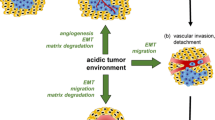
Abstract
Acidic extracellular pH is a major feature of tumor tissue, extracellular acidification being primarily considered to be due to lactate secretion from anaerobic glycolysis. Clinicopathological evidence shows that transporters and pumps contribute to H+ secretion, such as the Na+/H+ exchanger, the H+-lactate co-transporter, monocarboxylate transporters, and the proton pump (H+-ATPase); these may also be associated with tumor metastasis. An acidic extracellular pH not only activates secreted lysosomal enzymes that have an optimal pH in the acidic range, but induces the expression of certain genes of pro-metastatic factors through an intracellular signaling cascade that is different from hypoxia. In addition to lactate, CO2 from the pentose phosphate pathway is an alternative source of acidity, showing that hypoxia and extracellular acidity are, while being independent from each other, deeply associated with the cellular microenvironment. In this article, the importance of an acidic extracellular pH as a microenvironmental factor participating in tumor progression is reviewed.
Similar content being viewed by others
Introduction
The extracellular pH (pH e ) of tumor tissues is often acidic [1], and acidic metabolites, e.g. lactic acid caused by anaerobic glycolysis in hypoxia, seem to be the main cause. Accumulating evidence shows that an acidic microenvironment is a regulator of cellular phenotype. Whereas Na+-HCO3- co-transporter and Cl-/HCO3- exchanger contribute a fall in intracellular pH, the Na+/H+ exchanger (NHE) [2], the H+-lactate co-transporter, monocarboxylate transporters (MCTs), and the H+-ATPase (H+ pump) are responsible for the secretion of H+[3]. Because carbonic anhydrase (CA) is widely distributed and can form H+ by catalyzing hydration of CO2, an excess amount of CO2 production through the pentose phosphate pathway in tumor cells is an alternative cause of a lower pH [4]. Acidic pH e increases not only the activation of some lysosomal enzymes with acidic optimal pH, but also the expression of some genes involved with pro-metastatic factors. When melanoma cells pretreated with an acidic medium were injected into the tail vein of mice, a significantly higher frequency of them metastasized to the lungs [5]. Thus, an acidic microenvironment is closely associated with tumor metastasis.
Acidity is found at the surface of skin and in inflammatory sites. It is also associated with bone resorption. Thus, an acidic microenvironment plays a role of homeostasis and the immune defense system. We will review the roles of acidic pH e in tumor progression along with other physiological and pathological conditions.
Lactate and tumor
The “Warburg effect” is a well-accepted theory that says that tumors tend to produce lactate by using the anaerobic glycolytic pathway, even in the presence of sufficient oxygen, rather than oxidative phosphorylation for energy production [1]. High lactate levels indicate metastases, tumor recurrence, and prognosis in some cancer patients [6–9]. In the molecular mechanism relating to these clinical contributions, lactate from tumor cells contributes to their immune escape. High lactate secretion from tumor cells inhibits its export from T cells, thereby disturbing their metabolism and function [10]. Tumor-derived lactate affects inflammation and immune deficiency of tumor cells. Lactate itself functions as an intrinsic inflammatory mediator that increases interleukin (IL)-17A production by T-cells and macrophages, resulting in the promotion of chronic inflammation in tumor microenvironments [11]. Lactate inhibits dendritic cell activation during antigen-specific autologous T-cell stimulation [12]. It also enhances the motility of tumor cells and inhibits monocyte migration and cytokine release [13]. It can contribute to angiogenesis through induction of IL-8 via nuclear factor-κB (NF-κB) [14] and induction of vascular endothelial growth factor (VEGF/VEGF-A) via hypoxia-inducible transcription factor (HIF)-1 [15]. Furthermore, lactate production contributes to radio-resistance of tumors due to its antioxidant properties [16].
Inhibition of the lactate transporter has been considered a potential new therapeutic strategy. For example, α-cyano-4-hydroxycinnamate, a specific inhibitor of the lactate transporter MCT1, suppresses tumor angiogenesis [17]. Quercetin (CYP2C9), which is an inhibitory flavonoid, inhibits lactate transport and acts as a hyperthermic sensitizer of HeLa cells [18].
Appearance of acidic microenvironments under physiological and pathological conditions
An oncogenic transformation assay by oncogenic-virus infection shows that lactate production is correlated with an increase in the number of transformed foci by viral infection in a presence of 5% CO2 in 95% air [19]. Since high lactate corresponds to a high proton concentration, an acidic pH e is a major feature of the solid tumor tissue [1, 20–22]. Lactic acid is a product of the anaerobic glycolysis including the activity of lactate dehydrogenase (LDH) 5 that generates lactic acid from pyruvate and the expression of which has been strongly associated with the poor prognosis of patients with non-small cell lung [23, 24] and colorectal cancers [25–27].
CO2 is a major source of acid in glycolytically impaired mice [4]. The pentose phosphate pathway is seen as a major productive pathway for CO2 which can be processed to H+ and HCO3- by the catalytic activity of CA. In osteoclasts, CA II, a CA isozyme, is a major enzyme producing H+ to decalcify bone hydroxyapatite. Osteoclasts secrete H+ and create an acidic microenvironment below pH 5.5, which is critical for the bone resorption [28, 29] and the proton can be secreted through H+-ATPase [30]. Induction of CA II expression itself is also induced by an acidic pH e [31]. Thus, secretion of acidic metabolites and/or the pentose phosphate pathway-mediated CO2 production, and CA-mediated production of H2CO3 form acidic microenvironments.
Extracellular acidity is a pathological feature of inflammation [32] and solid tumor tissue [1, 20–22]. Acidity in inflammatory tissue is due to production of proton from macrophages, whereas tumor tissue acidity is due to acidic metabolites, e.g., lactate, caused by anaerobic glycolysis under the hypoxia [20–22, 33]. The acidic microenvironment acts as a trigger for pain in both inflammation [34, 35] and in cancer patients [36].
Ovarian cancer G-protein-coupled receptor 1 (OGR1), a receptor for sphingosylphosphorylcholine, and GPR4, a close relative of OGR1, also act as a proton-sensing receptor in osteosarcoma cells and primary human osteoblast precursors [37]. OGR1 (GPR68) stimulates cyclooxygenase-2 expression and prostaglandin (PG) E2 production in response to acidic pH e in a human osteoblastic cell line [38]. Because PGE2 is involved in osteoclastic differentiation of precursor cells [39], inhibition of the OGR1 signaling negatively regulates osteoclastogenesis [40]. Another type of G-protein-coupled receptor, TDAG8 (GPR65), also senses pH e [41, 42].
Breast cancer frequently metastasizes to bone. Osteoclasts can be activated by breast cancer-derived H+ such that osteolysis occurs when cancer cells metastasize to bone [36]. During this process, patients feel pain through acid-sensing ion channels (ASIC) 1a, 1b and 3 [36, 43, 44].
