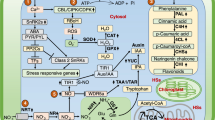
Abstract
Background
Traditional agriculture is on the front line of climate change, being most impacted by the increase in the intensity and frequency of extreme events, such as floods, drought and rising temperatures. Local ecological knowledge is a recognized keystone of successfully managed socioecological systems, but loss of soil fertility, water scarcity, incidence of diseases and decreased production due to climate change are linked to the greater vulnerability experienced by traditional farmers. Plant biostimulants are natural products used to stimulate nutrient uptake and efficiency by crops, increase tolerance to abiotic/biotic stress and improve quality without negative impacts on the environment if obtained from renewed sources. Humic substances are some of the most used plant biostimulants in agriculture and play a central role in plant adaptation.
Materials and methods
We reviewed and discussed a sample set of papers (n = 52) about humic substances to mitigate abiotic stress in crops using data basis from Web of Science (Clarivate Analytics), Scopus—IBM (International Business Machines Corporation), and Scielo (Scientific Electronic Library Online).
Results
The predominance of authors in the global south is notable, but it is not a coincidence, since this is where the effects of climate change will have the greatest impact. The mechanisms involved in the stress mitigation involve the activation of signaling factors, gene response induction, the accumulation of osmoprotective and anti-oxidant compounds, the induction of antioxidative metabolism, ion homeostasis, membrane transport and adjustment of hormonal balance. The intriguing question is: how can a complex mixture of molecules affect so many distinct effects on plants responsible for plant adaptation?
Conclusions
The complexity of humic substances challenges our knowledge method, but supramolecular chemistry may provide answers that enable us to broaden our understanding of the plant defense mechanisms modulated by these substances.
Graphical Abstract

Similar content being viewed by others
Introduction
Small-scale producers represent 80% of global rural properties and produce more than 80% of the food consumed in developing countries [1]. They are on the front line of climate change [2], being most impacted by the reduction in crop productivity and other damages resulting from the increase in intensity and frequency of extreme events, such as floods, droughts, and frosts [3]. Extreme weather conditions have shaken the lives of small rural communities, a trend attributed to climate change and human actions (https://news.un.org/pt/story/2021/08/1760132). For example, high temperatures and lack of rainfall in the Alto Rio Negro region, in the west of Amazonas, have changed the centuries-old practices of working in the fields, putting the food security of entire communities at risk (https://www.bbc.com/portuguese/articles/c6pjn19jw9xo). The intensity and frequency of extratropical cyclones have caused floods with devastating consequences for agricultural production in southern Brazil (https://www.bbc.com/portuguese/articles/c2qlypx3k1wo). The planet is getting warmer, consequently so are the ocean waters, and El Niño is evolving. This combination is causing the atmospheric conditions that make meteorological events more intense.
Traditional family-based agriculture is more vulnerable to climate extremes. This, in turn, leads to greater poverty levels, food inflation and unemployment, posing serious risks to food and nutritional security, especially where agriculture is the most relevant economic activity [3]. In Brazil, 33 million people (15% of the population) is considered to be in serious food and nutritional insecurity in 2022, the majority of them living in rural areas [4]. The vulnerability is even more dramatic as traditional knowledge based on experience and on the observation of nature has been put to the test by ongoing climate change. Adapting traditional crops to unexpected impacts is an important achievement.
The frequency and severity of climate events increased plant performance constraints, reducing crop productivity and quality [5]. Nowadays, abiotic stresses in plants are the leading cause of severe yield losses ranging between 50 and 80%, depending on the crop and geographical location [6]. The intensification of agricultural production is expected to increase productivity in affected areas [7]. However, agricultural intensification is among the main drivers of climate change and soil contamination, making food production both the culprit and the victim [8]. Innovative technologies are essential to reduce agricultural pressure on the environment by decreasing water use and chemical inputs, while maintaining productivity [9]. The debate around sustainable food production should include appropriate goals to favor an agroecological transition. Biological inputs play a prominent role in this context since they promote plant growth, improve crop productivity and potentially reduce damages from climate change, allowing reduced employment of agrochemicals and of products from non-renewable origins.
Plant biostimulants (PB) can be considered an environmentally friendly agronomic tool to enhance abiotic stress tolerance, address environmental concerns, and fulfil the need to develop sustainable agriculture [10]. Humic substances are one of the most used PB. They are part of a market that grows 10% per year, which is expected to make US$ 4.14 billion by 2025 (https://www.grandviewresearch.com/press-release/global-biostimulants-market). Traditional knowledge about organic matter management allowed us to evolve from gatherers and hunters to drivers of social development [11]. In this regard, the use of humic substances (HS), a fraction of soil organic matter and organic residues, can enhance production, and this effect is significantly higher under stress conditions regardless of the sources of extraction [12, 13]. Humic products (HP) from renewable sources (e.g., raw materials from agro-industrial processing or animal husbandry) are highly bioactive and can be used to substitute commercial products based on coal, lignite or peat [14, 15]. Aspects related to the use of HPs to mitigate the effects of stress on plants will be addressed in this review.
Abiotic stress and humic substances
Under natural conditions, plants usually face different environmental stresses, such as drought, salinity, extreme temperatures, heavy metals, and UV radiation [5]. Previous studies have demonstrated the promising potential of HPs, as well as their functions and possible challenges to mitigate different abiotic stresses and improve quality and yield [10, 16, 17]. Table 1 summarizes the scientific reports found using the terms “humic”, “plant”, “heavy metals”, “salinity”, “drought”, “extreme temperature = heat/cold”, and “UV radiation”, published in the last 6 years (2018–2023). For this purpose, three data bases (Web of Science, Google Scholar and Scopus) were employed. The purpose of this bibliographic review was not to thoroughly explore the available databases but rather to provide a few examples on how HP can contribute to reduce abiotic stress damages.
General damage promoted by abiotic stress and general plant response
In general terms, all forms of biotic and abiotic stress promote the generation of reactive oxygen species (ROS). The production of ROS in cells under normal growth conditions is low (240 µM s−1 O2− and a steady-state level of 0.5 µM H2O2 in chloroplasts), but different stresses including drought, salinity, high CO2 concentration, heavy metals and nutrient deficiency can disrupt the cellular homeostasis, enhancing the production of ROS (240–720 µM s−1 O2− and a steady-state level of 5–15 µM H2O2) [63]. The cellular response against the resulting oxidative stress is critical for plant growth and adaptation [64]. Oxidative stress generally occurs when the balance between the production of ROS and the quenching activity is upset by a stressful event. If the stress level reaches a threshold, the excessive accumulation of ROS will trigger gene response, including programmed cell suicide pathways [65]. Stress-induced ROS production is part of a multilayered reduction/oxidation (redox) response system in which stress sensing and adaptation are synchronized with plant metabolism and phytohormone pathways [64]. Moreover, any physical, chemical, or metabolic shock activates the plasma-membrane-bound NADPH oxidases and apoplastic peroxidases, leading to an oxidative burst. A complex anti-oxidant network has evolved in plant cells to scavenge ROS and regulate their levels according to the requirements of cell signaling. The main mechanism of stress tolerance modulated direct or indirectly by HS are shown in Box 1. Certain aspects of the following topics are discussed.
Box 1. Some of the general abiotic stress tolerance mechanisms induce by humic substances -Activation of signaling factors; -Gene response induction; -Accumulation of osmoprotective and anti-oxidant compounds; -Induction of antioxidative metabolism; -Ion homeostasis and membrane transport; -Adjustment of hormonal balance |
Activation of signaling factors under stress: three masters cell messages are influenced by humic substances
Stress perception involves cell receptor activation, and the amplification of signal recruits second messengers, so-called because they represent intracellular signals being translated from the primary external signal. Other signaling components interpret these intracellular messengers further, resulting in the activation of downstream pathways that may have multiple outputs. The protein phosphorylation pathway activates transcription factors (TF), inducing the expression of stress-responsive genes [66]. Several intracellular signaling molecules are involved in stress signal transduction. Reactive oxygen species, cytosolic Ca2+ concentrations, cell pH changes and phytohormones are among them. HS interact with many of these messengers, indicating their involvement in a regulation process that we do not entirely understand.
