polyamide amine-PEG dendrimers into the pancreatic tumors [282]. Stimuli responsive dendrimers enhance therapeutic efficiency and diminish the side effects. These are responsive to pH, temperature, enzyme, light, the concentration of glutathione [283].
From the above discussion, it is evident that dendrimers are nanoplatforms which can be tuned for therapeutic applications, and show great promise in the treatment of various cancers. The challenge of bench-to-bedside translation of dendrimers, however, remains a significant challenge.
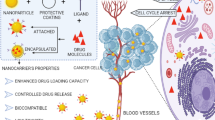
5 Challenges in nano drug delivery
The use of diverse nanomaterials with desired properties and recent progress in the drug delivery arena have revealed outstanding challenges in cancer therapy and management. It is anticipated that the nanomaterials will revolutionize the entire health care system based on the dramatic developments made in drug delivery sector over the past few decades. However, the design of effective cancer nanotherapeutics remains a great challenge, and only a few nanoformulations have entered clinical trials. A schematic representation of the major challenges in the delivery of cancer nanotherapeutics is depicted in Fig. 9. The physicochemical properties of nanomaterials play a significant role in the biocompatibility, and toxicity in the biological systems [284, 285]. Therefore, synthesis and characterization of the nanomaterials for drug delivery need to be carefully performed to avoid the potential unwanted toxicity of nanocarriers to healthy cells [23]. Additionally, since these nanocarriers interact with the biomolecules and may tend to aggregate forming a protein corona, disturbing the regular function of nanomedicine formulations and rendering them ineffective in controlling the cancer cell growth [286]. In conjunction to physicochemical properties, the nanomaterial storage and stability may also have an influence on their pharmacological performance [287, 288].
Another challenge in drug delivery is the safety for human health, as issues may be associated with nanomaterial, and may not have immediate impact or may not be noticeable quickly. The use of nanocarriers in the treatment of cancer may result in unwanted toxicity through unfavourable interactions with biological entities [289]. Several studies have revealed the detrimental properties of nanocarriers due to their toxicity [290, 291]. Therefore, ‘Nanotoxicology’ a branch of nanomedicine has emerged as an essential field of research, paving the way for the assessment of toxicity of nanoparticles. In addition to all the above, a significant setback in nanomedicine commercialization is the clinical translation due to the lack of in-depth understanding of nano-bio interfacial interactions. Specifically, the lack of in vitro/in vivo correlation of drug release profiles is a major lingering issue. Furthermore, the manufacturing of nanomedicine products for commercialization is a key obstacle, as large scale-production is technically challenging. Generally, only small quantities of nanomedicine are used for pre-clinical and clinical trial studies. The large-scale production of nanoformulations, however, is quite challenging as their physicochemical properties may vary from batch to batch. Moreover, the involvement of complicated multi-stage processes of production of nanotherapeutics and the high cost of raw materials renders these nanotherapeutics an expensive option. Consequently, the use of well-planned and -designed manufacturing processes are essential, and the clinical benefit must be huge which can justify the manufacturing costs.
Another key issue is the challenge of regulatory approval of nanomedicines, as there are no specific guidelines set by FDA for the products with nanomaterials. Currently used criteria have been borrowed directly from guidelines pertaining to bulk materials. The regulatory verdicts on the nanoformulated drugs are based on the individual assessment of paybacks and perils, making evaluations a time-consuming affair that causes delays in commercialization. Also, difficulties in the approval will tend to increase due to the development of multifunctional nanoplatforms. Thus, to mitigate the problems associated with nanomaterial-based therapeutic agents for cancer treatment, design and development strategies need to be employed before they are used in medicine for better treatment and human life. Understanding the complications involved in cancer cell physiology and the tumor microenvironment, along with drug and carrier pharmacokinetics is essential for the development of successful new cancer therapeutics. Alongside, case-by-case basis investigations are required to harness the tremendous potential of cancer nanotherapeutics. A comprehensive set of guidelines for regulatory approval is urgently needed to expedite the evaluation and approval of cancer nanotherapeutics.
6 Conclusions
Progress in materials science and nanotechnology have brought nanomaterials-based formulations/drugs to the forefront of medical research, emerging as potential tools for cancer treatment and management. The smart design and synthesis of a library of nanomaterials, precise control over their physicochemical properties and ease of their surface functionalization to increase specificity is indeed necessary for the success of cancer nanotherapeutics. An understanding of nano-bio interfacial interactions and targeting of nanoparticles to the tumor cells is essential for cancer therapy and management. All these strategies can reduce the systemic toxicity at the tumor sites by ensuring that healthy cells are not affected. Also, several nanoplatforms have already been developed to release the cargos in response to various stimuli, offering multifunctionality and specificity. Despite the numerous advantages of the nano-based cancer therapeutics, clinical translation of these nanomedicines remains to be a challenging mission. Due to the lack of understanding of toxicity and in vivo behaviour of nanoformulations, clinical trials are experiencing major setbacks. Therefore, only a few numbers of nano-drugs available in market for the treatment of cancer. However, further advancements in nanomedicine will provide breakthroughs that represent a paradigm shift in the treatment of cancer, and can significantly contribute to an improved patient outcome. At this stage, it can be envisioned that improvement in materials is possible for next‐generation nanomedicine through smart design, and new developments can provide better cancer managment strategies.
Availability of data and materials
The review is based on the published data and sources of data upon which conclusions have been drawn can be found in the reference list.