An acidic pH e is also found in the epidermis and plays an important protective role against bacterial infection [45–47]. Using the conditional knockout (KO) mice for focal adhesion kinase (FAK) in keratinocytes, Ilic et al. [47] showed that the stratum corneum pH e gradient of keratinocytes in these mice had significantly more neutral pH values, and that NHE1 failed to localize to the plasma membrane [47]. Thus, FAK controls pH-dependent epidermal barrier homeostasis by regulating actin-directed NHE1 plasma membrane localization [47].
Lung liquid is acidic [48], which is worse in patients with cystic fibrosis [49], although the airway pH is not known for certain because different detecting methods have been used [50].
CA expression in cancer
CA isoforms are associated with tumor malignancy, including CA I [51], CA II [51, 52], CA IX [53, 54], CA XII [55], and CA XIII [56]. Among them, CA IX in particular has been well studied in association with hypoxia and tumor survival through regulating intracellular pH [53, 57]. In ovarian cancer, high expression of CA IX with a concomitant increase in VEGF-A is associated with overall survival rates positively [58]. Overexpression of CA IX increases tumor cell migration and invasion [59]. CA inhibitor suppresses invasion of renal cancer cells in vitro[60]. Based on the accumulated evidence, a new therapeutic strategy targeting CA has been considered [61–63].
Acidic pH e activates proteinase activity and induces gene expression
Acidic pH e activates some proteinases. Although caries is due to some bacterial acidic metabolites, Tjäderhane et al. [64] found that host-derived pro-matrix metalloproteinase-9 (proMMP-9), proMMP-2 and proMMP-8 in saliva could be activated by acid, and thereby suggested that these MMPs contribute to the disruption of dentin in caries. Alternatively, host derived proMMP-9 could be activated in the stomach, and this suggests it functions as a digestive enzyme for collagenous foods [65, 66]. Activation of proMMP-9 by an acidic pH e also occurs in a human melanoma model [67].
Lysosomal enzymes have an acidic optimal pH. Some tumor cells have the ability to secrete them, such as cathepsin B and cathepsin L [5]. Cathepsin K plays an important role in osteoclast-mediated bone resorption [68, 69]; its inhibition prevents breast cancer-induced osteolysis and skeletal tumor burden [70]. Thus, osteoclast-mediated acidic pH e leads to mineral dissolution and activation of cathepsins to digest bone matrix, such as type I collagen. Podgorski et al. [71] reported that SPARC/osteonectin, a major non-collagenous protein in bone, is digested by cathepsin K and its fragments are associated with bone-metastasis. Another lysosomal enzyme, heparanase, has an acidic optimal pH; it degrades heparan sulfate in the basement membrane and contributes to tumor invasion and metastasis [72, 73].
Also, acidic microenvironments affect the expression of some genes, such as MMP-9 [74, 75] and acidic sphingomyelinase in mouse B16 melanoma [74], platelet-derived endothelial cell growth factor (thymidine phosphorylase) in human breast cancer cells [76], the inducible isoform of nitric oxide synthase (iNOS) in macrophages [77], VEGF-A in glioma [78] and glioblastoma [79] cells, and IL-8 expression in human pancreatic adenocarcinoma [80–82] and ovarian carcinoma cells [83].
Acidic pH e signal transduction pathway
Thus, although acidic pH e occurs in several physiological and pathological conditions, information on its signaling remains limited. Transcription factors AP-1 and NF-κB, independent of hypoxia, have important roles in the acidic pH e -induced expression of VEGF-A [78, 84] and IL-8 [80–83, 85]. p38 mitogen-activated protein kinase (MAPK) is involved in acidic pH e signaling that induces IL-8 [85].
We also found involvement of phospholipase D (PLD) in the acidic pH e -intracellular signaling to induce MMP-9 production [75, 86]. Acidic pH e -induced PLD activation was prolonged for at least for 24 h, different from general growth factor signaling. Inhibition of PLD activity by 1-butanol and Myr-ARF6 suppresses acidic pH e -induced MMP-9 expression [87]. Acidic pH e increases the steady-state levels of phosphorylated ERK1/2 and p38, and PLD inhibitors prevent these increases. Using 5′-deleted constructs of the MMP-9 promoter, we found that the acidic pH e -responsive region was located at nucleotides -670 to -531, a region containing the NF-κB binding site. A mutation in the NFκB binding site reduced acidic pH e -induced MMP-9 promoter activity, and NF-κB activity was induced by acidic pH e . Pharmacological inhibitors specific for MEK1/2 (PD098059) and p38 (SB203580) attenuated acidic pH e -induced NF-κB activity and MMP-9 expression. The data suggest that PLD, MAPKs including ERK 1/2 and p38, and NF-κB mediate acidic pH e signaling thereby inducing MMP-9 expression. Activation of ERK1/2 and p38, followed by the NF-κB axis, which is stimulated by tumor necrosis factor-α (TNF-α), also occurs in cholangiocarcinoma [88]. This suggests that acidic pH e signaling is, at least in part, the signaling pathway for TNF-α. However, it has been reported that acidic pH e activates p38, but not ERK1/2, in T-cell receptor signaling in Jurkat cells [89]. This may be cell-type specific. In a further contribution dealing with the intracellular substances of acidic pH e , we have found that calcium influx triggers acidic pH e -induced PLD activation and that acidic sphingomyelinase mediates acidic pH e signaling to activate NF-κB independently of the PLD-MAPK pathway [74].
OGR1 stimulates cyclooxygenase-2 expression and PGE2 production in response to an acidic pH e in a human osteoblastic cell line through G(q/11)/phospholipase C/protein kinase C pathway [38] and in human aortic smooth muscle cells through the phospholipase C/cyclooxygenase/PGI2 pathway [90].
Acidic pH directly affects transcription factor activity; DNA binding activity of the transcription factor, SP1, is enhanced by intracellular acidic pH [91]. Intracellular pH is maintained a constitutively neutral state but known to become transiently acidic when pH e decreases to acidic. Therefore an acidic pH can activate SP1.
Acidic pH e stimulates disruption of adherence junctions
When tumor cells move into their surrounding tissue, cell-cell junctions become dissociated. Acidic pH disrupts adherence junction by Src activation, resulting in E-cadherin degradation through the protein kinase Cδ pathway [92, 93]. Acidic pH e also induces motility of tumor cells, and inhibits monocyte migration and cytokine release [13].
Acidic pH e stimulates metastatic potential
Brockton et al. [54] have shown that high stromal CA IX expression is associated with nodal metastasis. The high activity produces an acidic microenvironment that leads to increased metastatic ability of the tumor cells. We have reported that induction rate of MMP-9 secretion correlates with metastatic potential of mouse B16 melanoma clones, and an acidic pH e stimulates invasion through a type-IV collagen barrier [75, 86]. In human melanoma models, an acidic pH e increases both migration and invasiveness in vitro, accompanied by MMP-9 activation [67]. NHE1 is also associated with the metastatic ability of tumor cells; it is accumulated in leading edge of the cell and is activated by CD44 (a hyaluronan (HA) receptor) -binding to HA [94]. Because HA directs membrane-type 1 matrix metalloproteinase (MT1-MMP) to the invasion front (invadopodia) [95, 96], NHE1 might interact with MT1-MMP through CD44 at an acidic pH e [97, 98].