ROS play important signaling roles in the early stages of the stress response, activating stress-responsive genes that encode enzymes in anti-oxidant biosynthesis or enzymes that directly detoxify reactive oxidative radicals, mitigating stress damage [67]. Reactive oxygen species are demonstrated to regenerate Ca2+ signals to short and long distances by coordinating Ca2+and ROS signals via Ca2+-dependent phosphorylation of NADPH oxidases [68]. Upon exposure to various environmental stimuli, the cytosolic Ca2+ concentration [(Ca2+)cyt] increases rapidly, reaching micromolar levels; the transient influx of Ca2+ generates unique signatures that initiate cellular responses to diverse developmental cues and environmental challenges [69]. Because high (Ca2+)cyt is cytotoxic, (Ca2+)cyt is recovered within a range of 50–200 μM by Ca2+-ATPases and H+/Ca2+−antiporters [73] Lee and Seo. Then, Ca2+ is sequestered into vacuoles through an H+/Ca2+ antiport system driven by the proton-motive force of the tonoplast H+-translocating ATPase [70]. Felle [71] showed that pH also could act as a signal and/or a messenger of abiotic (changes in light intensity, drought, lack of oxygen) and biotic (presence of symbiotic partners or microbial attackers) factors. As a stress signal, pH involves transmembrane Ca2+/pH interaction as a general principle of cellular signaling following the first encounter with defense-related substances. Perception of stress receptor activates G-protein, inducing Ca2+ influx, elevating cytosolic Ca2+ activity with the following consequences described by Felle [71]: (1) Ca2+ activates anion channels–a fraction of the anions that leave the cells and depolarize the plasma membrane are organic acids, which bind protons and thus alkalize the apoplast, leading to other transporters modulation; (2) elevated cytosolic Ca2+ activates an NADH oxidase, contributing to cytosolic acidification and external alkalization; and (3) elevated cytosolic Ca2+ and decreased pH are involved in gene activation.
The multiple messengers act simultaneously to activate stress signaling, and the most studied ones (ROS, Ca2+ and pH) can be influenced by the exogenous application of HS. Previous works have shown that HS can induce stress alleviation, promoting ROS accumulation and metabolism [16, 74, 75]. Applying HA extracted from vermicompost to rice seedlings increased the concentration of both H2O2 and O2− in specific root zones, accompanied by increased activity and gene expression of the main enzymes involved in ROS metabolism [74]. Rice root seedlings exposed to humic acids (HA) showed a clear peak of Ca2+ influx in the same root zone of H+ efflux coupled with very large anion exudation [76], unveiling evidence that HS influence H+-Ca2+ cell signaling. Phosphokinase Ca2+ dependent activity was monitored using differential gene expression, while voltage gate Ca2+ channels were also overexpressed in the presence of HP [76]. Moreover, Zandonadi et al. [77] showed that HA induce a concerted plasmalemma and tonoplast H+ pumps activation in a typical control of cell electric environment mediated by phytohormones. Direct evidence of cytoplasmatic pH changes induced by HP was obtained by Baia et al. [78] using a specific cytosolic pH (pHcyt) dye. The pHcyt was changed as a typical short chain organic acid stress triggered by HA.
The immediate consequence of stress signal transduction by secondary cell messengers is the activation of TF for stress gene response codification. The main TF related to abiotic stress response can be oversimplified into two categories: (1) Abscisic acid (ABA)-dependent including myeloblastosis oncogene (MYB) and myocytomatosis oncogene (MYC) regulon, ABA-responsive element binding protein (AREB) and ABA-binding factor (ABF) and (2) ABA-independent TF including NAC (NAM, ATAF1/2, and CUC2) family a plant-specific transcription factor involved in multiple abiotic-stress responses and zinc-finger homeodomain (ZFHD) regulon [79]. All these TF were induced in the maize seedlings treated with HA isolated from vermicompost [80]. Therefore, plants treated with HA showed high-stress response genes (drought, salinity, extreme temperature, heavy metals and pathogen response) transcription even without these stressors [62]. We do not know exactly how this occurs, but it is possible to offer certain explanations. Perhaps the most convincing one, because it is general and integrative, is the fact that HS can emulate the action of various plant hormones [81, 82], working as a key regulatory hub in plant responses integrating hormonal signaling and stress response pathways [80]. The following section provides examples of plant stress responses modified by HS.
Induction of low-molecular-weight anti-oxidant metabolites by humic substances
Plants accumulate large amounts of low-molecular-weight, anti-oxidant metabolites, such as ascorbate, glutathione, and tocopherol, and they have an extensive network of enzymatic anti-oxidants, such as superoxide dismutases, ascorbate peroxidases (APX), catalases, glutaredoxins (GRXs) and peroxiredoxins [83]. The evolution of this complex oxidant (ROS)/anti-oxidant network allows flexible control of cellular ROS levels. Tocopherol detoxifies ROS produced during oxidative stresses and its biosynthesis take place through different pathways: the methylerythritol 4-phosphate, the shikimate (SK) and tocopherol-core pathways that are regulated by different enzymes [84]. The SK pathway is significantly modulated by HP, as demonstrated by Schiavon et al. [85]. Plants treated with HP showed high content of tocopherol and ascorbate [86, 87].
Lipid peroxidation is one of the most explored outcomes of ROS on membrane structure and function, and anti-oxidant metabolites protect the membrane by inhibiting lipids peroxidation [88]. Ascorbate (AsA) plays a vital role in stress physiology, especially in protecting lipids peroxidation [89]. It was observed that total AsA content was higher in plants treated with HA [57]. Ascorbate also maintains the membrane-bound anti-oxidant α-tocopherol in the reduced state [90]. The non-protein, water-soluble and low molecular weight tripeptide thiol glutathione (GSH; α-glutamyl cysteinyl glycine) plays a pivotal role in minimizing cellular dysfunction arising through stress-induced redox perturbation. Successive oxidation and reduction of Asa, glutathione and NADPH would enhance the potential scavenging of H2O2 generated through photooxidative stress in the chloroplast. These reactions are collectively called the ascorbate–glutathione cycle [91]. Increased activation of superoxide dismutase (SOD), catalase (CAT) and NADPH-oxidase (NOX) enzymes and ascorbate, glutathione (GSH) and GSH/GSSG ratio (The ratio of reduced GSH to oxidized GSH: GSSG) was observed in the presence of HA under Cd stress [57].
Promotion of compatible solutes biosynthesis
The availability of water for its biological roles as a solvent and a transport medium, an electron donor in the Hill reaction, and an evaporative coolant, is often impaired by environmental conditions. Although plant species display varying degrees of sensitivity to reduced soil water potential, low temperature or high salinity, it is assumed that all plants, at some level, have encoded capability for stress perception, signaling and response [92]. Osmoprotectants or compatible solutes are small molecules that act as osmolytes and help organisms survive extreme osmotic stress [93]. The main compatible solutes induced by stress include proline, citrulline, glycine betaine, 3-dimethylsulfoniopropionate, monosaccharide (fructose), sugar alcohols (mannitol and pinitol), and di- and oligo-saccharides (sucrose, trehalose and fructan) [94]. Metabolic acclimation via the accumulation of compatible solutes is often regarded as a primary strategy for the protection and survival of plants under abiotic stress [94]. Compatible solutes contribute to stress tolerance by acting as osmoregulators, since their high solubility in water substitutes for water molecules released from leaves. In some cases, compatible solutes act as active oxygen scavengers or thermostabilizers [95]. The reports showing proline accumulation in plants treated with HS are abundant and mainly related to drought and salinity [96,97,98,99,100,101,102,103,104,105]. Additional changes that HS promotes on the carbohydrate profile include the production of non-reducing sugar trehalose [86], whose concentration increases under abiotic or biotic stress, acting in the cell osmoregulation [106] Hassan et al. Aguiar et al. [103] also observed changes in carbohydrate profiles in sugarcane treated with HA after drought stress using a metabolomic approach. Three compounds linked to ascorbate metabolism–catabolism (vitamin C), threonic, isothreonic and oxalic acids, were also observed in greater concentrations in both maize and sugarcane leaves treated with HA in the presence of plant-growth-promoting bacteria [87]. The pathways by which ascorbate is catabolized to form oxalic, threonic and isothreonic acids have been previously reported, as well as their roles in many aspects of redox control and anti-oxidant activities in plant cells [104].