Abbreviations
- EPR:
-
enhanced permeability and retention
- FDA:
-
Food and Drug Administration
- PEG:
-
polyethylene glycol
- DOX:
-
doxorubicin
- IGF1:
-
insulin-like growth factor 1
- IONPs:
-
iron oxide nanoparticles
- PDX:
-
patient-derived xenograft
- MRI:
-
magnetic resonance imaging
- AMF:
-
alternating magnetic field
- EGFR:
-
epidermal growth factor receptor
- FR:
-
folate receptor
- MSNs:
-
mesoporous silica nanoparticles
- siRNA:
-
small interfering RNA
- FITC:
-
fluorescein isothiocyanate
- RITC:
-
rhodamine B isothiocyanate
- PLGA:
-
poly(lactic-co-glycolic acid)
- PVA:
-
polyvinyl alcohol
- PDDAC:
-
poly(diallyldimethylammonium chloride)
- PDT:
-
photodynamic therapy
- PSMA:
-
prostate-specific membrane antigen
- CTAB:
-
cetyltrimethylammonium bromide
- CNTs:
-
carbon nanotubes
- MWCNTs:
-
multi-walled carbon nanotubes
- NIR:
-
near-IR
- BSA:
-
bovine serum albumin
- SCID:
-
severe combined immunodeficiency
- GO:
-
graphene oxide
- CD:
-
β-cyclodextrin
- DEN:
-
dendrimer
- CPT:
-
camptothecin
- PpIX:
-
protoporphyrin IX
- OSCC:
-
oral squamous cell carcinoma
- MUC1:
-
Mucin 1
- Lf:
-
lactoferrin
- PLA:
-
polylactic acid
- DTX:
-
docetaxel
- TMZ:
-
temozolomide
- BBB:
-
blood–brain-barrier
- PAMAM:
-
polyamide amine
- DHA:
-
docosahexaenoic acid
- HEP-mPEG:
-
heparin-functionalized monomethoxy PEG
References
W. You, M. Henneberg, Cancer incidence increasing globally: the role of relaxed natural selection. Evol. Appl. 11(2), 140–152 (2017)
M.U.R. Naidu et al., Chemotherapy-induced and/or radiation therapy-induced oral mucositis-complicating the treatment of cancer. Neoplasia 6(5), 423–431 (2004)
Y.H. Bae, Drug targeting and tumor heterogeneity. J. Control. Release 133(1), 2–3 (2009)
X. Gao et al., In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969 (2004)
S. Bae et al., Doxorubicin-loaded human serum albumin nanoparticles surface-modified with TNF-related apoptosis-inducing ligand and transferrin for targeting multiple tumor types. Biomaterials 33(5), 1536–1546 (2012)
M. De Palma et al., Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat. Med. 9, 789 (2003)
P.N. Navya, A. Kaphle, H.K. Daima, Nanomedicine in sensing, delivery, imaging and tissue engineering: advances, opportunities and challenges. Nanoscience 5, 30–56 (2019)
R.A. Revia, M. Zhang, Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater. Today 19(3), 157–168 (2016)
A. Bajaj et al., Detection and differentiation of normal, cancerous, and metastatic cells using nanoparticle-polymer sensor arrays. Proc. Natl. Acad. Sci. 106(27), 10912 (2009)
J. Shi et al., Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 10(9), 3223–3230 (2010)
P.N. Navya et al., Single step formation of biocompatible bimetallic alloy nanoparticles of gold and silver using isonicotinylhydrazide. Mater. Sci. Eng. C 96, 286–294 (2019)
D.D. Lasic, D. Papahadjopoulos, Liposomes revisited. Science 267(5202), 1275 (1995)
A. Bajaj et al., Array-based sensing of normal, cancerous, and metastatic cells using conjugated fluorescent polymers. J. Am. Chem. Soc. 132(3), 1018–1022 (2010)
P. Ghosh et al., Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 60(11), 1307–1315 (2008)
O.R. Miranda et al., Array-based sensing of proteins using conjugated polymers. J. Am. Chem. Soc. 129(32), 9856–9857 (2007)
G. Han, P. Ghosh, V.M. Rotello, Functionalized gold nanoparticles for drug delivery. Nanomedicine 2(1), 113–123 (2007)
G. Han et al., Drug and gene delivery using gold nanoparticles. NanoBiotechnology 3(1), 40–45 (2007)
A. Verma, V.M. Rotello, Surface recognition of biomacromolecules using nanoparticle receptors. Chem. Commun. 3, 303–312 (2005)
Q. Liu et al., Differentiation of cancer cell type and phenotype using quantum dot-gold nanoparticle sensor arrays. Cancer Lett. 334(2), 196–201 (2013)
K. Saha et al., Gold nanoparticles in chemical and biological sensing. Chem. Rev. 112(5), 2739–2779 (2012)
D. Peer et al., Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751 (2007)
V.M. Rotello, Sniffing out cancer using “chemical nose” sensors. Cell Cycle 8(22), 3615–3616 (2009)
P.N. Navya, H.K. Daima, Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 3(1), 1 (2016)
A. Wicki et al., Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J. Control. Release 200, 138–157 (2015)
C.K. Kim et al., Entrapment of hydrophobic drugs in nanoparticle monolayers with efficient release into cancer cells. J. Am. Chem. Soc. 131(4), 1360–1361 (2009)
S.S. Agasti et al., Photoregulated release of caged anticancer drugs from gold nanoparticles. J. Am. Chem. Soc. 131(16), 5728–5729 (2009)
W. Du, O. Elemento, Cancer systems biology: embracing complexity to develop better anticancer therapeutic strategies. Oncogene 34, 3215 (2014)
J. Zhao, V. Castranova, Toxicology of nanomaterials used in nanomedicine. J. Toxicol. Environ. Health B 14(8), 593–632 (2011)
H. Maeda, H. Nakamura, J. Fang, The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 65(1), 71–79 (2013)
R.K. Jain, T. Stylianopoulos, Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653 (2010)
S.K. Hobbs et al., Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 95(8), 4607–4612 (1998)
N. Bertrand et al., Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 66, 2–25 (2014)
D. Rosenblum et al., Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 9(1), 1410 (2018)
J. Shi et al., Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17(1), 20 (2017)
J.E. Lancet et al., Final results of a phase III randomized trial of CPX-351 versus 7 + 3 in older patients with newly diagnosed high risk (secondary) AML. J. Clin. Oncol. 34, 7000 (2016)
V.P. Chauhan, R.K. Jain, Strategies for advancing cancer nanomedicine. Nat. Mater. 12(11), 958 (2013)
H. Lee et al., In vivo distribution of polymeric nanoparticles at the whole-body, tumor, and cellular levels. Pharm. Res. 27(11), 2343–2355 (2010)
M.J. Ernsting et al., Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 172(3), 782–794 (2013)
C. Wong et al., Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. 108, 2426–2431 (2011)
L.K. Bogart et al., Nanoparticles for imaging, sensing, and therapeutic intervention (ACS Publications, Washington DC, 2014)
J.D. Byrne, T. Betancourt, L. Brannon-Peppas, Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 60(15), 1615–1626 (2008)
A. Varki, Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 446(7139), 1023 (2007)
A. Verma, F. Stellacci, Effect of surface properties on nanoparticle–cell interactions. Small 6(1), 12–21 (2010)
R. Mout et al., Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 41(7), 2539–2544 (2012)
R. Weissleder et al., Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotechnol. 23(11), 1418–1423 (2005)