Pretreatment of the tumor cells in an acidic medium induces production of proteinases (MMPs and cathepsins) and proangiogenic factors (VEGF-A and IL-8) and promotes experimental metastasis to the lung after injection into the tail vein of nude mice [5]; elevation of pH by one unit following injection of sodium bicarbonate prevents spontaneous metastases [99]. Furthermore, using P-31 magnetic resonance spectroscopic evaluation, it was found that acidic pH e in spontaneous soft tissue sarcomas predicts metastasis in dogs [100].
Acidic pH e sensing systems
ASICs are voltage-independent and proton-activated channels found in tumor cells and associated tumor malignancy [101]. Transient receptor potential (TRP) V isoforms, TRPV1, TRPV5 and TRPV6, also act as acid-sensitive channels [102, 103]. ERK1/2 plays as a downstream target of ASICs and TRPVs [104–106]. Another subfamily of TRP, TRPM7 has proton conductivity [107]. TRPM7 regulates EGF signaling to induce STAT3 activation and vimentin expression during epithelial-mesenchymal transition [108]. OGR1 also acts as a proton-sensing receptor, stimulating inositol phosphate formation [37].
pH e gradient formation by H+ pumps and exchangers
NHE1 accumulates at the leading edge to make a pH e gradient associated with cell migration [109]. The Rho-ROCK pathway contributes to NHE1 activation and focal adhesions [110, 111]. Protons stabilize the collagen–α2β1 integrin bond, but alkalosis, a lack of protons or an inhibited NHE activity, prevents adhesion [112]. Furthermore, the cell forms an individual pH e gradient to facilitate movement: i.e. at leading edge or invadopodia, cells preferentially attach to the substrate due to the acidic pH e induced by NHE1, while cell-matrix interaction at the rear end is weak due to a mid-alkaline pH e [113]. Mutation studies clearly showed that downregulation of NHE1 function suppresses cell polarity, migration, and invasion through matrigel™ [111]. Inhibition of NHE1 activity by HOE642 (cariporide) reduced migration and adhesion activities [109].
To secrete acidic metabolites, NHE1 and the H+-lactate co-transporter are involved [114]. H+-ATPase (the H+ pump) and cell surface ATP synthase also play a role in extracellular acidification [115, 116], thereby contributing to tumor metastasis [3]. Therefore, inhibition of the H+ pump can be a new strategy for cancer treatment [117–119]. Angiostatin has anti-tumor efficacy by inhibiting cell surface ATP synthase activity through binding its β subunit [116]. In particular, treatment of the cells with angiostatin proved more cytotoxicity at an acidic pH e than a neutral pH e .
Drug efficacy and acidic pH e
Two analogues of camptothecin (CPT), topotecan (TPT) and irinotecan (CPT-11), have significant anti-tumor activity in the clinic, although their abilities depend on the CPT E ring lactone, which forms an inactive hydroxy acid at physiological pH. The reaction is reversible at an acidic pH e , which provides a rationale for selectivity because many solid tumors, while creating an acidic extracellular environment, maintain a normal intracellular pH [120]. An acidic pH e inhibits cellular uptake of mitoxantrone and topotecan, so that elevation of pH e in tumor tissue enhances those drugs’ efficacy [120, 121]. Because the buffer action is weaker in tumor tissue than normal tissue, NaHCO3 has much potential to raise pH e relatively specifically in tumor tissue [122, 123]. Acidic pH e also plays a role in the resistance of tumor cells to drugs by increasing the expression of p-glycoprotein, thereby increasing drug efflux [124, 125]. Recently, an acidic pH e -specific drug-releasing system has been developed [126, 127]. A novel polymeric micelle constituted of 2 block copolymers of poly (L-lactic acid)-b-poly(ethylene glycol) b-poly (L-histidine) - TAT (transactivator of transcription) and poly(L-histidine)-b-poly(ethylene glycol) increases the cytotoxicity of doxorubicin in several multidrug-resistant tumor cell lines [127]. To measure pH e , a magnetic resonance image technique has been developed using acidic pH e specific probes [128, 129]. Thus, clinicians should pay attention to tumor pH e in selecting drugs and helping to maximize their chemotherapeutic action. Vasodilating drugs, such as hydralazine and captopril, inhibit tumor growth rate in vivo by reducing tumor blood flow [130]. Although the reduction in tumor growth by those drugs also reduces the oxygen supply, it reduces pH e . In patients given vasodilating drugs, anti-tumor drugs with weak acidic pK a value, such as 5-fluorouracil (5FU) and cyclophosphamide, may have increased efficacy at an acidic pH e . In contrast, the anti-tumor drugs with weak base pKa values, such as doxorubicin, mitoxantrone and daunorubicin, may not be fully functioned because acidic pH e reduces their cytotoxicity [121, 131]. In early-stage breast cancer, high CAIX is a predictive marker of doxorubicin resistance [132].
Because cis-diamminedichloroplatinum (II) (CDDP) solution has an acidic pH, NaHCO3 is used to prevent the angialgia in the cancer patients coming from the acidic pH solution injection because it increases pH [133, 134]. However, CDDP is frequently used for co-injection with other chemotherapeutic drugs, such as 5FU. In some cases, co-injection of NaHCO3 (depends on the concentration) may reduce the clinical efficacy of 5FU + CDDP regimen.
Hyperthermia and acidic pH e
Hyperthermic treatment (42.5°C) for JB-1-E plasmacytoma tumor cells in vitro enhances the colony formation index when cells are maintained at pH 6.4, regardless oxygen tensions [135]. Melanoma cells growing at low pH are sensitized to hyperthermia because of the altered intracellular pH threshold for the heat sensitization in vitro[136, 137].
Conclusion
Acidic pH e is toxic to many cells, including tumors [138]. However, if tumors have successfully adapted to their condition, and use it for their own cellular activation, this increases drug resistance and leads to more aggressive behavior. Therefore, management of tumor pH e and inhibition of blockade of proton-sensing system are important in not only raising drug efficacy, e.g. mitoxantrone, but in preventing metastasis.
Abbreviations
- pHe:
-
Extracellular pH
- NHE:
-
Na+/H+ exchanger
- MCT:
-
Monocarboxylate transporter
- CA:
-
Carbonic anhydrase
- IL:
-
Interleukin
- NF-κB:
-
Nuclear factor κB
- VEGF:
-
Vascular endothelial growth factor
- HIF:
-
Hypoxia inducible factor
- LDH:
-
Lactate dehydrogenase
- OGR:
-
Ovarian cancer G-protein-coupled receptor
- PG:
-
Prostaglandin
- ASIC:
-
Acid-sensing ion channel
- KO:
-
Knockout
- FAK:
-
Focal adhesion kinase
- MMP:
-
Matrix-metalloproteinase
- iNOS:
-
Nitric oxide synthase
- PLD:
-
Phospholipase D
- MAPK:
-
Mitogen-activated protein kinase
- TNF-α:
-
Tumor necrosis factor-α
- HA:
-
Hyaluronan
- MT1-MMP:
-
Membrane-type 1 matrix metalloproteinase
- TRP:
-
Transient receptor potential
- CDDP:
-
cis-Diamminedichloroplatinum (II).