Activation of enzymatic anti-oxidant metabolism
Superoxide is scavenged via the disproportionation reaction catalyzed by SOD that produces hydrogen peroxide. Three major types of SOD differ mainly in their prosthetic metals: Cu/Zn, Mn, and Fe. Plants usually have a Cu/ZnSOD in the cytosol, an MnSOD in the mitochondria, and Cu/Zn and/or FeSOD in the chloroplast [105].
Hydrogen peroxide, for the most part, is scavenged by either CAT (H2O2 + H2O2 → O2 + 2H2O) or peroxidase (H2O2 + AS2 → 2H2O + AS). The peroxidative mechanism generally requires a reductant in chloroplasts, and the cytoplasm is ascorbate. The APX is part of the ascorbate–glutathione cycle, which involves successive enzymatic oxidations and reductions of Asa, glutathione and NADP. Enzymatic anti-oxidants, such as SOD, CAT and glutathione peroxidase (GPX) are designed to minimize the concentration of H2O2 and superoxide and induced by different stress [106].
Cordeiro et al. [72], using a specific fluorescent dye for cell H2O2 detection, observed that root maize seedlings treated with HA increased their ROS content, stimulating gene expression of CAT, thus resulting in increased activity of this enzyme and minimizing the oxidative effect of ROS. The induction of ROS production and the consequent enhancement of enzymatic anti-oxidant metabolism by HA were further observed by García et al. [16]. Aguiar et al. [103] submitted sugarcane to water restriction and, immediately after, observed during the rehydration period that the activity of anti-oxidant enzymes CAT, SOD and APX remained higher in leaf and root tissues of HA-treated plants, when compared to the control plants. Humic acids can protect lipids from peroxidation, one of the most dangerous ROS cell effects, by activating an anti-oxidant enzyme protection system [75]. Under salt stress conditions, applying HA increased antioxidant enzyme activities. Foliar and soil + foliar application increased SOD and glutathione reductase (GR) activities, and decreased CAT and APX activities in beans [107].
Ion homeostasis and membrane transport
The intracellular ion homeostasis disruption causes oxidative stress in plants. Maintaining ion homeostasis requires an electrophysiological adjustment that can be sustained by plasma membrane (PM) proton pump stimulation. Figure 1 summarizes the ion homeostasis and the modifications on membrane transport induced by HA, shown by Souza et al. [108]. Activation of PM H+-ATPase by HP improves the electrochemical proton gradient that drives ion transport across cell membranes [109], modulating the cellular electrical environment and ion fluxes [76, 110]. According to Khaleda et al. [111], Na+ can be removed from cytosol by efflux systems, such as Na+/H+ antiporters, which transport Na+ across the PM and the Salt-Overly Sensitive (SOS) pathway. Na+ that enters the root cell and is transported to leaf tissue must be compartmentalized in the vacuole to avoid cytosolic accumulation. This process is mediated by the vacuolar Na+/H+ antiporter, NHX, which moves Na+ into the vacuole in exchange for H+.
NaCl stress-signaling pathways and interactions with humic acids. Enzymes or transcripts in cyan were evaluated by Souza et al. The influence of HA on V-ATPase and H-PPase was described by Zandonadi et al. [77] and Ca2 + (cyt) pulse, voltage gate Ca2 + channel and Ca2 + -dependent protein kinases (CDPK) activity by Ramos et al. [76]. The HKT1 transporter was evaluated by Khaleda et al. [111], and TOR expression by Trevisan et al. [112] and Canellas et al. [113]. Figure 1 was adapted from reference 117
High-affinity potassium transporter (HKT) family members recover Na+ from the xylem to reduce its transport or accumulation in the shoot. It has also been reported that HA promoted the activity of HKT1 transporters helping Arabidopsis to survive salt effects [111]. However, a large part of the machinery responsible for ion homeostasis is recruited by the HA treatment even without the absence of Na+ at toxic levels [108].
Hormonal balance
The ROS production and signaling are integrated with the action of auxin (AUX), brassinosteroids (BRA), gibberellins (GIB), ABA, ethylene (ET), strigolactones (SLS), salicylic acid (SA) and jasmonic acid (JA) in the coordinate regulation of plant growth and stress tolerance [114]. The multiple points of reciprocal control and integration nodes involve Ca2+-dependent processes and mitogen-activated protein kinase phosphorylation cascade [114]. Plant hormones are vital in linking gene transcription to stress response. Humic substance’s hormone-like activity has been documented in great detail [81]. Molecules released from humic superstructures may then access cell membranes and induce different physiological responses, such as hormone auxins AUX, GIB, CK (cytokinins), alkamides (ALK), nitric oxide (NO), ABA and ET [115,116,117,118,119,120,121,122,123]. However, it is possible that, in addition to the presence of chemical homologues to plant hormones present in the more than 10,000 molecules in the humic supramolecules, the exogenous application of HS changes the plant hormonal balance, by acting as a key regulatory hub in plant responses, integrating hormonal signaling and response pathways.
Stress-induced changes in growth are regulated by phytohormones, such as ABA and ET, which control ROS production and function through synergistic or antagonistic interactions [64]. Despite the relatively large number of research reporting the hormonal effect of HP, there is a gap between the analysis of the hormone balance induced by HP and the anti-stress response. For example, AUXs, CKs, Gas, ET, ABA and SLS balance regulate the P starvation response, one of the most limiting nutritional stresses in highly weathered soils. Their interaction with HP was mentioned by Jindo et al. [124]. Olaetxea et al. [125] found significant increases in ABA root concentration in seedlings treated with HA, showing that the HA-mediated enhancement of root hydraulic conductivity and shoot growth depended on ABA signaling pathways. The AUX-like effect is HS's most well-known phytohormonal behavior. It has been examined in the literature for over half a century [126], indicating that abiotic stress can alter AUX metabolism. According to Potters et al. [127], stress can impact various aspects of auxin homeostasis, including AUX redistribution via effects on the expression of PIN genes, which mediate polar auxin transport. Abiotic stresses can also impede AUX transport by altering the pH in the plant apoplast or by altering the concentrations of phenolics, such as quercetin and kaempferol, which can act as endogenous inhibitors of auxin transport. A higher transcriptional level of PIN genes was found in plants treated with HA [110].
Concluding remarks
Stresses in plants caused by salt, drought, temperature and toxic compounds are the reason behind reduced crop yields. Plants respond to these abiotic stresses partly by activating the expression of stress-responsive genes, increasing tolerance. Based on the literature, it is clear that HS may contribute to plant adaptation to abiotic stresses. How can this happen? We still do not fully understand how this occurs, but the results from research carried out in recent years, it is possible to see clarity in the middle of the fog. We can simplify the responses of plants to HS and abiotic stresses in a typical physiological response that includes increased generation of ROS (1), the promotion of proton pump activities (2) and changes in plant hormonal balance (3). All of these master variables can act as cell signals that induce other secondary messengers, such as changes on (Ca2+)cyt (4), promoting a downstream phosphorylation cascade triggered by Ca2+-dependent protein kinases (CDPK) (5), resulting in gene response by activation of TF (6). These steps may be modified by applying HS (Fig. 2).