D. Peer et al., Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2(12), 751 (2007)
D.B. Kirpotin et al., Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 66(13), 6732–6740 (2006)
H. Zhou et al., IGF1 receptor targeted theranostic nanoparticles for targeted and image-guided therapy of pancreatic cancer. ACS Nano 9(8), 7976–7991 (2015)
C.S. Kumar, F. Mohammad, Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 63(9), 789–808 (2011)
S. Sigismund, D. Avanzato, L. Lanzetti, Emerging functions of the EGFR in cancer. Mol. Oncol. 12(1), 3–20 (2018)
S.-I. Ohno et al., Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 21(1), 185–191 (2013)
W.A. Messersmith, D.J. Ahnen, Targeting EGFR in colorectal cancer. N. Engl. J. Med. 359(17), 1834 (2008)
X. Li et al., Enhancement of cell recognition in vitro by dual-ligand cancer targeting gold nanoparticles. Biomaterials 32(10), 2540–2545 (2011)
S. Bhattacharyya et al., Efficient delivery of gold nanoparticles by dual receptor targeting. Adv. Mater. 23(43), 5034–5038 (2011)
J. Gao, S.-S. Feng, Y. Guo, Antibody engineering promotes nanomedicine for cancer treatment. Nanomedicine 5(8), 1141–1145 (2010)
D.R. Elias et al., Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomed. Nanotechnol. Biol. Med. 9(2), 194–201 (2013)
P.M. Valencia et al., Effects of ligands with different water solubilities on self-assembly and properties of targeted nanoparticles. Biomaterials 32(26), 6226–6233 (2011)
L. Vroman, Effect of adsorbed proteins on the wettability of hydrophilic and hydrophobic solids. Nature 196(4853), 476 (1962)
S.K. Hobbs et al., Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. 95(8), 4607–4612 (1998)
G.-H. Son, B.-J. Lee, C.-W. Cho, Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Invest. 47(4), 287–296 (2017)
A. Prasanna et al., Smart drug delivery systems for cancer treatment using nanomaterials. Mater. Today Proc. 5(10), 21047–21054 (2018)
L. Zhang, Y. Li, C.Y. Jimmy, Chemical modification of inorganic nanostructures for targeted and controlled drug delivery in cancer treatment. J. Mater. Chem. B 2(5), 452–470 (2014)
H. Alimoradi, et al., in Nanostructures for drug delivery. Redox activated polymeric nanoparticles in tumor therapy (Elsevier, Amsterdam, 2017). pp. 327–354
C.-C. Song, F.-S. Du, Z.-C. Li, Oxidation-responsive polymers for biomedical applications. J. Mater. Chem. B 2(22), 3413–3426 (2014)
C.D. Vo, G. Kilcher, N. Tirelli, Polymers and sulfur: what are organic polysulfides good for? Preparative strategies and biological applications. Macromol. Rapid Commun. 30(4–5), 299–315 (2009)
Y. Kato et al., Acidic extracellular microenvironment and cancer. Cancer Cell Int. 13(1), 89 (2013)
M. Karimi et al., Smart external stimulus-responsive nanocarriers for drug and gene delivery (Morgan & Claypool Publishers, San Rafael, 2015)
Y. Gao et al., A multifunctional nanocarrier based on nanogated mesoporous silica for enhanced tumor-specific uptake and intracellular delivery. Macromol. Biosci. 12(2), 251–259 (2012)
A. Agostini et al., A photoactivated molecular gate. Chem. Eur. J. 18(39), 12218–12221 (2012)
S.-F. Lee et al., Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl. Mater. Interfaces 5(5), 1566–1574 (2013)
Z. Jin et al., Electrochemically controlled drug-mimicking protein release from iron–alginate thin-films associated with an electrode. ACS Appl. Mater. Interfaces 4(1), 466–475 (2012)
H. Yu et al., pH- and NIR light-responsive micelles with hyperthermia-triggered tumor penetration and cytoplasm drug release to reverse doxorubicin resistance in breast cancer. Adv. Funct. Mater. 25(17), 2489–2500 (2015)
Y. Chen et al., Multifunctional envelope-type mesoporous silica nanoparticles for pH-responsive drug delivery and magnetic resonance imaging. Biomaterials 60, 111–120 (2015)
W. She et al., Dendronized heparin–doxorubicin conjugate based nanoparticle as pH-responsive drug delivery system for cancer therapy. Biomaterials 34(9), 2252–2264 (2013)
L. Xing et al., Coordination polymer coated mesoporous silica nanoparticles for pH-responsive drug release. Adv. Mater. 24(48), 6433–6437 (2012)
P. Shi et al., pH-responsive NIR enhanced drug release from gold nanocages possesses high potency against cancer cells. Chem. Commun. 48(61), 7640–7642 (2012)
R. Vivek et al., pH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloids Surf. B Biointerfaces 111, 117–123 (2013)
S. Aryal, C.-M.J. Hu, L. Zhang, Polymer–cisplatin conjugate nanoparticles for acid-responsive drug delivery. ACS Nano 4(1), 251–258 (2010)
H. Zhang et al., Daunorubicin-TiO2 nanocomposites as a “smart” pH-responsive drug delivery system. Int. J. Nanomed. 7, 235–242 (2012)
D. Xiao et al., A redox-responsive mesoporous silica nanoparticle capped with amphiphilic peptides by self-assembly for cancer targeting drug delivery. Nanoscale 7(22), 10071–10077 (2015)
Y. Su et al., Redox-responsive polymer–drug conjugates based on doxorubicin and chitosan oligosaccharide-g-stearic acid for cancer therapy. Mol. Pharm. 12(4), 1193–1202 (2015)
S. Zhai et al., Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials 121, 41–54 (2017)
Z. Tang et al., Redox-responsive star-shaped magnetic micelles with active-targeted and magnetic-guided functions for cancer therapy. Acta Biomater. 42, 232–246 (2016)
C. Gao et al., pH/redox responsive core cross-linked nanoparticles from thiolated carboxymethyl chitosan for in vitro release study of methotrexate. Carbohyd. Polym. 111, 964–970 (2014)
M. Ghorbani, H. Hamishehkar, Redox and pH-responsive gold nanoparticles as a new platform for simultaneous triple anti-cancer drugs targeting. Int. J. Pharm. 520(1), 126–138 (2017)
C.T. Nguyen et al., Redox-sensitive nanoparticles from amphiphilic cholesterol-based block copolymers for enhanced tumor intracellular release of doxorubicin. Nanomed. Nanotechnol. Biol. Med. 11(8), 2071–2082 (2015)
K. Hayashi et al., Magnetically responsive smart nanoparticles for cancer treatment with a combination of magnetic hyperthermia and remote-control drug release. Theranostics 4(8), 834–844 (2014)
M. Li et al., Enhanced synergism of thermo-chemotherapy for liver cancer with magnetothermally responsive nanocarriers. Theranostics 8(3), 693–709 (2018)
G. Wang et al., Theranostic hyaluronic acid-iron micellar nanoparticles for magnetic-field-enhanced in vivo cancer chemotherapy. ChemMedChem 13(1), 78–86 (2017)
E. Voulgari et al., Synthesis, characterization and in vivo evaluation of a magnetic cisplatin delivery nanosystem based on PMAA-graft-PEG copolymers. J. Control. Release 243, 342–356 (2016)
S. Sabnis et al., Superparamagnetic reconstituted high-density lipoprotein nanocarriers for magnetically guided drug delivery. Int. J. Nanomed. 12, 1453–1464 (2017)