References
Warburg O, Posener K, Negelein E: Über den Stoffwechsel der Tumoren (On metabolism of tumors). Biochem Z. 1924, 152: 319-344.
Chesler M, Nicholson C: Regulation of intracellular pH in vertebrate central neurons. Brain Res. 1985, 325 (1–2): 313-316.
Nishisho T, Hata K, Nakanishi M, Morita Y, Sun-Wada GH, Wada Y, Yasui N, Yoneda T: The a 3 isoform vacuolar type H+-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res. 2011, 9 (7): 845-855. 10.1158/1541-7786.MCR-10-0449.
Helmlinger G, Sckell A, Dellian M, Forbes NS, Jain RK: Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res. 2002, 8 (4): 1284-1291.
Rofstad EK, Mathiesen B, Kindem K, Galappathi K: Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006, 66 (13): 6699-6707. 10.1158/0008-5472.CAN-06-0983.
Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, Mueller-Klieser W: Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001, 51 (2): 349-353. 10.1016/S0360-3016(01)01630-3.
Walenta S, Chau TV, Schroeder T, Lehr HA, Kunz-Schughart LA, Fuerst A, Mueller-Klieser W: Metabolic classification of human rectal adenocarcinomas: a novel guideline for clinical oncologists?. J Cancer Res Clin Oncol. 2003, 129 (6): 321-326. 10.1007/s00432-003-0450-x.
Walenta S, Salameh A, Lyng H, Evensen JF, Mitze M, Rofstad EK, Mueller-Klieser W: Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol. 1997, 150 (2): 409-415.
McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O: Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008, 283 (33): 22700-22708. 10.1074/jbc.M801765200.
Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S: Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007, 109 (9): 3812-3819. 10.1182/blood-2006-07-035972.
Yabu M, Shime H, Hara H, Saito T, Matsumoto M, Seya T, Akazawa T, Inoue N: IL-23-dependent and -independent enhancement pathways of IL-17A production by lactic acid. Int Immunol. 2011, 23 (1): 29-41. 10.1093/intimm/dxq455.
Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M: Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006, 107 (5): 2013-2021. 10.1182/blood-2005-05-1795.
Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W: Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011, 39 (2): 453-463.
Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O: Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011, 71 (7): 2550-2560. 10.1158/0008-5472.CAN-10-2828.
Hunt TK, Aslam RS, Beckert S, Wagner S, Ghani QP, Hussain MZ, Roy S, Sen CK: Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal. 2007, 9 (8): 1115-1124. 10.1089/ars.2007.1674.
Sattler UG, Meyer SS, Quennet V, Hoerner C, Knoerzer H, Fabian C, Yaromina A, Zips D, Walenta S, Baumann M: Glycolytic metabolism and tumour response to fractionated irradiation. Radiother Oncol. 2010, 94 (1): 102-109. 10.1016/j.radonc.2009.11.007.
Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, Moon EJ, Dhup S, Danhier P, Frerart F: Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012, 7 (3): e33418-10.1371/journal.pone.0033418.
Kim JH, Kim SH, Alfieri AA, Young CW: Quercetin, an inhibitor of lactate transport and a hyperthermic sensitizer of HeLa cells. Cancer Res. 1984, 44 (1): 102-106.
Hatanaka M, Hanafusa H: Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970, 41 (4): 647-652. 10.1016/0042-6822(70)90429-0.
Kallinowski F, Vaupel P: Concurrent measurements of O2 partial pressures and pH values in human mammary carcinoma xenotransplants. Adv Exp Med Biol. 1986, 200: 609-621. 10.1007/978-1-4684-5188-7_74.
Martin GR, Jain RK: Noninvasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluorescence ratio imaging microscopy. Cancer Res. 1994, 54 (21): 5670-5674.
Helmlinger G, Yuan F, Dellian M, Jain RK: Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997, 3 (2): 177-182. 10.1038/nm0297-177.
Kayser G, Kassem A, Sienel W, Schulte-Uentrop L, Mattern D, Aumann K, Stickeler E, Werner M, Passlick B, Zur Hausen A: Lactate-dehydrogenase 5 is overexpressed in non-small cell lung cancer and correlates with the expression of the transketolase-like protein 1. Diagn Pathol. 2010, 5: 22-10.1186/1746-1596-5-22.
Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL: Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003, 89 (5): 877-885. 10.1038/sj.bjc.6601205.
Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E: Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005, 22 (1): 25-30. 10.1007/s10585-005-2343-7.
Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL: Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway–a report of the Tumour Angiogenesis Research Group. J Clin Oncol. 2006, 24 (26): 4301-4308. 10.1200/JCO.2006.05.9501.
Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Trarbach T, Folprecht G, Shi MM, Lebwohl D, Jalava T, Laurent D: Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin Cancer Res. 2011, 17 (14): 4892-4900. 10.1158/1078-0432.CCR-10-2918.
Baron R, Neff L, Louvard D, Courtoy PJ: Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol. 1985, 101 (6): 2210-2222. 10.1083/jcb.101.6.2210.
Lehenkari P, Hentunen TA, Laitala-Leinonen T, Tuukkanen J, Vaananen HK: Carbonic anhydrase II plays a major role in osteoclast differentiation and bone resorption by effecting the steady state intracellular pH and Ca2+. Exp Cell Res. 1998, 242 (1): 128-137. 10.1006/excr.1998.4071.
Mobasheri A, Golding S, Pagakis SN, Corkey K, Pocock AE, Fermor B, O’Brien MJ, Wilkins RJ, Ellory JC, Francis MJ: Expression of cation exchanger NHE and anion exchanger AE isoforms in primary human bone-derived osteoblasts. Cell Biol Int. 1998, 22 (7–8): 551-562.
Biskobing DM, Fan D: Acid pH increases carbonic anhydrase II and calcitonin receptor mRNA expression in mature osteoclasts. Calcif Tissue Int. 2000, 67 (2): 178-183. 10.1007/s00223001107.
Häbler C: Über den K- und Ca-Gehalt von eiter und Exsudaten und seine Beziehungen zum Entzündungsschmerz. Klin Wochenschrift. 1929, 8: 1569-1572. 10.1007/BF01849103.
Dellian M, Helmlinger G, Yuan F, Jain RK: Fluorescence ratio imaging of interstitial pH in solid tumours: effect of glucose on spatial and temporal gradients. Br J Cancer. 1996, 74 (8): 1206-1215. 10.1038/bjc.1996.518.
Rocha-Gonzalez HI, Herrejon-Abreu EB, Lopez-Santillan FJ, Garcia-Lopez BE, Murbartian J, Granados-Soto V: Acid increases inflammatory pain in rats: Effect of local peripheral ASICs inhibitors. Eur J Pharmacol. 2009, 603 (1–3): 56-61.