Humic products can act as elicitors and induce: (1) the production of reactive oxygen in species [16, 72, 74, 75]; (2) activity of proton pumps [128,129,130,131,132,133,134,135] and (3) hormonal activities [80, 81]. The first signals are amplified by secondary messengers, with the calcium pulse in the cytoplasm [4] being among the primary ones [76], triggering a cascade response [5], the most common of which being phosphorylation of proteins that activate transcription factors (TF, 6)
When it comes to the general response of the plant to stress and the involvement of HS in these adaptations, the primary focus is the maintenance of cellular homeostasis, more specifically, the redox balance in the case of different abiotic stresses that have the generation of ROS in common. Regarding this balance, Lamar [136] draws attention to an interesting aspect:
“HS possess pro-oxidants (i.e., quinone moieties), in addition to anti-oxidants (phenolic hydroxyls) within their chemical structures, which allow them to take part in redox reactions. Thus, the ability of HS to enhance plant growth may, in part, be redox-based and be influenced by the ratio of pro- to anti-oxidants in the HS chemical structure. Pro-oxidant moieties could be involved in ROS production leading to apoplastic oxidative bursts. In contrast, the anti-oxidant moieties (i.e., polyphenolics) could moderate the oxidative burst in addition to the plant upregulating its anti-oxidant system to counterbalance the overproduction of ROS and re-establish redox homeostasis, protection of membranes, proteins and nucleic acids resulting in stress tolerance or enhanced growth and productivity”.
The determination of the mode of action and the efficiency of HS as a plant-growth promoter is rendered difficult due to the complex nature of their components. The HS have multiple biological activities, affecting various metabolic processes simultaneously. The chemical nature of HS was unveiled by Piccolo and collaborators, describing it as the supramolecular association of thousands of molecules held together by predominantly weak and hydrophobic forces. The total dissection of humic composition was assessed by the sequential chemical fractionation proposed by Nebbioso and Piccolo [137], named as “humeomic”. If the inventory of each molecular component allows detailed knowledge about the composition, the biological activity seems to result from the interaction between them in a solution. In a seminal work [138], Piccolo indicates that the reactivity of HS in the environment is a product of the acid functional groups (OH and COOH) and of the balance between the amount of hydrophilic and hydrophobic components. Spectroscopic methods such as nuclear magnetic resonance and infrared can obtain these characteristics. The lateral root induction and activity of PM H+-ATPase were significantly corelated with the hydrophobic/hydrophilic carbon ratio [139, 140]. In addition, the progressive removal of the humic components using sequential chemical fractionation showed that, when strongly bound components were removed by breaking the ester and ether bonds, the humic residues lost their ability to induce the like-auxin activity (DR5::GUS) and lateral root emergence [141].
However, these capacities were retained in the free or weakly bound molecules [141]. These findings confirm that auxin-like activity in HP is associated with complex hydrophobic structures. The control of these processes seems to be regulated by root exudation, which is significantly larger in plants treated with HA [142]. This process is typical of interaction between complex systems, in which the components interact producing new reactions that encourage the release of bioactive compounds to plant use and the thermodynamic stabilization of the suprastructure. Other characteristics can be used to map the relationship between HS and plant traits, such as Lamar's electron shuttling capacity [136]. In this way, the bioactivity of HS can be put in the perspective of Yakhin et al. [143], who defined a biostimulant as a product of biological origin that improves plant productivity as a consequence of the novel or emergent properties of the complex of constituents and not as a sole consequence of the presence of essential plant nutrients, plant growth regulators, or plant protective compounds. This may be considered odd by those who are used to describing biological mechanisms as machines that achieve specific targets and goals.
The concept of emergence, introduced by the definition of Yakhin et al. [143], was questioned by du Jardin et al. [144], who proposed the following issue:
“What do we know about the underlying mechanisms of action and how relevant are the concepts of emergence and interaction to explain biostimulation of plants? Little experimental evidence is available to address this question”.
According to du Jardin et al. [144]:
“Data should be generated which would demonstrate that a plant biostimulant product is a holistic and unitary system of molecules, i.e. that the biostimulatory effect cannot be reproduced by any possible combination of its constituents unless it reconstitutes the complete mixture”.
Data from research [145] showed that the simplification of chemical complexity did not represent a loss of bioactivity until an indefinable chemical level. In other words, in the 1980s and 1990s, the pharmacological approach using chemical inhibitors did not allow definitive answers; the omics approach from the 2000s shuffled the cards showing a much more holistic response in plants than expected, creating effort to give practical meaning to the paradigm break proposed by Piccolo [138] concerning the conformation of HS in solution. The self-assemble driving force to the supramolecular arrangement of HS is the decrease in the total surface area of small molecules with amphiphilic character exposed to water. This surface decrease releases the water molecules from an energetically unfavorable contact with the hydrophobic part. As a result, a global increase in entropy due to the release of water molecules makes the process thermodynamically favorable.
Spontaneous formation of local order assisted (humic aggregate) by an entropy increase and the formation of more or less spherical compartments in microphases (with internal hydrophobic phase) resemble the dissipative structures originally described by Prigogine [145]. The surfactant behavior of HS promotes the aggregation in water in a spontaneous and initially slow manner, which can become faster over time, since a larger layer on the active surface facilitates the aggregation of newly added components. The humic supra-aggregated in the solution can be considered as dissipative structures and described as islands of order surrounded by a sea of disorder with the maintenance or even an increase of order at the expense of greater disorder in the surrounding environment. These islands can be modified by mass and energy from the external environment, such as the exudation of low molecular-weight organic acids, as shown by Piccolo [138]. Considering these structures as complex chemical systems operating out of equilibrium, their characterization presents an inherent difficulty. Instead of the classic determination of structure (of which a convincing model has never been reached), the objective is to map relationships and to study patterns, that is, to abandon the quantitative approach to qualitative analysis, to change the perspective from structure to process analysis, from interactions with the environment and with the plant.
In this perspective, the characterization of the relationship between hydrophilic and hydrophobic components [139] makes sense for the evaluation of the environmental reactivity of HS, as originally shown by Piccolo [138]. Reactivity can be understood as a result of the organization process resulting from the aggregation of humic matter in a macroscopic set. They are, therefore, not sensitive to microscopic details. It is a collective state with ordered behavior in large samples but imprecise on a small scale. Large samples make it possible to evaluate emerging phenomena; that is, it is at the macro level that the principles of the supramolecular organization gain relevance; self-organization is a process through which a system comes to exhibit global-scale patterns and structures that emerge from the numerous local interactions between its components. The pattern is an emergent property of the system and not imposed on the system by an outside influence. The constitution of an ordered system through self-organization is understood as a primary tendency of complex systems, in contrast to the former emphasis on the degradation of order associated with the entropy principle. The self-organization of humic matter in supramolecular arrangement requires a review of the humification theory, which has already been done by Piccolo et al. [146, 147].
However, considering the physiological aspects of humic matter and its relevant aspects in mitigating plant abiotic stress damage, many questions can be made. Among them, one could consider the supramolecular arrangement as a set of chemical compounds whose key to accessing the various bioactive compartments would lie with the plant and its system for recognizing the environment. The organization pattern of the humic arrangement, described by the hydrophilic/hydrophobic ratio and the electron shuttling capacity [136], could be used as behavior descriptors of suprahumic properties? A consistent answer is not available at the time, but the HS remains as a tool used by many farmers to mitigate plant stress.
Availability of data and materials
Not applicable.