X. Hua et al., Magnetically triggered drug release from nanoparticles and its applications in anti-tumor treatment. Drug Deliv. 24(1), 511–518 (2017)
X. Dong et al., Mesoporous bamboo charcoal nanoparticles as a new near-infrared responsive drug carrier for imaging-guided chemotherapy/photothermal synergistic therapy of tumor. Adv. Healthc. Mater. 5(13), 1627–1637 (2016)
F. Li et al., Near-infrared light stimuli-responsive synergistic therapy nanoplatforms based on the coordination of tellurium-containing block polymer and cisplatin for cancer treatment. Biomaterials 133, 208–218 (2017)
H. Zhang et al., Visible-light-sensitive titanium dioxide nanoplatform for tumor-responsive Fe2+ liberating and artemisinin delivery. Oncotarget 8(35), 58738–58753 (2017)
L. Meng et al., Chitosan-based nanocarriers with pH and light dual response for anticancer drug delivery. Biomacromolecules 14(8), 2601–2610 (2013)
A. Jose et al., Temperature-sensitive liposomes for co-delivery of tamoxifen and imatinib for synergistic breast cancer treatment. J. Liposome Res. 29, 153–162 (2018)
X. Fan et al., Thermoresponsive supramolecular chemotherapy by “V”-shaped armed β-cyclodextrin star polymer to overcome drug resistance. Adv. Healthc. Mater. 7(7), 1701143 (2017)
Y. Wen, J.K. Oh, Intracellular delivery cellulose-based bionanogels with dual temperature/pH-response for cancer therapy. Colloids Surf. B Biointerfaces 133, 246–253 (2015)
A. Kaphle, N.P. Nagraju, H.K. Daima, Contemporary developments in nanobiotechnology: applications, toxicity, sustainability and future perspective, in Nanobiotechnology: human health and the environment, ed. by A. Dhawan (CRC Press, Boca Raton, 2018), pp. 1–34
A. Umapathi et al., Impact of physicochemical properties and surface chemistry of nanomaterials on toxicity, in Nanotoxicology: toxicity evaluation, risk assessment and management, ed. by V. Kumar, N. Dasgupta, S. Ranjan (CRC Press, Boca Raton, 2018), pp. 35–61
T. Sun et al., Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 53(46), 12320–12364 (2014)
J. Yue et al., Gold nanoparticle size and shape effects on cellular uptake and intracellular distribution of siRNA nanoconstructs. Bioconjug. Chem. 28(6), 1791–1800 (2017)
K. Huang et al., Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 6(5), 4483–4493 (2012)
F. Lu et al., Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 5(12), 1408–1413 (2009)
B.D. Chithrani, A.A. Ghazani, W.C.W. Chan, Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6(4), 662–668 (2006)
P. Decuzzi et al., Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 141(3), 320–327 (2010)
X. Huang et al., The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 31(3), 438–448 (2010)
E.C. Cho et al., Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI Etchant. Nano Lett. 9(3), 1080–1084 (2009)
C. He et al., Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31(13), 3657–3666 (2010)
K. Xiao et al., The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 32(13), 3435–3446 (2011)
O. Harush-Frenkel et al., Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem. Biophys. Res. Commun. 353(1), 26–32 (2007)
F. Zhao et al., Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 7(10), 1322–1337 (2011)
S. Krasnici et al., Effect of the surface charge of liposomes on their uptake by angiogenic tumor vessels. Int. J. Cancer 105(4), 561–567 (2003)
R.B. Campbell et al., Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 62(23), 6831 (2002)
V. Angeles et al., The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology 20(11), 115103 (2009)
J.-Z. Du et al., Tailor-made dual pH-sensitive polymer–doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 133(44), 17560–17563 (2011)
Y.Y. Yuan et al., Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv. Mater. 24(40), 5476–5480 (2012)
Z.J. Zhu et al., Surface properties dictate uptake, distribution, excretion, and toxicity of nanoparticles in fish. Small 6(20), 2261–2265 (2010)
Y.B. Patil et al., Single-step surface functionalization of polymeric nanoparticles for targeted drug delivery. Biomaterials 30(5), 859–866 (2009)
Y.-I. Chung et al., The effect of surface functionalization of PLGA nanoparticles by heparin- or chitosan-conjugated Pluronic on tumor targeting. J. Control. Release 143(3), 374–382 (2010)
S.A. Kulkarni, S.-S. Feng, Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 30(10), 2512–2522 (2013)
J. Wu et al., Robust, responsive, and targeted PLGA anticancer nanomedicines by combination of reductively cleavable surfactant and covalent hyaluronic acid coating. ACS Appl. Mater. Interfaces 9(4), 3985–3994 (2017)
K. Yin Win, S.-S. Feng, Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for ral delivery of anticancer drugs. Biomaterials 26(15), 2713–2722 (2005)
D.N. Heo et al., Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials 33(3), 856–866 (2012)
A. Kumar et al., Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials 33(4), 1180–1189 (2012)
Y. Qiu et al., Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials 31(30), 7606–7619 (2010)
S.D. Brown et al., Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 132(13), 4678–4684 (2010)
I. Slowing, B.G. Trewyn, V.S.Y. Lin, Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 128(46), 14792–14793 (2006)
Z. Liu et al., Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 1(1), 50–56 (2007)
R.P. Feazell et al., Soluble single-walled carbon nanotubes as longboat delivery systems for platinum(IV) anticancer drug design. J. Am. Chem. Soc. 129(27), 8438–8439 (2007)
N.W.S. Kam, Z. Liu, H. Dai, Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J. Am. Chem. Soc. 127(36), 12492–12493 (2005)
Z. Liu et al., Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Res. 2(2), 85–120 (2009)
A. Accardo, G. Morelli, Review peptide-targeted liposomes for selective drug delivery: advantages and problematic issues. Pept. Sci. 104(5), 462–479 (2015)
D. Kim, Y.Y. Jeong, S. Jon, A drug-loaded aptamer–gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano 4(7), 3689–3696 (2010)
M.E. Werner et al., Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials 32(33), 8548–8554 (2011)
C.H.J. Choi et al., Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl. Acad. Sci. USA 107(3), 1235–1240 (2010)
C. Zhang et al., Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 67(4), 1555 (2007)
S.M. Hillier et al., Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 69(17), 6932 (2009)
J. Wan et al., Docetaxel-decorated anticancer drug and gold nanoparticles encapsulated apatite carrier for the treatment of liver cancer. J. Photochem. Photobiol. B Biol. 185, 73–79 (2018)