Steen KH, Issberner U, Reeh PW: Pain due to experimental acidosis in human skin: evidence for non-adapting nociceptor excitation. Neurosci Lett. 1995, 199 (1): 29-32. 10.1016/0304-3940(95)12002-L.
Nagae M, Hiraga T, Yoneda T: Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab. 2007, 25 (2): 99-104. 10.1007/s00774-006-0734-8.
Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K: Proton-sensing G-protein-coupled receptors. Nature. 2003, 425 (6953): 93-98. 10.1038/nature01905.
Tomura H, Wang JQ, Liu JP, Komachi M, Damirin A, Mogi C, Tobo M, Nochi H, Tamoto K, Im DS: Cyclooxygenase-2 expression and prostaglandin E2 production in response to acidic pH through OGR1 in a human osteoblastic cell line. J Bone Miner Res. 2008, 23 (7): 1129-1139. 10.1359/jbmr.080236.
Kobayashi Y, Mizoguchi T, Take I, Kurihara S, Udagawa N, Takahashi N: Prostaglandin E2 enhances osteoclastic differentiation of precursor cells through protein kinase A-dependent phosphorylation of TAK1. J Biol Chem. 2005, 280 (12): 11395-11403. 10.1074/jbc.M411189200.
Iwai K, Koike M, Ohshima S, Miyatake K, Uchiyama Y, Saeki Y, Ishii M: RGS18 acts as a negative regulator of osteoclastogenesis by modulating the acid-sensing OGR1/NFAT signaling pathway. J Bone Miner Res. 2007, 22 (10): 1612-1620. 10.1359/jbmr.070612.
Ihara Y, Kihara Y, Hamano F, Yanagida K, Morishita Y, Kunita A, Yamori T, Fukayama M, Aburatani H, Shimizu T: The G protein-coupled receptor T-cell death-associated gene 8 (TDAG8) facilitates tumor development by serving as an extracellular pH sensor. Proc Natl Acad Sci U S A. 2010, 107 (40): 17309-17314. 10.1073/pnas.1001165107.
He XD, Tobo M, Mogi C, Nakakura T, Komachi M, Murata N, Takano M, Tomura H, Sato K, Okajima F: Involvement of proton-sensing receptor TDAG8 in the anti-inflammatory actions of dexamethasone in peritoneal macrophages. Biochem Biophys Res Commun. 2011, 415 (4): 627-631. 10.1016/j.bbrc.2011.10.122.
Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwata K, Yoneda T: Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone. 2006, 39 (5): 1107-1115. 10.1016/j.bone.2006.04.033.
Jasti J, Furukawa H, Gonzales EB, Gouaux E: Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007, 449 (7160): 316-323. 10.1038/nature06163.
Marples MJ: The ecology of human skin. 1965, Springfield, IL: Charles C. Thomas
Behne MJ, Barry NP, Hanson KM, Aronchik I, Clegg RW, Gratton E, Feingold K, Holleran WM, Elias PM, Mauro TM: Neonatal development of the stratum corneum pH gradient: localization and mechanisms leading to emergence of optimal barrier function. J Invest Dermatol. 2003, 120 (6): 998-1006. 10.1038/jid.2003.11.
Ilic D, Mao-Qiang M, Crumrine D, Dolganov G, Larocque N, Xu P, Demerjian M, Brown BE, Lim ST, Ossovskaya V: Focal adhesion kinase controls pH-dependent epidermal barrier homeostasis by regulating actin-directed Na+/H+ exchanger 1 plasma membrane localization. Am J Pathol. 2007, 170 (6): 2055-2067. 10.2353/ajpath.2007.061277.
Adamson TM, Boyd RD, Platt HS, Strang LB: Composition of alveolar liquid in the foetal lamb. J Physiol. 1969, 204 (1): 159-168.
Tate S, MacGregor G, Davis M, Innes JA, Greening AP: Airways in cystic fibrosis are acidified: detection by exhaled breath condensate. Thorax. 2002, 57 (11): 926-929. 10.1136/thorax.57.11.926.
Ricciardolo FL, Gaston B, Hunt J: Acid stress in the pathology of asthma. J Allergy Clin Immunol. 2004, 113 (4): 610-619. 10.1016/j.jaci.2003.12.034.
Bekku S, Mochizuki H, Yamamoto T, Ueno H, Takayama E, Tadakuma T: Expression of carbonic anhydrase I or II and correlation to clinical aspects of colorectal cancer. Hepatogastroenterology. 2000, 47 (34): 998-1001.
Leppilampi M, Koistinen P, Savolainen ER, Hannuksela J, Parkkila AK, Niemela O, Pastorekova S, Pastorek J, Waheed A, Sly WS: The expression of carbonic anhydrase II in hematological malignancies. Clin Cancer Res. 2002, 8 (7): 2240-2245.
Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J: Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009, 69 (1): 358-368. 10.1158/0008-5472.CAN-08-2470.
Brockton NT, Klimowicz AC, Bose P, Petrillo SK, Konno M, Rudmik L, Dean M, Nakoneshny SC, Matthews TW, Chandarana S: High stromal carbonic anhydrase IX expression is associated with nodal metastasis and decreased survival in patients with surgically-treated oral cavity squamous cell carcinoma. Oral Oncol. 2012, 48 (7): 615-622. 10.1016/j.oraloncology.2012.01.018.
Parkkila S, Parkkila AK, Saarnio J, Kivela J, Karttunen TJ, Kaunisto K, Waheed A, Sly WS, Tureci O, Virtanen I: Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. J Histochem Cytochem. 2000, 48 (12): 1601-1608. 10.1177/002215540004801203.
Kummola L, Hamalainen JM, Kivela J, Kivela AJ, Saarnio J, Karttunen T, Parkkila S: Expression of a novel carbonic anhydrase, CA XIII, in normal and neoplastic colorectal mucosa. BMC cancer. 2005, 5: 41-10.1186/1471-2407-5-41.
Swietach P, Wigfield S, Cobden P, Supuran CT, Harris AL, Vaughan-Jones RD: Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J Biol Chem. 2008, 283 (29): 20473-20483. 10.1074/jbc.M801330200.
Williams E, Martin S, Moss R, Durrant L, Deen S: Co-expression of VEGF and CA9 in ovarian high-grade serous carcinoma and relationship to survival. Virchows Arch. 2012, 461 (1): 33-39. 10.1007/s00428-012-1252-9.
Shin HJ, Rho SB, Jung DC, Han IO, Oh ES, Kim JY: Carbonic anhydrase IX (CA9) modulates tumor-associated cell migration and invasion. J Cell Sci. 2011, 124 (Pt 7): 1077-1087.
Parkkila S, Rajaniemi H, Parkkila AK, Kivela J, Waheed A, Pastorekova S, Pastorek J, Sly WS: Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci U S A. 2000, 97 (5): 2220-2224. 10.1073/pnas.040554897.