Abbreviations
- NAC:
-
NAM, ATAF1,2 and CUC2
- MYB:
-
MYB proto-oncogene
- MYC:
-
Proto-oncogene
- bZIP:
-
Basic leucine zipper
- WRKY:
-
WRKY family
- AP2/ERF:
-
APETALA2/Ethylene-responsive element-binding protein family
- B3:
-
B3 domain-containing proteins
- ARR:
-
Two-component response regulator
- REMORIN:
-
Remorin family protein
- AUX/indole-3-acetic acid:
-
Auxin/indole-3-acetic acid protein family
- ARF:
-
Auxin Response Factor family
- 12) SUVR5:
-
Histone–lysine N–methyltransferase
- bHLH:
-
Basic Helix-Loop-Helix family
- HDAC:
-
Histone deacetylase
- PPR:
-
Putative pentatricopeptide repeat-containing proteins
- F-box:
-
F-box protein
- LSD:
-
Lysine-specific demethylase
- PLATZ:
-
Plant AT-rich sequence and zinc-binding protein
- CAMTA:
-
Calmodulin-binding transcription activators
- Ring-type E3:
-
RING-type E3 ubiquitin transferase
- PAT:
-
S-Acyltransferase; 22)
- GRAS:
-
GRAS transcription factor family (scarecrow)
- CRF:
-
Chromatin remodeling factors
- AGO:
-
Argonaute
- GTE:
-
Transcription factor group E
- MTREF:
-
Mitochondrial transcription termination factor family protein
- G-box:
-
G-box protein
- MOB:
-
MOB kinase activator-like
- UBX2:
-
Ubiquitin regulatory X domain-containing protein 2
- SWI/SNF:
-
SWItch/sucrose non-fermentable
- EIN3:
-
EIN3-like (EIL) transcription factor family
- FREE1:
-
Free domain protein required for endosomal sorting 1
- TCP:
-
TCP protein domain
- LOB:
-
LOB domain-containing protein
- Alba:
-
Alba DNA/RNA-binding protein
- SEUSS:
-
Transcriptional corepressor SEUSS
- C3H Type:
-
C3H-type transcription factor
- GRFs:
-
GRF transcription factor
- ABH:
-
Alpha/beta hydrolase
- CtBP:
-
C-terminal-binding protein
- ALF:
-
Alfin-like transcription factor; 40)
- HB:
-
Homeobox transcription factor family
References
IFAD-International Fund for Agricultural Development. Smallholders, food security and the environment. 2013. https://www.ifad.org/documents/38714170/39135645/smallholders_report.pdf/>. Accessed 20 Sep 2023.
Soubry B, Sherren K, Thornton T. Are we taking farmers seriously? a review of the literature on farmer perceptions and climate change, 2007–2018. J Rural Stud. 2020;74:210–22. https://doi.org/10.1016/j.jrurstud.2019.09.005.
IPCC—Intergovernmental Panel on Climate Change. Climate change 2014 synthesis report, 2014. Working Groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva: IPCC; 2014. p. 151.
Rede PENSSAN II Inquérito nacional sobre insegurança alimentar no contexto da pandemia da covid-19 no Brasil. 2022. https://olheparaafome.com.br. Accessed 29 Dec 2023.
Zandalinas SI, Fritschi FB, Mittler R. Global warming, climate change, and environmental pollution: recipe for a multifactorial stress combination disaster. Trend Plant Sci. 2021;26:588–99. https://doi.org/10.1016/j.tplants.2021.02.011.
Zhang H, Li Y, Zhu JK. Developing naturally stress-resistant crops for a sustainable agriculture. Nat Plant. 2018;4:989–96. https://doi.org/10.1038/s41477-018-0309-4.
Keating BA, Carberry PS, Bindraban PS, Asseng S, Meinke H, Dixon J. Eco-efficient agriculture: concepts, challenges, and opportunities. Crop Sci. 2010;50:109–19. https://doi.org/10.2135/cropsci2009.10.0594.
Struik PC, Kuyper TW, Brussaard L, Leeuwis C. Deconstructing and unpacking scientific controversies in intensification and sustainability: why the tensions in concepts and values? Curr Op Environ Sust. 2014;8:80–8. https://doi.org/10.1016/j.cosust.2014.10.002.
De Schutter O. Report submitted by the special rapporteur on the right to food. United Nations General Assembly. 2010. http://www.srfood.org/images/stories/pdf/officialreports/20110308. Accessed 26 June 2023.
Ma Y, Freitas H, Dias MC. Strategies and prospects for biostimulants to alleviate abiotic stress in plants. Front Plant Sci. 2022;13:1024243. https://doi.org/10.3389/fpls.2022.102424.
Zong Y, Chen Z, Innes JB, Chen C, Wang Z, Wang H. Fire and flood management of coastal swamp enabled first rice paddy cultivation in east China. Nature. 2007;449:459–62. https://doi.org/10.1038/nature06135.
Rose MT, Patti AF, Little KR, Brown AL, Jackson WR, Cavagnaro TR. A meta-analysis and review of plant-growth response to humic substances: practical implications for agriculture. Adv Agron. 2014;124:37–89. https://doi.org/10.1016/B978-0-12-800138-7.00002-4.
Li J, van Gerrewey T, Geelen D. A meta-analysis of biostimulant yield effectiveness in field trials. Front Plant Sci. 2022;13: 836702. https://doi.org/10.3389/fpls.2022.836702.
Zaller JG. Foliar spraying of vermicompost extracts: effects on fruit quality and indications of late-blight suppression of field-grown tomatoes. Biol Agric Hortic. 2006;24:165–80. https://doi.org/10.1080/01448765.2006.9755017.
Arancon NQ, Owens JD, Converse C. The effects of vermicompost tea on the growth and yield of lettuce and tomato in a non-circulating hydroponics system. J Plant Nut. 2019;42:2447–58. https://doi.org/10.1080/01904167.2019.1655049.
García AC, Santos LA, Izquierdo FG, Sperandio MVL, Castro RN, Berbara RLL. Vermicompost humic acids as an ecological pathway to protect rice plant against oxidative stress. Ecol Eng. 2012;47:203–8. https://doi.org/10.1016/j.ecoleng.2012.06.011.
van Oosten MJ, Pepe O, de Pascale S, Siletti S. Maggio a the role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem Biol Technol Agric. 2017;4:5. https://doi.org/10.1186/s40538-017-0089-5.
Ramadan KMA, El-Beltagi HS, El-Mageed TAA, Saudy HS, Al-Otaibi HH, Mahmoud MAA. The changes in various physio-biochemical parameters and yield traits of Faba bean due to humic acid plus 6-benzylaminopurine application under deficit irrigation. Agronomy. 2023;13:1227. https://doi.org/10.3390/agronomy13051227.
El-Hashash EF, Abou El-Enin MM, Abd El-Mageed TA, Attia MAE-H, El-Saadony MT, El-Tarabily KA, Shaaban A. Bread wheat productivity in response to humic acid supply and supplementary irrigation mode in three northwestern coastal sites of Egypt. Agronomy. 2022;12:1499. https://doi.org/10.3390/agronomy12071499.
Makhlouf BSI, KhalilSaudy SRAEHS. Efficacy of humic acids and chitosan for enhancing yield and sugar quality of sugar beet under moderate and severe drought. J Soil Sci Plant Nutr. 2022;22:1676–91. https://doi.org/10.1007/s42729-022-00762-7.
Matuszak-Slamani R, Bejger R, Włodarczyk M, Kulpa D, Sienkiewicz M, Gołębiowska D, Skórska E, Ukalska-Jaruga A. Effect of humic acids on soybean seedling growth under polyethylene-glycol-6000-induced drought stress. Agronomy. 2022;12:1109. https://doi.org/10.3390/agronomy12051109.
Chen Q, Qu Z, Ma G, Wang W, Dai J, Zhang M, Wei Z, Liu Z. Humic acid modulates growth, photosynthesis, hormone and osmolytes system of maize under drought conditions. Agric Water Manag. 2022;263: 107447. https://doi.org/10.1016/j.agwat.2021.107447.
Pačuta V, Rašovský M, Michalska-Klimczak B, Wyszyňski Z. Grain yield and quality traits of durum wheat (Triticum durum Desf.) treated with seaweed- and humic acid-based biostimulants. Agronomy. 2021;11:1270. https://doi.org/10.3390/agronomy11071270.
Forotaghe Z, Souri M, Jahromi M, Torkashvand A. Physiological and biochemical responses of onion plants to deficit irrigation and humic acid application. Open Agricult. 2021;6:728–37. https://doi.org/10.1515/opag-2021-0050.
Man-hong Y, Lei Z, Sheng-tao X. Effect of water-soluble humic acid applied to potato foliage on plant growth, photosynthesis characteristics and fresh tuber yield under different water deficits. Sci Rep. 2020;10:7854. https://doi.org/10.1038/s41598-020-63925-5.
Mehdiniyaafra J, Niknejad Y, Amoli H, Tari DB. Effects of drought stress on some phytochemical characteristics of rice cultivars under different chemical and organic nutritional sources. J Plant Nut. 2021;44:1193–206. https://doi.org/10.1080/01904167.2020.1862196.