J.D. Mangadlao et al., Prostate-specific membrane antigen targeted gold nanoparticles for theranostics of prostate cancer. ACS Nano 12(4), 3714–3725 (2018)
M.A. Safwat et al., Fluorouracil-loaded gold nanoparticles for the treatment of skin cancer: development, in vitro characterization, and in vivo evaluation in a mouse skin cancer xenograft model. Mol. Pharm. 15(6), 2194–2205 (2018)
M.U. Farooq et al., Gold nanoparticles-enabled efficient dual delivery of anticancer therapeutics to HeLa cells. Sci. Rep. 8(1), 2907 (2018)
S. Patra et al., Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 53, 298–309 (2015)
M. Ramar et al., Synthesis of silver nanoparticles using Solanum trilobatum fruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim. Acta A Mol. Biomol. Spectrosc. 140, 223–228 (2015)
M. Jannathul Firdhouse, P. Lalitha, Apoptotic efficacy of biogenic silver nanoparticles on human breast cancer MCF-7 cell lines. Prog. Biomater. 4(2), 113–121 (2015)
B. Kumar et al., In vitro evaluation of silver nanoparticles cytotoxicity on Hepatic cancer (Hep-G2) cell line and their antioxidant activity: green approach for fabrication and application. J. Photochem. Photobiol. B Biol. 159, 8–13 (2016)
N. Soni et al., Noscapinoids bearing silver nanocrystals augmented drug delivery, cytotoxicity, apoptosis and cellular uptake in B16F1, mouse melanoma skin cancer cells. Biomed. Pharmacother. 90, 906–913 (2017)
V.R. Netala et al., Biogenesis of silver nanoparticles using leaf extract of Indigofera hirsuta L. and their potential biomedical applications (3-in-1 system). Artif. Cells Nanomed. Biotechnol. 46, 1138–1148 (2018)
H. Banu et al., Gold and silver nanoparticles biomimetically synthesized using date palm pollen extract-induce apoptosis and regulate p53 and Bcl-2 expression in human breast adenocarcinoma cells. Biol. Trace Elem. Res. 186, 122–134 (2018)
L. Qiu et al., Silver nanoparticles covered with pH-sensitive camptothecin-loaded polymer prodrugs: switchable fluorescence “off” or “on” and drug delivery dynamics in living cells. ACS Appl. Mater. Interfaces 9(46), 40887–40897 (2017)
Y. Tang et al., Co-delivery of trichosanthin and albendazole by nano-self-assembly for overcoming tumor multidrug-resistance and metastasis. ACS Appl. Mater. Interfaces 9(32), 26648–26664 (2017)
H. Nosrati et al., Theranostic nanoparticles based on magnetic nanoparticles: design, preparation, characterization and evaluation as novel anticancer drug carrier and MRI contrast agent. Drug Dev. Ind. Pharm. 44, 1668–1678 (2018)
A.S. Semkina et al., Multimodal doxorubicin loaded magnetic nanoparticles for VEGF targeted theranostics of breast cancer. Nanomed. Nanotechnol. Biol. Med. 14(5), 1733–1742 (2018)
H.-P. Yeh et al., A new photosensitized oxidation-responsive nanoplatform for controlled drug release and photodynamic cancer therapy. ACS Appl. Mater. Interfaces 10, 21160–21172 (2018)
N. Guldris et al., Orthogonal clickable iron oxide nanoparticle platform for targeting, imaging, and on-demand release. Chem. Eur. J. 24, 8624–8631 (2018)
D.C. Manatunga et al., Effective delivery of hydrophobic drugs to breast and liver cancer cells using a hybrid inorganic nanocarrier: a detailed investigation using cytotoxicity assays, fluorescence imaging and flow cytometry. Eur. J. Pharm. Biopharm. 128, 18–26 (2018)
M.S.U. Ahmed et al., Double-receptor-targeting multifunctional iron oxide nanoparticles drug delivery system for the treatment and imaging of prostate cancer. Int. J. Nanomed. 12, 6973–6984 (2017)
N. Gao et al., Tumor penetrating theranostic nanoparticles for enhancement of targeted and image-guided drug delivery into peritoneal tumors following intraperitoneal delivery. Theranostics 7(6), 1689–1704 (2017)
Y. Sun et al., Temperature-sensitive gold nanoparticle-coated pluronic-PLL nanoparticles for drug delivery and chemo-photothermal therapy. Theranostics 7(18), 4424–4444 (2017)
T. Santiago et al., Surface-enhanced Raman scattering investigation of targeted delivery and controlled release of gemcitabine. Int. J. Nanomed. 12, 7763–7776 (2017)
Y. Li et al., Polyethylenimine-functionalized silver nanoparticle-based co-delivery of paclitaxel to induce HepG2 cell apoptosis. Int. J. Nanomed. 11, 6693–6702 (2016)
S.A. Sadat Shandiz et al., Novel imatinib-loaded silver nanoparticles for enhanced apoptosis of human breast cancer MCF-7 cells. Artif. Cells Nanomed. Biotechnol. 45(6), 1082–1091 (2017)
Z. Muhammad et al., PEG capped methotrexate silver nanoparticles for efficient anticancer activity and biocompatibility. Eur. J. Pharm. Sci. 91, 251–255 (2016)
S. Kumar et al., PEG coated and doxorubicin loaded multimodal Gadolinium oxide nanoparticles for simultaneous drug delivery and imaging applications. Int. J. Pharm. 527(1), 142–150 (2017)
Y. Huang et al., Superparamagnetic iron oxide nanoparticles conjugated with folic acid for dual target-specific drug delivery and MRI in cancer theranostics. Mater. Sci. Eng. C 70, 763–771 (2017)
S. Bano et al., “Smart” nickel oxide based core–shell nanoparticles for combined chemo and photodynamic cancer therapy. Int. J. Nanomed. 11, 3159–3166 (2016)
P.-C. Liang et al., Doxorubicin-modified magnetic nanoparticles as a drug delivery system for magnetic resonance imaging-monitoring magnet-enhancing tumor chemotherapy. Int. J. Nanomed. 11, 2021–2037 (2016)
K. Vimala et al., Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process Biochem. 49(1), 160–172 (2014)
P.K.B. Nagesh et al., PSMA targeted docetaxel-loaded superparamagnetic iron oxide nanoparticles for prostate cancer. Colloids Surf. B Biointerfaces 144, 8–20 (2016)
S.-H. Tseng, M.-Y. Chou, I.M. Chu, Cetuximab-conjugated iron oxide nanoparticles for cancer imaging and therapy. Int. J. Nanomed. 10, 3663–3685 (2015)
A.A. Bhirde et al., Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomedicine 5(10), 1535–1546 (2010)
T. Feng et al., Charge-convertible carbon dots for imaging-guided drug delivery with enhanced in vivo cancer therapeutic efficiency. ACS Nano 10(4), 4410–4420 (2016)
Y. Qin et al., Near-infrared light remote-controlled intracellular anti-cancer drug delivery using thermo/pH sensitive nanovehicle. Acta Biomater. 17, 201–209 (2015)
A. Al Faraj, A.P. Shaik, A.S. Shaik, Magnetic single-walled carbon nanotubes as efficient drug delivery nanocarriers in breast cancer murine model: noninvasive monitoring using diffusion-weighted magnetic resonance imaging as sensitive imaging biomarker. Int. J. Nanomed. 10, 157–168 (2015)
M. Ajmal et al., Synthesis, characterization and in vitro evaluation of methotrexate conjugated fluorescent carbon nanoparticles as drug delivery system for human lung cancer targeting. J. Photochem. Photobiol. B Biol. 153, 111–120 (2015)