Zatovicova M, Jelenska L, Hulikova A, Csaderova L, Ditte Z, Ditte P, Goliasova T, Pastorek J, Pastorekova S: Carbonic anhydrase IX as an anticancer therapy target: preclinical evaluation of internalizing monoclonal antibody directed to catalytic domain. Curr Pharm Des. 2010, 16 (29): 3255-3263. 10.2174/138161210793429832.
Muselaers S, Mulders P, Oosterwijk E, Oyen W, Boerman O: Molecular imaging and carbonic anhydrase IX-targeted radioimmunotherapy in clear cell renal cell carcinoma. Immunotherapy. 2013, 5 (5): 489-495. 10.2217/imt.13.36.
Oosterwijk-Wakka JC, Boerman OC, Mulders PF, Oosterwijk E: Application of Monoclonal Antibody G250 Recognizing Carbonic Anhydrase IX in Renal Cell Carcinoma. Int J Mol Sci. 2013, 14 (6): 11402-11423. 10.3390/ijms140611402.
Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T: The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998, 77 (8): 1622-1629. 10.1177/00220345980770081001.
Davis GE, Martin BM: A latent Mr 94,000 gelatin-degrading metalloprotease induced during differentiation of HL-60 promyelocytic leukemia cells: a member of the collagenase family of enzymes. Cancer Res. 1990, 50 (4): 1113-1120.
Davis GE: Identification of an abundant latent 94-kDa gelatin-degrading metalloprotease in human saliva which is activated by acid exposure: implications for a role in digestion of collagenous proteins. Arch Biochem Biophys. 1991, 286 (2): 551-554. 10.1016/0003-9861(91)90078-W.
Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ: Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996, 14 (2): 176-186. 10.1007/BF00121214.
Karsdal MA, Henriksen K, Sorensen MG, Gram J, Schaller S, Dziegiel MH, Heegaard AM, Christophersen P, Martin TJ, Christiansen C: Acidification of the osteoclastic resorption compartment provides insight into the coupling of bone formation to bone resorption. Am J Pathol. 2005, 166 (2): 467-476. 10.1016/S0002-9440(10)62269-9.
Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Moritz JD, Schu P, Von Figura K: Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A. 1998, 95 (23): 13453-13458. 10.1073/pnas.95.23.13453.
Le Gall C, Bellahcene A, Bonnelye E, Gasser JA, Castronovo V, Green J, Zimmermann J, Clezardin P: A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Res. 2007, 67 (20): 9894-9902. 10.1158/0008-5472.CAN-06-3940.
Podgorski I, Linebaugh BE, Koblinski JE, Rudy DL, Herroon MK, Olive MB, Sloane BF: Bone marrow-derived cathepsin K cleaves SPARC in bone metastasis. Am J Pathol. 2009, 175 (3): 1255-1269. 10.2353/ajpath.2009.080906.
Nakajima M, Irimura T, Di Ferrante D, Di Ferrante N, Nicolson GL: Heparan sulfate degradation: relation to tumor invasive and metastatic properties of mouse B16 melanoma sublines. Science. 1983, 220 (4597): 611-613. 10.1126/science.6220468.
Nakajima M, Irimura T, Di Ferrante N, Nicolson GL: Metastatic melanoma cell heparanase. Characterization of heparan sulfate degradation fragments produced by B16 melanoma endoglucuronidase. J Biol Chem. 1984, 259 (4): 2283-2290.
Kato Y, Ozawa S, Tsukuda M, Kubota E, Miyazaki K, St-Pierre Y, Hata R: Acidic extracellular pH increases calcium influx-triggered phospholipase D activity along with acidic sphingomyelinase activation to induce matrix metalloproteinase-9 expression in mouse metastatic melanoma. FEBS J. 2007, 274 (12): 3171-3183. 10.1111/j.1742-4658.2007.05848.x.
Kato Y, Nakayama Y, Umeda M, Miyazaki K: Induction of 103-kDa gelatinase/type IV collagenase by acidic culture conditions in mouse metastatic melanoma cell lines. J Biol Chem. 1992, 267 (16): 11424-11430.
Griffiths L, Dachs GU, Bicknell R, Harris AL, Stratford IJ: The influence of oxygen tension and pH on the expression of platelet-derived endothelial cell growth factor/thymidine phosphorylase in human breast tumor cells grown in vitro and in vivo. Cancer Res. 1997, 57 (4): 570-572.
Bellocq A, Suberville S, Philippe C, Bertrand F, Perez J, Fouqueray B, Cherqui G, Baud L: Low environmental pH is responsible for the induction of nitric-oxide synthase in macrophages. Evidence for involvement of nuclear factor-κB activation. J Biol Chem. 1998, 273 (9): 5086-5092. 10.1074/jbc.273.9.5086.
Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK: Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001, 61 (16): 6020-6024.
Xu L, Fukumura D, Jain RK: Acidic extracellular pH induces vascular endothelial growth factor (VEGF) in human glioblastoma cells via ERK1/2 MAPK signaling pathway: mechanism of low pH-induced VEGF. J Biol Chem. 2002, 277 (13): 11368-11374. 10.1074/jbc.M108347200.
Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K: Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res. 1999, 5 (11): 3711-3721.
Shi Q, Le X, Wang B, Xiong Q, Abbruzzese JL, Xie K: Regulation of interleukin-8 expression by cellular pH in human pancreatic adenocarcinoma cells. J Interferon Cytokine Res. 2000, 20 (11): 1023-1028. 10.1089/10799900050198471.
Shi Q, Xiong Q, Le X, Xie K: Regulation of interleukin-8 expression by tumor-associated stress factors. J Interferon Cytokine Res. 2001, 21 (8): 553-566. 10.1089/10799900152547812.
Xu L, Fidler IJ: Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Res. 2000, 60 (16): 4610-4616.
Shi Q, Le X, Wang B, Abbruzzese JL, Xiong Q, He Y, Xie K: Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001, 20 (28): 3751-3756. 10.1038/sj.onc.1204500.
Bischoff DS, Zhu JH, Makhijani NS, Yamaguchi DT: Acidic pH stimulates the production of the angiogenic CXC chemokine, CXCL8 (interleukin-8), in human adult mesenchymal stem cells via the extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and NF-κB pathways. J Cell Biochem. 2008, 104 (4): 1378-1392. 10.1002/jcb.21714.
Kato Y, Ozono S, Shuin T, Miyazaki K: Slow induction of gelatinase B mRNA by acidic culture conditions in mouse metastatic melanoma cells. Cell Biol Int. 1996, 20 (5): 375-377. 10.1006/cbir.1996.0044.
Kato Y, Lambert CA, Colige AC, Mineur P, Noël A, Frankenne F, Foidart JM, Baba M, Hata RI, Miyazaki K: Acidic extracellular pH induces matrix metalloproteinase-9 expression in mouse metastatic melanoma cells through the phospholipase D-mitogen-activated protein kinase signaling. J Biol Chem. 2005, 280 (12): 10938-10944. 10.1074/jbc.M411313200.