Shen J, Guo M, Wang Y, Yuan X, Dong S, Song XE. An investigation into the beneficial effects and molecular mechanisms of humic acid on foxtail millet under drought conditions. PLoS ONE. 2020;15: e0234029. https://doi.org/10.1371/journal.pone.0234029.
Qin K, Leskovar DI. Humic substances improve vegetable seedling quality and post-transplant yield performance under stress conditions. Agriculture. 2020;10:254. https://doi.org/10.3390/agriculture10070254.
Khodadadi S, Chegini MA, Soltani A. Influence of foliar-applied humic acid and some key growth regulators on sugar beet (Beta vulgaris L.) under drought stress: anti-oxidant defense system, photosynthetic characteristics and sugar yield. Sugar Tech. 2020;22:765–72. https://doi.org/10.1007/s12355-020-00839-6.
Kıran S, Furtana GB, Talhouni M, Ellialtıoğlu ŞŞ. Drought stress mitigation with humic acid in two Cucumis melo L. genotypes differ in their drought tolerance. Bragantia. 2019;78:490–7. https://doi.org/10.1590/1678-4499.20190057.
Khorasaninejad S, Ahmadabadi AA, Hemmati K. The effect of humic acid on leaf morphophysiological and phytochemical properties of Echinacea purpurea L. under water deficit stress. Sci Hortic. 2018;239:314–23. https://doi.org/10.1016/j.scienta.2018.03.015.
Rekaby SA, Al-Huqail AA, Gebreel M. Compost and humic acid mitigate the salinity stress on quinoa (Chenopodium quinoa Willd L.) and improve some sandy soil properties. J Soil Sci Plant Nutr. 2023;23:2651–61. https://doi.org/10.1007/s42729-023-01221-7.
Abu-Ria M, Shukry W, Abo-Hamed S, Albaqami M, Almuqadam L, Ibraheem F. Humic acid modulates ionic homeostasis, osmolytes content, and anti-oxidant defense to improve salt tolerance in rice. Plants. 2023;12:1834. https://doi.org/10.3390/plants12091834.
Guo Y, Liu H, Gong P, Li P, Tian R, Zhang Y, Xu Y, Xue B. Preliminary studies on how to reduce the effects of salinity. Agronomy. 2022;12:3006. https://doi.org/10.3390/agronomy12123006.
Targino VA, Lopes AS, de Sousa VF O, Henschel JM, da Silva JHB, Rodrigues LS. Crescimento e fisiologia de mudas de mamoeiro ‘Sunrise’ em resposta à salinidade e ácido húmico. Rev Bras Eng Agríc Ambient. 2023;27:352–8. https://doi.org/10.1590/1807-1929/agriambi.v27n5p352-358.
Shukry WM, Abu-Ria ME, Abo-Hamed SA. The efficiency of humic acid for improving salinity tolerance in salt-sensitive rice (Oryza sativa): growth responses and physiological mechanisms. Gesunde Pflanzen. 2023;1:15. https://doi.org/10.1007/s10343-023-00885-6.
Yang F, Yuan Y, Liu Q, Zhang X, Gai S, Jin Y, Cheng K. Artificial humic acid promotes growth of maize seedling under alkali conditions. Environ Pollut. 2023;327: 121588. https://doi.org/10.1016/j.envpol.2023.121588.
Huang R. The effect of humic acid on the desalinisation of coastal clayey saline soil. W Suppl. 2022;22:7242–55. https://doi.org/10.2166/ws.2022.311.
Dias TJ, da Silva Leal MP, do Nascimento ES, Veras MLM, Silva TI, Lopes AS. Morphological and physiological changes in papaya seedlings irrigated with saline water and application of humic substances. Com Sci. 2020;4(11):3290. https://doi.org/10.1429/cs.v11i0.3290.
El-Kady AFY, Borham TI. Sustainable cultivation under saline irrigation water: alleviating salinity stress using different management treatments on Terminalia arjuna (Roxb). Agric Water Manage. 2020;229:105902. https://doi.org/10.1016/j.agwat.2019.105902.
Li B, Zhang T, Zhang Q, Zhu Q, Huang D, Zhu H, Xu C, Su S, Zeng X. Influence of straw-derived humic acid-like substance on the availability of Cd/As in paddy soil and their accumulation in rice grain. Chemosphere. 2022;300: 134368. https://doi.org/10.1016/j.chemosphere.2022.134368.
Turhan A, Aşık BB, Kuşçu H. The influence of irrigation water salinity and humic acid on nutrient contents of onion (Allium cepa L.). J Agric Sci. 2020;26:147–53. https://doi.org/10.15832/ankutbd.459907.
Shalaby OS, EL-Messairy MM. Humic acid and boron treatment to mitigate salt stress on the melon plant. AAS. 2018;111:349–56. https://doi.org/10.14720/aas.2018.111.2.10.
Cha JY, Kang SH, Ali I. Humic acid enhances heat stress tolerance via transcriptional activation of heat-shock proteins in arabidopsis. Sci Rep. 2020;10:15042. https://doi.org/10.1038/s41598-020-71701-8.
Han L, Zhao Z, Li J, Ma X, Zheng X, Yue H, Sun G, Lin Z, Guan S. Application of humic acid and hydroxyapatite in Cd-contaminated alkaline maize cropland: a field trial. Sci Total Environ. 2023;859: 160315. https://doi.org/10.1016/j.scitotenv.2022.160315.
Hassanein RA, Hussein OS, Abdelkader AF. Metabolic activities and molecular investigations of the ameliorative impact of some growth biostimulators on chilling-stressed coriander (Coriandrum sativum L.) plant. BMC Plant Biol. 2021;21:361. https://doi.org/10.1186/s12870-021-03021-6.
Huang S, Wang Z, Song Q, Hong J, Jin T, Huang H, Zheng Z. Potential mechanism of humic acid attenuating toxicity of Pb2+ and Cd2+ in Vallisneria natans. Sci Total Environ. 2023;864: 160974. https://doi.org/10.1016/j.scitotenv.2022.160974.
Li S, Huang X, Li G, Zhang K, Bai L, He H, Chen S, Dai J. Effects of mineral-based potassium humate on cadmium accumulation in rice (Oryza sativa L) under three levels of cadmium-contaminated alkaline soils. Sustainability. 2023;15:2836. https://doi.org/10.3390/su15032836.
El-Sayed S, Abdel-Aziz N, Mazhar A. Anti-oxidant isoenzymes, chemical constituents and growth parameters of cadmium-stressed Dimorphotheca ecklonis plant and affected by humic acid. Egypt J Chem. 2022;65:519–32. https://doi.org/10.21608/ejchem.2022.119441.5370.
Dogan M, Bolat I, Karakas S, Dikilitas M, Gutiérrez-Gamboa G, Kaya O. Remediation of cadmium stress in strawberry plants using humic acid and silicon applications. Life. 2022;12:1962. https://doi.org/10.3390/life12121962.
Ran S, He T, Zhou X, Yin D. Effects of fulvic acid and humic acid from different sources on Hg methylation in soil and accumulation in rice. J Environ Sci. 2022;119:93–105. https://doi.org/10.1016/j.jes.2022.02.023.
Li S, Huang X, Li G, Zhang K, Bai L, He H, Chen S, Dai J. Effects of mineral-based potassium humate on cadmium accumulation in rice (Oryza sativa L.) under three levels of cadmium-contaminated alkaline soils. Sustainability. 2023;15:2836. https://doi.org/10.3390/su15032836.
Boysan CS, Bozkurt MA, Yılmaz H. The effect of humic acid on rapeseed (Brassica napus L.) plant growth, heavy metal uptake, phytoremediation parameters (BCF, TF and TI), and anti-oxidant activity in heavy metal-polluted soil. Yuzuncu Yıl Univ J Agric Sci. 2022;32:237–48. https://doi.org/10.2913/yyutbd.997850.
Yildirim E, Ekinci M. Turan M Humic + Fulvic acid mitigated Cd adverse effects on plant growth, physiology and biochemical properties of garden cress. Sci Rep. 2020;11:8040. https://doi.org/10.1038/s41598-021-86991-9.