W. Shao et al., A new carbon nanotube-based breast cancer drug delivery system: preparation and in vitro analysis using paclitaxel. Cell Biochem. Biophys. 71(3), 1405–1414 (2015)
Q. Pan et al., Lactobionic acid and carboxymethyl chitosan functionalized graphene oxide nanocomposites as targeted anticancer drug delivery systems. Carbohyd. Polym. 151, 812–820 (2016)
J. Chen et al., One-step reduction and PEGylation of graphene oxide for photothermally controlled drug delivery. Biomaterials 35(18), 4986–4995 (2014)
L. Guo et al., Prostate cancer targeted multifunctionalized graphene oxide for magnetic resonance imaging and drug delivery. Carbon 107, 87–99 (2016)
Q. Zhang et al., Biocompatible, uniform, and redispersible mesoporous silica nanoparticles for cancer-targeted drug delivery in vivo. Adv. Funct. Mater. 24(17), 2450–2461 (2013)
M. Ma et al., Bi2S3-embedded mesoporous silica nanoparticles for efficient drug delivery and interstitial radiotherapy sensitization. Biomaterials 37, 447–455 (2015)
R. Chakravarty et al., Hollow mesoporous silica nanoparticles for tumor vasculature targeting and PET image-guided drug delivery. Nanomedicine 10(8), 1233–1246 (2015)
Y. Zhang et al., Polymer-coated hollow mesoporous silica nanoparticles for triple-responsive drug delivery. ACS Appl. Mater. Interfaces 7(32), 18179–18187 (2015)
L. Xiong et al., Cancer-cell-specific nuclear-targeted drug delivery by dual-ligand-modified mesoporous silica nanoparticles. Small 11(44), 5919–5926 (2015)
E. Niemelä et al., Sugar-decorated mesoporous silica nanoparticles as delivery vehicles for the poorly soluble drug celastrol enables targeted induction of apoptosis in cancer cells. Eur. J. Pharm. Biopharm. 96, 11–21 (2015)
X. Xie et al., EpCAM aptamer-functionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. Eur. J. Pharm. Sci. 83, 28–35 (2016)
Y. Zhao et al., Iron oxide nanoparticles-based vaccine delivery for cancer treatment. Mol. Pharm. 15(5), 1791–1799 (2018)
D. Cui et al., Gastrin-releasing peptide receptor-targeted gadolinium oxide-based multifunctional nanoparticles for dual magnetic resonance/fluorescent molecular imaging of prostate cancer. Int. J. Nanomed. 12, 6787–6797 (2017)
S.-B. Ghaffari et al., Functionalization of ZnO nanoparticles by 3-mercaptopropionic acid for aqueous curcumin delivery: synthesis, characterization, and anticancer assessment. Mater. Sci. Eng. C 79, 465–472 (2017)
V. Gnanavel, V. Palanichamy, S.M. Roopan, Biosynthesis and characterization of copper oxide nanoparticles and its anticancer activity on human colon cancer cell lines (HCT-116). J. Photochem. Photobiol. B Biol. 171, 133–138 (2017)
A.A. Bhirde et al., Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano 3(2), 307–316 (2009)
S.R. Ji et al., Carbon nanotubes in cancer diagnosis and therapy. Biochim. Biophys. Acta 1806(1), 29–35 (2010)
J.T. Robinson et al., High performance in vivo near-IR (> 1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res. 3(11), 779–793 (2010)
K. Yang, L. Feng, Z. Liu, Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 105, 228–241 (2016)
O. Akhavan et al., The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J. Mater. Chem. 22(27), 13773–13781 (2012)
Y. Wang et al., Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials 34(31), 7715–7724 (2013)
M. Kumar et al., N-desmethyl tamoxifen and quercetin-loaded multiwalled CNTs: a synergistic approach to overcome MDR in cancer cells. Mater. Sci. Eng. C 89, 274–282 (2018)
D. Wang et al., Facile preparation of doxorubicin-loaded and folic acid-conjugated carbon nanotubes@poly(N-vinyl pyrrole) for targeted synergistic chemo-photothermal cancer treatment. Bioconjug. Chem. 28(11), 2815–2822 (2017)
V. Karthika et al., Biocompatible properties of nano-drug carriers using TiO2–Au embedded on multiwall carbon nanotubes for targeted drug delivery. Mater. Sci. Eng. C 90, 589–601 (2018)
N.H. Levi-Polyachenko et al., Rapid photothermal intracellular drug delivery using multiwalled carbon nanotubes. Mol. Pharm. 6(4), 1092–1099 (2009)
D. Wang et al., Multi-layered tumor-targeting photothermal-doxorubicin releasing nanotubes eradicate tumors in vivo with negligible systemic toxicity. Nanoscale 10(18), 8536–8546 (2018)
N. Singh, A. Sachdev, P. Gopinath, Polysaccharide functionalized single walled carbon nanotubes as nanocarriers for delivery of curcumin in lung cancer cells. J. Nanosci. Nanotechnol. 18(3), 1534–1541 (2018)
X. Zhao et al., PEGylated multi-walled carbon nanotubes as versatile vector for tumor-specific intracellular triggered release with enhanced anti-cancer efficiency: optimization of length and PEGylation degree. Colloids Surf. B Biointerfaces 168, 43–49 (2018)
A. Kavosi et al., The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci. Rep. 8, 8375 (2018)
L. Zhang et al., Delivery of a chemotherapeutic drug using novel hollow carbon spheres for esophageal cancer treatment. Int. J. Nanomed. 12, 6759–6769 (2017)
N. Karki et al., Functionalized graphene oxides for drug loading, release and delivery of poorly water soluble anticancer drug: a comparative study. Colloids Surf. B Biointerfaces 169, 265–272 (2018)
A. Mohammadi Gazestani et al., In vivo evaluation of the combination effect of near-infrared laser and 5-fluorouracil-loaded PLGA-coated magnetite nanographene oxide. Artif. Cells Nanomed. Biotechnol. 46, 25–33 (2018)
Z. Wei et al., Antitumor effect of a Pt-loaded nanocomposite based on graphene quantum dots combats hypoxia-induced chemoresistance of oral squamous cell carcinoma. Int. J. Nanomed. 13, 1505–1524 (2018)
S. Malekmohammadi et al., Immobilization of gold nanoparticles on folate-conjugated dendritic mesoporous silica-coated reduced graphene oxide nanosheets: a new nanoplatform for curcumin pH-controlled and targeted delivery. Soft Matter 14(12), 2400–2410 (2018)
A. Siriviriyanun et al., Cyclodextrin- and dendrimer-conjugated graphene oxide as a nanocarrier for the delivery of selected chemotherapeutic and photosensitizing agents. Mater. Sci. Eng. C 89, 307–315 (2018)
C. Huang et al., Amphiphilic prodrug-decorated graphene oxide as a multi-functional drug delivery system for efficient cancer therapy. Mater. Sci. Eng. C 89, 15–24 (2018)
P. Li et al., Lanthanide-doped upconversion nanoparticles complexed with nano-oxide graphene used for upconversion fluorescence imaging and photothermal therapy. Biomater. Sci. 6(4), 877–884 (2018)
Y.-J. Lu et al., Magnetic graphene oxide for dual targeted delivery of doxorubicin and photothermal therapy. Nanomaterials 8(4), 193 (2018)