Itatsu K, Sasaki M, Harada K, Yamaguchi J, Ikeda H, Sato Y, Ohta T, Sato H, Nagino M, Nimura Y: Phosphorylation of extracellular signal-regulated kinase 1/2, p38 mitogen-activated protein kinase and nuclear translocation of nuclear factor-κB are involved in upregulation of matrix metalloproteinase-9 by tumour necrosis factor-α. Liver Int. 2009, 29 (2): 291-298. 10.1111/j.1478-3231.2008.01858.x.
Hirata S, Fukamachi T, Sakano H, Tarora A, Saito H, Kobayashi H: Extracellular acidic environments induce phosphorylation of ZAP-70 in Jurkat T cells. Immunol Lett. 2008, 115 (2): 105-109. 10.1016/j.imlet.2007.10.006.
Tomura H, Wang JQ, Komachi M, Damirin A, Mogi C, Tobo M, Kon J, Misawa N, Sato K, Okajima F: Prostaglandin l2 production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J Biol Chem. 2005, 280 (41): 34458-34464. 10.1074/jbc.M505287200.
Torigoe T, Izumi H, Yoshida Y, Ishiguchi H, Okamoto T, Itoh H, Kohno K: Low pH enhances Sp1 DNA binding activity and interaction with TBP. Nucleic Acids Res. 2003, 31 (15): 4523-4530. 10.1093/nar/gkg487.
Chen Y, Chen CH, Tung PY, Huang SH, Wang SM: An acidic extracellular pH disrupts adherens junctions in HepG2 cells by Src kinases-dependent modification of E-cadherin. J Cell Biochem. 2009, 108 (4): 851-859. 10.1002/jcb.22313.
Chen Y, Kung HN, Chen CH, Huang SH, Chen KH, Wang SM: Acidic extracellular pH induces p120-catenin-mediated disruption of adherens junctions via the Src kinase-PKCδ pathway. FEBS Lett. 2011, 585 (4): 705-710. 10.1016/j.febslet.2011.01.022.
Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E: CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004, 279 (26): 26991-27007. 10.1074/jbc.M311838200.
Mori H, Tomari T, Koshikawa N, Kajita M, Itoh Y, Sato H, Tojo H, Yana I, Seiki M: CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J. 2002, 21 (15): 3949-3959. 10.1093/emboj/cdf411.
Suenaga N, Mori H, Itoh Y, Seiki M: CD44 binding through the hemopexin-like domain is critical for its shedding by membrane-type 1 matrix metalloproteinase. Oncogene. 2005, 24 (5): 859-868. 10.1038/sj.onc.1208258.
Lin Y, Chang G, Wang J, Jin W, Wang L, Li H, Ma L, Li Q, Pang T: NHE1 mediates MDA-MB-231 cells invasion through the regulation of MT1-MMP. Exp Cell Res. 2011, 317 (14): 2031-2040. 10.1016/j.yexcr.2011.05.026.
Lin Y, Wang J, Jin W, Wang L, Li H, Ma L, Li Q, Pang T: NHE1 mediates migration and invasion of HeLa cells via regulating the expression and localization of MT1-MMP. Cell Biochem Funct. 2012, 1: 41-46.
Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA: Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009, 69 (6): 2260-2268. 10.1158/0008-5472.CAN-07-5575.
Lora-Michiels M, Yu D, Sanders L, Poulson JM, Azuma C, Case B, Vujaskovic Z, Thrall DE, Charles HC, Dewhirst MW: Extracellular pH and P-31 magnetic resonance spectroscopic variables are related to outcome in canine soft tissue sarcomas treated with thermoradiotherapy. Clin Cancer Res. 2006, 12 (19): 5733-5740. 10.1158/1078-0432.CCR-05-2669.
Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL: Acid-sensing ion channels in malignant gliomas. J Biol Chem. 2003, 278 (17): 15023-15034. 10.1074/jbc.M300991200.
Ohta T, Imagawa T, Ito S: Novel gating and sensitizing mechanism of capsaicin receptor (TRPV1): tonic inhibitory regulation of extracellular sodium through the external protonation sites on TRPV1. J Biol Chem. 2008, 283 (14): 9377-9387. 10.1074/jbc.M709377200.
Nakaya K, Harbidge DG, Wangemann P, Schultz BD, Green ED, Wall SM, Marcus DC: Lack of pendrin HCO3- transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am J Physiol Renal Physiol. 2007, 292 (5): F1314-F1321. 10.1152/ajprenal.00432.2006.
Chen Y, Willcockson HH, Valtschanoff JG: Vanilloid receptor TRPV1-mediated phosphorylation of ERK in murine adjuvant arthritis. Osteoarthritis Cartilage. 2009, 17 (2): 244-251. 10.1016/j.joca.2008.06.015.
Chen Y, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, Parekh PK, Moore C, Gereau RW, Taylor AB: Temporomandibular joint pain: A critical role for Trpv4 in the trigeminal ganglion. Pain. 2013, 154 (8): 1295-1304. 10.1016/j.pain.2013.04.004.
Wang G, Su J, Li L, Feng J, Shi L, He W, Liu Y: Edaravone alleviates hypoxia-acidosis/reoxygenation-induced neuronal injury by activating ERK1/2. Neurosci Lett. 2013, 543: 72-77.
Numata T, Okada Y: Proton conductivity through the human TRPM7 channel and its molecular determinants. J Biol Chem. 2008, 283 (22): 15097-15103. 10.1074/jbc.M709261200.
Davis FM, Azimi I, Faville RA, Peters AA, Jalink K, Putney JW, Goodhill GJ, Thompson EW, Roberts-Thomson SJ, Monteith GR: Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2013, in press
Stüwe L, Müller M, Fabian A, Waning J, Mally S, Noël J, Schwab A, Stock C: pH dependence of melanoma cell migration: protons extruded by NHE1 dominate protons of the bulk solution. J Physiol. 2007, 585 (Pt 2): 351-360.
Tominaga T, Barber DL: Na-H exchange acts downstream of RhoA to regulate integrin-induced cell adhesion and spreading. Mol Biol Cell. 1998, 9 (8): 2287-2303. 10.1091/mbc.9.8.2287.
Denker SP, Barber DL: Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002, 159 (6): 1087-1096. 10.1083/jcb.200208050.
Stock C, Gassner B, Hauck CR, Arnold H, Mally S, Eble JA, Dieterich P, Schwab A: Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J Physiol. 2005, 567 (Pt 1): 225-238.
Krahling H, Mally S, Eble JA, Noel J, Schwab A, Stock C: The glycocalyx maintains a cell surface pH nanoenvironment crucial for integrin-mediated migration of human melanoma cells. Pflugers Arch. 2009, 458 (6): 1069-1083. 10.1007/s00424-009-0694-7.
Cardone RA, Casavola V, Reshkin SJ: The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005, 5 (10): 786-795. 10.1038/nrc1713.
Montcourrier P, Silver I, Farnoud R, Bird I, Rochefort H: Breast cancer cells have a high capacity to acidify extracellular milieu by a dual mechanism. Clin Exp Metastasis. 1997, 15 (4): 382-392. 10.1023/A:1018446104071.