Duan D, Tong J, Xu Q, Dai L, Ye J, Wu H, Xu C, Shi J. Regulation mechanisms of humic acid on Pb stress in tea plant (Camellia sinensis L.). Environ Pollut. 2020;267:115546. https://doi.org/10.1016/j.envpol.2020.115546.
Evren Y, Ceyda OK, Fevzi E, Aysegul Y, Mustafa K. Humic acid protects against oxidative damage induced by cadmium toxicity in wheat (Triticum aestivum) roots through water management and the anti-oxidant defence system. Bot Serbica. 2019;4:161–73. https://doi.org/10.2298/botserb1902161y.
Ozfidan-Konakci C, Yildiztugay E, Bahtiyar M, Kucukoduk M. The humic acid-induced changes in the water status, chlorophyll fluorescence and anti-oxidant defense systems of wheat leaves with cadmium stress. Ecotoxicol Environ Saf. 2018;155:66–75. https://doi.org/10.1016/j.ecoenv.2018.02.071.
Dobbss LB, dos Santos TC, Pittarello M, de Souza SB, Ramos AC, Busato JG. Alleviation of iron toxicity in Schinus terebinthifolius raddi (anacardiaceae) by humic substances. Environ Sci Pollut Res Int. 2018;25:9416–25. https://doi.org/10.1007/s11356-018-1193-1.
Sergiev I, Todorova D, Katerova Z. Polyamines and amino acids in triticale plants grown on humic acids enriched nutrient solution and treated with UV-B irradiation. Theor Exp Plant Physiol. 2018;30:153–63. https://doi.org/10.1007/s40626-018-0110-9.
Khan MA, Asaf S, Khan AL, Jan R, Kang SM, Kim KM. Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE. 2020;15(4): e0232228. https://doi.org/10.1371/journal.pone.0232228.
Alsamadany H. Physiological, biochemical and molecular evaluation of mungbean genotypes for agronomical yield under drought and salinity stresses in the presence of humic acid. Saudi J Biol Sci. 2022;29: 103385. https://doi.org/10.1016/j.sjbs.2022.103385.
Canellas LP, Canellas NOA, Irineu LES, Olivares FL, Piccolo A. A Plant chemical priming by humic acids. Chem Biol Technol Agr. 2020;7:12. https://doi.org/10.1186/s40538-020-00178-4.
Mittler R. Oxidative stress, anti-oxidants and stress tolerance. Trend Plant Sci. 2022;7:405–10. https://doi.org/10.1016/s1360-1385(02)02312-9.
Considine MJ, Foyer CH. Stress effects on the reactive oxygen species-dependent regulation of plant growth and development. J Exp Bot. 2021;72:05795–806. https://doi.org/10.1093/jxb/erab265.
Foyer CH, Noctor G. Redox homeostasis and anti-oxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–75. https://doi.org/10.1105/tpc.105.033589.
Xiong L, Ishitani M. Stress signal transduction: components, pathways and network integration. In: Rai AK, Takabe T, editors. Abiotic stress tolerance in plants. Dordrecht: Springer; 2006. p. 3–29.
Mittler R, Zandalinas SI, Fichman van BF. Reactive oxygen species signalling in plant stress responses. Nat Rev Mol Cell Biol. 2022;23:663–79. https://doi.org/10.1038/s41580-022-00499-2.
Waszczak C, Carmody M, Kangasjärvi J. Reactive oxygen species in plant signaling. Ann Rev Plant Biol. 2018;69:209–36. https://doi.org/10.1146/annurev-arplant-042817-040322.
Lee HJ, Seo PJ. Ca2+talyzing initial responses to environmental stresses. Trend Plant Sci. 2021;26:849–70. https://doi.org/10.1016/j.tplants.2021.02.007.
Schumaker KS, Sze H. Calcium transport into the vacuole of oat roots characterisation of H+/Ca2+ exchange activity. J Biol Chem. 1986;261:12172–8. https://doi.org/10.1016/S0021-.
Felle HH. pH: signal and messenger in plant cell. Plant Biol. 2001;3:577–91. https://doi.org/10.1055/s-2001-19372.
Cordeiro FC, Santa-Catarina C, Silveira V, de Souza SR. Humic acid effect on catalase activity and the generation of reactive oxygen species in corn (Zea mays L). Biosci Biotechnol Biochem. 2011;75(1):70–4. https://doi.org/10.1271/bbb.100553.
Berbara RL, García AC. Humic substances and plant defense metabolism, physiological mechanisms and adaptation strategies in plants under changing environment. In: Parvaiz A, Mohd RW, editors. Humic substances and plant defense metabolism. New York: Springer; 2014. p. 297–319.
García AC, Santos LA, Ambrósio de Souza LG, Tavares OCH, Zonta E, Gomes ETM, García-Mina JM, Berbara RLL. Vermicompost humic acids modulate the accumulation and metabolism of ROS in rice plants. J Plant Physiol. 2016;192:56–63. https://doi.org/10.1016/j.jplph.2016.01.008.
García AC, Santos LA, Izquierdo FG, Rumjanek VM, Castro RN, dos Santos FS, Souza LGA, Berbara RLL. Potentialities of vermicompost humic acids to alleviate water stress in rice plants (Oryza sativa L.). J Geochem Explor. 2014;136:48–54. https://doi.org/10.1016/j.gexplo.2013.10.005.
Ramos AC, Olivares FL, Silva LS, Aguiar NO, Canellas LP. Humic matter elicits proton and calcium fluxes and signaling dependent on Ca2+-dependent protein kinase (CDPK) at early stages of lateral plant root development. Chem Biol Technol Agric. 2015;2:3. https://doi.org/10.1186/s40538-014-0030-0.
Zandonadi DB, Canellas LP, Façanha AR. Indolacetic and humic acids induce lateral root development through a concerted plasmalemma and tonoplast H+ pumps activation. Planta. 2007;225:1583–95. https://doi.org/10.1007/s00425-006-0454-2.
Baia DC, Olivares FL, Zandonadi DB, de Paula SC, Spaccini R, Canellas LP. Humic acids trigger the weak acids stress response in maize seedlings. Chem Biol Technol Agric. 2020;7:1–13. https://doi.org/10.1186/s40538-020-00193-5.
Lata C. Yadav, Prasad M. Role of plant transcription factors in abiotic stress tolerance. In: Shanker A, Venkateswarlu B, editors. Abiotic stress response in plants—physiological, biochemical and genetic perspectives. London: NTECH Open Access Publishers; 2011. p. 269–96.
Souza AC, Olivares FL, Peres LEP, Piccolo A, Canellas LP. Plant hormone crosstalk mediated by humic acids. Chem Biol Technol Agric. 2022;9:29. https://doi.org/10.1186/s40538-022-00295-2.
Nardi S, Pizzeghello D, Ertani A. Hormone-like activity of the soil organic matter. Appl Soil Ecol. 2018;123:517–20. https://doi.org/10.1016/j.apsoil.2017.04.020.
Olaetxea M, Hita D, García AC, Fuentes M, Baigorri R, Mora V, Garnica M, Urrutia O, Erro J, Zamarreño AM, Berbara RL, Garcia-Mina JM. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root- and shoot growth. Appl Soil Ecol. 2018;123:521–37. https://doi.org/10.1016/j.apsoil.2017.06.007.
Mittler R, Vanderauwera S, Gollery M, van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–8. https://doi.org/10.1016/j.tplants.2004.08.009.
Ali E, Hussain S, Hussain N, Kakar KU, Shah JM, Zaidi SHR, Jan M, Zhang K, Khan MA, Imtiaz M. Tocopherol as plant protector: an overview of tocopherol biosynthesis enzymes and their role as anti-oxidant and signaling molecules. Acta Physiol Plant. 2022;44:1–11. https://doi.org/10.1007/s11738-021-03350-x.
Schiavon M, Pizzeghello D, Muscolo A, Vaccaro S, Francioso O, Nardi S. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J Chem Ecol. 2010;36:662–9. https://doi.org/10.1007/s10886-010-9790-6.