X. Liu et al., Targeted delivery of SNX-2112 by polysaccharide-modified graphene oxide nanocomposites for treatment of lung cancer. Carbohyd. Polym. 185, 85–95 (2018)
C. Wang et al., Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J. Colloid Interface Sci. 516, 332–341 (2018)
M. Manzano, M. Vallet-Regí, Mesoporous silica nanoparticles in nanomedicine applications. J. Mater. Sci. Mater. Med. 29(5), 65 (2018)
W. Cheng et al., A drug-self-gated and tumor microenvironment-responsive mesoporous silica vehicle: “four-in-one” versatile nanomedicine for targeted multidrug-resistant cancer therapy. Nanoscale 9(43), 17063–17073 (2017)
X. Xu, F. Hu, Q. Shuai, Facile synthesis of highly biocompatible folic acid-functionalised SiO2 nanoparticles encapsulating rare-earth metal complexes, and their application in targeted drug delivery. Dalton Trans. 46(44), 15424–15433 (2017)
H. Fan et al., Triple function nanocomposites of porous silica-CoFe2O4-MWCNTs as a carrier for pH-sensitive anti-cancer drug controlled delivery. Dalton Trans. 46(43), 14831–14838 (2017)
N. Li et al., Curcumin-loaded redox-responsive mesoporous silica nanoparticles for targeted breast cancer therapy. Artif. Cells Nanomed. Biotechnol. 46, 921–935 (2018)
W. Xu et al., Macroporous silica nanoparticles for delivering Bcl2-function converting peptide to treat multidrug resistant-cancer cells. J. Colloid Interface Sci. 527, 141–150 (2018)
L. Pascual et al., MUC1 aptamer-capped mesoporous silica nanoparticles for controlled drug delivery and radio-imaging applications. Nanomed. Nanotechnol. Biol. Med. 13(8), 2495–2505 (2017)
Q. Liu et al., Facile synthesis by a covalent binding reaction for pH-responsive drug release of carboxylated chitosan coated hollow mesoporous silica nanoparticles. IET Nanobiotechnol. 12, 446–452 (2018)
C. Chen et al., pH-responsive nanoreservoirs based on hyaluronic acid end-capped mesoporous silica nanoparticles for targeted drug delivery. Int. J. Biol. Macromol. 111, 1106–1115 (2018)
L. Ansari et al., Improved anticancer efficacy of epirubicin by magnetic mesoporous silica nanoparticles: in vitro and in vivo studies. Artif. Cells Nanomed. Biotechnol. 46, 594–606 (2018)
M. Martínez-Carmona et al., Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 65, 393–404 (2018)
H.K. Daima et al., Complexation of plasmid DNA and poly(ethylene oxide)/poly(propylene oxide) polymers for safe gene delivery. Environ. Chem. Lett. 16(4), 1457–1462 (2018)
A.D. Bangham, M.M. Standish, J.C. Watkins, Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 13(1), 238–IN27 (1965)
J. Gubernator, Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 8(5), 565–580 (2011)
L. Sercombe et al., Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 6, 286 (2015)
B.S. Pattni, V.V. Chupin, V.P. Torchilin, New developments in liposomal drug delivery. Chem. Rev. 115(19), 10938–10966 (2015)
G. Bozzuto, A. Molinari, Liposomes as nanomedical devices. Int. J. Nanomed. 10, 975–999 (2015)
T. Zhang et al., Polysialic acid–polyethylene glycol conjugate-modified liposomes as a targeted drug delivery system for epirubicin to enhance anticancer efficiency. Drug Deliv. Transl. Res. 8(3), 602–616 (2018)
M. Wei et al., Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. Int. J. Nanomed. 10, 5123–5137 (2015)
Z.C. Soe et al., Folate receptor-mediated celastrol and irinotecan combination delivery using liposomes for effective chemotherapy. Colloids Surf. B Biointerfaces 170, 718–728 (2018)
A. Jhaveri et al., Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 277, 89–101 (2018)
Y.J. Kim et al., Co-eradication of breast cancer cells and cancer stem cells by cross-linked multilamellar liposomes enhances tumor treatment. Mol. Pharm. 12(8), 2811–2822 (2015)
I.M. Shaikh et al., Liposome co-encapsulation of synergistic combination of irinotecan and doxorubicin for the treatment of intraperitoneally grown ovarian tumor xenograft. J. Control. Release 172(3), 852–861 (2013)
S.K. Ramadass et al., Paclitaxel/epigallocatechin gallate coloaded liposome: a synergistic delivery to control the invasiveness of MDA-MB-231 breast cancer cells. Colloids Surf. B Biointerfaces 125, 65–72 (2015)
Y. Xia et al., pH sensitive liposomes delivering tariquidar and doxorubicin to overcome multidrug resistance of resistant ovarian cancer cells. Colloids Surf. B Biointerfaces 170, 514–520 (2018)
E. Heidarli, S. Dadashzadeh, A. Haeri, State of the art of stimuli-responsive liposomes for cancer therapy. Iran. J. Pharm. Res. 16(4), 1273–1304 (2017)
Y. Chi et al., Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J. Control. Release 261, 113–125 (2017)
X. Chen et al., Co-delivery of paclitaxel and anti-survivin siRNA via redox-sensitive oligopeptide liposomes for the synergistic treatment of breast cancer and metastasis. Int. J. Pharm. 529(1), 102–115 (2017)
J.N. Mock et al., Evidence for distinct mechanisms of uptake and antitumor activity of secretory phospholipase A2 responsive liposome in prostate cancer. Integr. Biol. 5(1), 172–182 (2013)
D. Bobo et al., Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm. Res. 33(10), 2373–2387 (2016)
J.-H. Park et al., Hyaluronic acid derivative-coated nanohybrid liposomes for cancer imaging and drug delivery. J. Control. Release 174, 98–108 (2014)
S. Lakkadwala, J. Singh, Dual functionalized 5-fluorouracil liposomes as highly efficient nanomedicine for glioblastoma treatment as assessed in an in vitro brain tumor model. J. Pharm. Sci. 107(11), 2902–2913 (2018)
L. Wang et al., A novel α-enolase-targeted drug delivery system for high efficacy prostate cancer therapy. Nanoscale 10(28), 13673–13683 (2018)
S. Singh, Liposome encapsulation of doxorubicin and celecoxib in combination inhibits progression of human skin cancer cells. Int. J. Nanomed. 13, 11–13 (2018)
M.B. Sokol et al., Development of novel PLGA nanoparticles with co-encapsulation of docetaxel and abiraterone acetate for a highly efficient delivery into tumor cells. J. Biomed. Mater. Res. B Appl. Biomater. 107, 1150–1158 (2019)
W. Zheng et al., Encapsulation of verapamil and doxorubicin by MPEG-PLA to reverse drug resistance in ovarian cancer. Biomed. Pharmacother. 108, 565–573 (2018)
A.M. Nassir et al., Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm. J. 26(6), 876–885 (2018)
S. Nicolas et al., Polymeric nanocapsules as drug carriers for sustained anticancer activity of calcitriol in breast cancer cells. Int. J. Pharm. 550(1), 170–179 (2018)
H. Gan et al., Enhanced delivery of sorafenib with anti-GPC3 antibody-conjugated TPGS-b-PCL/pluronic P123 polymeric nanoparticles for targeted therapy of hepatocellular carcinoma. Mater. Sci. Eng. C 91, 395–403 (2018)