Chi SL, Pizzo SV: Angiostatin is directly cytotoxic to tumor cells at low extracellular pH: a mechanism dependent on cell surface-associated ATP synthase. Cancer Res. 2006, 66 (2): 875-882. 10.1158/0008-5472.CAN-05-2806.
Spugnini EP, Citro G, Fais S: Proton pump inhibitors as anti vacuolar-ATPases drugs: a novel anticancer strategy. J Exp Clin Cancer Res. 2010, 29: 44-10.1186/1756-9966-29-44.
De Milito A, Canese R, Marino ML, Borghi M, Iero M, Villa A, Venturi G, Lozupone F, Iessi E, Logozzi M: pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer. 2010, 127 (1): 207-219. 10.1002/ijc.25009.
Wiedmann RM, Von Schwarzenberg K, Palamidessi A, Schreiner L, Kubisch R, Liebl J, Schempp C, Trauner D, Vereb G, Zahler S: The V-ATPase-inhibitor archazolid abrogates tumor metastasis via inhibition of endocytic activation of the Rho-GTPase Rac1. Cancer Res. 2012, 72 (22): 5976-5987. 10.1158/0008-5472.CAN-12-1772.
Adams DJ, Dewhirst MW, Flowers JL, Gamcsik MP, Colvin OM, Manikumar G, Wani MC, Wall ME: Camptothecin analogues with enhanced antitumor activity at acidic pH. Cancer Chemother Pharmacol. 2000, 46 (4): 263-271. 10.1007/s002800000157.
Vukovic V, Tannock IF: Influence of low pH on cytotoxicity of paclitaxel, mitoxantrone and topotecan. Br J Cancer. 1997, 75 (8): 1167-1172. 10.1038/bjc.1997.201.
Raghunand N, He X, Van Sluis R, Mahoney B, Baggett B, Taylor CW, Paine-Murrieta G, Roe D, Bhujwalla ZM, Gillies RJ: Enhancement of chemotherapy by manipulation of tumour pH. Br J Cancer. 1999, 80 (7): 1005-1011. 10.1038/sj.bjc.6690455.
Raghunand N, Mahoney B, Van Sluis R, Baggett B, Gillies RJ: Acute metabolic alkalosis enhances response of C3H mouse mammary tumors to the weak base mitoxantrone. Neoplasia. 2001, 3 (3): 227-235. 10.1038/sj.neo.7900151.
Lotz C, Kelleher DK, Gassner B, Gekle M, Vaupel P, Thews O: Role of the tumor microenvironment in the activity and expression of the p-glycoprotein in human colon carcinoma cells. Oncol Rep. 2007, 17 (1): 239-244.
Thews O, Dillenburg W, Rosch F, Fellner M: PET imaging of the impact of extracellular pH and MAP kinases on the p-glycoprotein (Pgp) activity. Adv Exp Med Biol. 2013, 765: 279-286. 10.1007/978-1-4614-4989-8_39.
Ko J, Park K, Kim YS, Kim MS, Han JK, Kim K, Park RW, Kim IS, Song HK, Lee DS: Tumoral acidic extracellular pH targeting of pH-responsive MPEG-poly(β-amino ester) block copolymer micelles for cancer therapy. J Control Release. 2007, 123 (2): 109-115. 10.1016/j.jconrel.2007.07.012.
Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH: Super pH-sensitive multifunctional polymeric micelle for tumor pH e specific TAT exposure and multidrug resistance. J Control Release. 2008, 129 (3): 228-236. 10.1016/j.jconrel.2008.04.024.
Garcia-Martin ML, Martinez GV, Raghunand N, Sherry AD, Zhang S, Gillies RJ: High resolution pH e imaging of rat glioma using pH-dependent relaxivity. Magn Reson Med. 2006, 55 (2): 309-315. 10.1002/mrm.20773.
Van Sluis R, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdan S, Galons JP, Gillies RJ: In vivo imaging of extracellular pH using 1H MRSI. Magn Reson Med. 1999, 41 (4): 743-750. 10.1002/(SICI)1522-2594(199904)41:4<743::AID-MRM13>3.0.CO;2-Z.
Adachi E, Tannock IF: The effects of vasodilating drugs on pH in tumors. Oncol Res. 1999, 11 (4): 179-185.
Raghunand N, Mahoney BP, Gillies RJ: Tumor acidity, ion trapping and chemotherapeutics. II. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem Pharmacol. 2003, 66 (7): 1219-1229. 10.1016/S0006-2952(03)00468-4.
Betof AS, Rabbani ZN, Hardee ME, Kim SJ, Broadwater G, Bentley RC, Snyder SA, Vujaskovic Z, Oosterwijk E, Harris LN: Carbonic anhydrase IX is a predictive marker of doxorubicin resistance in early-stage breast cancer independent of HER2 and TOP2A amplification. Br J Cancer. 2012, 106 (5): 916-922. 10.1038/bjc.2012.32.
Teh BG, Kobayashi W, Narita K, Fukui R, Kimura H: Superselective docetaxel-nedaplatin combined infusion concurrent with radiation thrapy in advanced oral cancers. Oral Oncol EXTRA. 2004, 40: 126-131. 10.1016/j.ooe.2004.08.002.
Shiga K, Yokoyama J, Hashimoto S, Saijo S, Tateda M, Ogawa T, Watanabe M, Kobayashi T: Combined therapy after superselective arterial cisplatin infusion to treat maxillary squamous cell carcinoma. Otolaryngol Head Neck Surg. 2007, 136 (6): 1003-1009. 10.1016/j.otohns.2006.12.018.
Overgaard J, Bichel P: The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiology. 1977, 123 (2): 511-514.
Coss RA, Storck CW, Daskalakis C, Berd D, Wahl ML: Intracellular acidification abrogates the heat shock response and compromises survival of human melanoma cells. Mol Cancer Ther. 2003, 2 (4): 383-388.
Coss RA, Storck CW, Wachsberger PR, Reilly J, Leeper DB, Berd D, Wahl ML: Acute extracellular acidification reduces intracellular pH, 42°C-induction of heat shock proteins and clonal survival of human melanoma cells grown at pH 6.7. Int J Hyperthermia. 2004, 20 (1): 93-106. 10.1080/02656730310001605519.
Lan A, Lagadic-Gossmann D, Lemaire C, Brenner C, Jan G: Acidic extracellular pH shifts colorectal cancer cell death from apoptosis to necrosis upon exposure to propionate and acetate, major end-products of the human probiotic propionibacteria. Apoptosis. 2007, 12 (3): 573-591. 10.1007/s10495-006-0010-3.
Acknowledgements
We thank Profs. Masaichi-Chang-Il Lee, Eiro Kubota, Kaoru Miyazaki, and Ryu-Ichiro Hata for their critical comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare no competing financial interests.
Authors’ contributions
YK designed the study. SO, CM, YM, AS and TM were involved in discussion. YK drafted the manuscript. YB revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kato, Y., Ozawa, S., Miyamoto, C. et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int 13, 89 (2013). https://doi.org/10.1186/1475-2867-13-89
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2867-13-89