G. Arya, M. Das, S.K. Sahoo, Evaluation of curcumin loaded chitosan/PEG blended PLGA nanoparticles for effective treatment of pancreatic cancer. Biomed. Pharmacother. 102, 555–566 (2018)
P. Gupta et al., Synthesis and in vitro studies of PLGA-DTX nanoconjugate as potential drug delivery vehicle for oral cancer. Int. J. Nanomed. 13, 67–69 (2018)
S.V.K. Rompicharla et al., Octa-arginine modified poly(amidoamine) dendrimers for improved delivery and cytotoxic effect of paclitaxel in cancer. Artif. Cells Nanomed. Biotechnol. 46, 847–859 (2018)
S.P. Kuruvilla et al., Dendrimer–doxorubicin conjugates exhibit improved anticancer activity and reduce doxorubicin-induced cardiotoxicity in a murine hepatocellular carcinoma model. PLoS ONE 12(8), e0181944–e0181944 (2017)
R.M. Iacobazzi et al., Targeting human liver cancer cells with lactobionic acid-G(4)-PAMAM-FITC sorafenib loaded dendrimers. Int. J. Pharm. 528(1), 485–497 (2017)
T. Lv et al., Role of generation on folic acid-modified poly(amidoamine) dendrimers for targeted delivery of baicalin to cancer cells. Mater. Sci. Eng. C 75, 182–190 (2017)
M. Alibolandi et al., Smart AS1411-aptamer conjugated pegylated PAMAM dendrimer for the superior delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int. J. Pharm. 519(1), 352–364 (2017)
Y. Zhao et al., Methotrexate nanoparticles prepared with codendrimer from polyamidoamine (PAMAM) and oligoethylene glycols (OEG) dendrons: antitumor efficacy in vitro and in vivo. Sci. Rep. 6, 28983 (2016)
J. Nie, Y. Wang, W. Wang, In vitro and in vivo evaluation of stimuli-responsive vesicle from PEGylated hyperbranched PAMAM-doxorubicin conjugate for gastric cancer therapy. Int. J. Pharm. 509(1), 168–177 (2016)
A. Kumari, S.K. Yadav, S.C. Yadav, Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 75(1), 1–18 (2010)
F. Masood, Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 60, 569–578 (2016)
Y. Zhong et al., Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromolecules 15(6), 1955–1969 (2014)
N. Kamaly et al., Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 41(7), 2971–3010 (2012)
D. Zhu et al., Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Acta Biomater. 30, 144–154 (2016)
M. Jin et al., Smart polymeric nanoparticles with pH-responsive and PEG-detachable properties for co-delivering paclitaxel and survivin siRNA to enhance antitumor outcomes. Int. J. Nanomed. 13, 2405–2426 (2018)
B. Xiao et al., Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J. Mater. Chem. B 3(39), 7724–7733 (2015)
J. Zhang et al., pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 58, 349–364 (2017)
Y. Peng et al., Codelivery of temozolomide and siRNA with polymeric nanocarrier for effective glioma treatment. Int. J. Nanomed. 13, 3467–3480 (2018)
J. Guo et al., Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 32(31), 8010–8020 (2011)
S. Mignani et al., Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 47(2), 514–532 (2018)
T. Dichwalkar et al., Omega-3 fatty acid grafted PAMAM-paclitaxel conjugate exhibits enhanced anticancer activity in upper gastrointestinal cancer cells. Macromol. Biosci. 17(8), 1600457 (2017)
K. Jain et al., Dendrimer toxicity: let’s meet the challenge. Int. J. Pharm. 394(1), 122–142 (2010)
R. Jevprasesphant et al., The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int. J. Pharm. 252(1), 263–266 (2003)
X. Du et al., Hyaluronic acid-functionalized half-generation of sectorial dendrimers for anticancer drug delivery and enhanced biocompatibility. Carbohyd. Polym. 202, 513–522 (2018)
V.M. Thanh et al., Low systemic toxicity nanocarriers fabricated from heparin-mPEG and PAMAM dendrimers for controlled drug release. Mater. Sci. Eng. C 82, 291–298 (2018)
C. Chittasupho, S. Anuchapreeda, N. Sarisuta, CXCR284 targeted dendrimer for anti-cancer drug delivery and breast cancer cell migration inhibition. Eur. J. Pharm. Biopharm. 119, 310–321 (2017)
K. Öztürk et al., Effective targeting of gemcitabine to pancreatic cancer through PEG-cored Flt-1 antibody-conjugated dendrimers. Int. J. Pharm. 517(1), 157–167 (2017)
M. Ghaffari et al., Surface functionalized dendrimers as controlled-release delivery nanosystems for tumor targeting. Eur. J. Pharm. Sci. 122, 311–330 (2018)
H.K. Daima et al., Synergistic influence of polyoxometalate surface corona towards enhancing the antibacterial performance of tyrosine-capped Ag nanoparticles. Nanoscale 6(2), 758–765 (2014)
H.K. Daima et al., Fine-tuning the antimicrobial profile of biocompatible gold nanoparticles by sequential surface functionalization using polyoxometalates and lysine. PLoS ONE 8(10), 1–14 (2013)
J. Shi et al., Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20 (2016)
B. Ruozi et al., PLGA nanoparticles loaded cerebrolysin: studies on their preparation and investigation of the effect of storage and serum stability with reference to traumatic brain injury. Mol. Neurobiol. 52(2), 899–912 (2015)
S. Ma et al., Highly stable fluorinated nanocarriers with iRGD for overcoming the stability dilemma and enhancing tumor penetration in an orthotopic breast cancer. ACS Appl. Mater. Interfaces 8(42), 28468–28479 (2016)
Y. Wang et al., An overview of nanotoxicity and nanomedicine research: principles, progress and implications for cancer therapy. J. Mater. Chem. B 3(36), 7153–7172 (2015)
R. Coradeghini et al., Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol. Lett. 217(3), 205–216 (2013)
Z. Ji et al., Designed synthesis of CeO2 nanorods and nanowires for studying toxicological effects of high aspect ratio nanomaterials. ACS Nano 6(6), 5366–5380 (2012)
Funding
Siddaganga Institute of Technology, Tumkur, India supported this work through TEQIP-II. Generous support of Japan Science and Technology (JST) Agency, Japan toward Asia Youth Exchange Program in Science (Sakura Exchange Program) is duly accredited by NPN and HKD. Further, HKD appreciates the Centre for Advanced Materials and Industrial Chemistry (CAMIC) in the School of Sciences, RMIT University, Australia for an Honorary Visiting Research Fellowship. AK acknowledges International Max Planck Research School (IMPRS) Fellowship (Molecular Biology) from the Max Planck Society, Germany (2016-18) and Melbourne Research Scholarship for the support of his research at the University of Melbourne. VR acknowledges the NIH (EB022641).
Author information
Authors and Affiliations
Contributions
All the authors have made substantial intellectual contribution in the preparation of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Navya, P.N., Kaphle, A., Srinivas, S.P. et al. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Convergence 6, 23 (2019). https://doi.org/10.1186/s40580-019-0193-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40580-019-0193-2