Aguiar NO, Olivares FL, Novotny EH, Canellas LP. Changes in metabolic profiling of sugarcane leaves induced by endophytic diazotrophic bacteria and humic acids. Peer J. 2018;6: e5445. https://doi.org/10.7717/peerj.5445.
Canellas NOA, Olivares FL, Canellas LP. Metabolite fingerprints of maize and sugarcane seedlings: searching for markers after inoculation with plant growth-promoting bacteria in humic acids. Chem Biol Technol Agric. 2019;6:14. https://doi.org/10.1186/s40538-019-0153-4.
Munné-Bosch S. The role of α-tocopherol in plant stress tolerance. J Plant Physiol. 2005;162:743–8. https://doi.org/10.1016/j.jplph.2005.04.022.
Veljović-Jovanović S, Vidović M, Morina F. Ascorbate as a key player in plant abiotic stress response and tolerance. In: Hossain M, Munné-Bosch S, Burritt D, Diaz-Vivancos P, Fujita M, Lorence A, editors. Ascorbic acid in plant growth, development and stress tolerance. Cham: Springer; 2017. p. 47–109.
Hasanuzzaman M, Bhuyan MHMB, Anee TI, Parvin K, Nahar K, Mahmud JA. Fujita M Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 2019;9:384. https://doi.org/10.3390/antiox8090384.
Bartoli CG, Buet A, Gergoff GGG, Galatro A. Simontacchi M ascorbate-glutathione cycle and abiotic stress tolerance in plants. In: Hossain M, Munné-Bosch S, Burritt D, Diaz-Vivancos P, Fujita M, Lorence A, editors. Ascorbic acid in plant growth, development and stress tolerance. New York: Springer; 2017. p. 177–200.
Bohnert HJ, Nelson DE, Jensen RG. Adaptations to environmental stresses. Plant Cell. 1995;7:1099–111. https://doi.org/10.1105/tpc.7.7.1099.
Bohnert HJ. Jensen RG Strategies for engineering water-stress tolerance in plants. Trend Biotechnol. 1996;14:89–97. https://doi.org/10.1016/0167-7799(96)80929-2.
Wani SH, Singh NB, Haribhushan A, Mir JI. Compatible solute engineering in plants for abiotic stress tolerance—role of glycine betaine. Curr Genom. 2013;14:157–65. https://doi.org/10.2174/1389202911314030001.
Ghosh UK, Islam MN, Siddiqui MN, Khan MAR. Understanding the roles of osmolytes for acclimatizing plants to changing environment: a review of potential mechanism. Plant Signal Behav. 2021;16:1913306. https://doi.org/10.1080/15592324.2021.1913306.
Merwad ARM. Effect of humic and fulvic substances and Moringa leaf extract on Sudan grass plants grown under saline conditions. Can J Soil Sci. 2017;97:703–16. https://doi.org/10.1139/cjss-2017-0050.
Akladious SA, Mohamed HI. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci Hortic. 2018;236:244–50. https://doi.org/10.1016/j.scienta.2018.03.047.
Benazzouk S, Djazouli ZE, Lutts S. Assessment of the preventive effect of vermicompost on salinity resistance in tomato (Solanum lycopersicum cv. Ailsa Craig). Acta Physiol Plant. 2018;40:121–9. https://doi.org/10.1007/s11738-018-2696-6.
Desoky ESM, Merwad ARM, Rady MM. Natural biostimulants improve saline soil characteristics and salt stressed-sorghum performance. Commun Soil Sci Plant Anal. 2018;49:967–83. https://doi.org/10.1080/00103624.2018.1448861.
Hatami E, Ali AS, Ali RG. Alleviating salt stress in almond rootstocks using of humic acid. Sci Hortic. 2018;237:296–302. https://doi.org/10.1016/j.scienta.2018.03.034.
Kaya C, Akram NA, Ashraf M, Sonmez O. Exogenous application of humic acid mitigates salinity stress in maize (Zea mays L.) plants by improving some key physico-biochemical attributes. Cereal Res Commun. 2018;46:67–78. https://doi.org/10.1556/0806.45.2017.064.
Hassan MU, Nawaz M, Shah AN, Raza A, Barbanti L, Skalicky M, Hashem M, Brestic M, Pandey S, Alamri S, Mostafa YS, L AE. Sameer HQ. trehalose: a key player in plant growth regulation and tolerance to abiotic stresses. J Plant Growth Regul. 2022;42:6. https://doi.org/10.1007/s00344-022-10851-7.
Aguiar NO, Medici LO, Olivares FL, Dobbss LB, Torres-Netto A, Silva SF, Novotny EH, Canellas LP. Metabolic profile and anti-oxidant responses during drought stress recovery in sugarcane treated with humic acids and endophytic diazotrophic bacteria. Ann Appl Biol. 2016;168:203–13. https://doi.org/10.1111/aab.12256.
Debolt S, Melino V, Ford CM. Ascorbate as a biosynthetic precursor in plants. Ann Bot. 2007;99:3–8. https://doi.org/10.1093/aob/mcl236.
Baker J, Orlandi EW. Sources and effects of reactive oxygen species in plants. In: Gilbert DL, Colton CA, editors. Ractive oxygen species in biological systems. New York: Kluwer Academic; 1995. p. 481–525.
Gratão PL, Polle A, Lea PJ, Azevedo RA. Making the life of heavy metal-stressed plants a little functional plant easier. Biol. 2005;32:481–94. https://doi.org/10.1071/FP05016.
Kutlu I, Gulmezoglu N. Suitable humic acid application methods to maintain physiological and enzymatic properties of bean plants under salt stress. Gesunde Pflanzen. 2023;75:1075–86. https://doi.org/10.1007/s10343-022-00766-4.
Souza AC, Zandonadi DB, Santos MP, Canellas NOA, Soares CP, Irineu LES, Rezende CE, Spaccini R, Piccolo A, Olivares FL, Canellas LP. Acclimation with humic acids enhances maize and tomato tolerance to salinity. Chem Biol Technol Agric. 2021;8:40. https://doi.org/10.1186/s40538-021-00239-2.
Nardi S, Carletti P, Pizzeghello D, Muscolo A. Biological activities of humic substances. In: Senesi N, Xing B, Huang PM, editors. Biophysicochemical processes involving natural non-living organic matter in environmental systems. Hoboken: Wiley; 2009. p. 305–40.
Azevedo IG, Olivares FLO, Ramos ACR, Bertolazi AA, Canellas LP. Humic acids and Herbaspirillum seropedicae change the extracellular H+ flux and gene expression in maize roots seedlings. Chem Biol Technol Agric. 2019;6:8. https://doi.org/10.1186/s40538-019-0149-0.
Khaleda L, Park HJ, Yun DJ, Jeon JR, Kim MG, Cha JY, Kim WY. Humic acid confers high-affinity K+ transporter 1-mediated salinity stress tolerance in arabidopsis. Mol Cells. 2017;40:966–75. https://doi.org/10.14348/molcells.2017.0229.
Trevisan S, Botton A, Vaccaro S, Vezzaro A, Quaggiotti S, Nardi S. Humic substances affect arabidopsis physiology by altering the expression of genes involved in primary metabolism, growth and development. Environ Exp Bot. 2011;74:45–55. https://doi.org/10.1016/j.envexpbot.2011.04.017.
Canellas LP, Canellas NOA, Soares TS, Olivares FL. Humic acids interfere with nutrient sensing in plants owing to the differential expression of TOR. J Plant Growth Regul. 2019;38:216–24.
Xia XJ, Zhou YH, Shi K, Zhou J, Foyer CH, Yu JQ. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot. 2015;66:2839–56. https://doi.org/10.1093/jxb/erv089.
Nardi S, Panuccio MR, Abenavoli MR, Muscolo A. Auxin-like effect of humic substances extracted from faeces of Allolobophora caliginosa and A. rosea.. Soil Biol Biochem. 1994;26:1341–6. https://doi.org/10.1016/0038-0717(94)90215-1.
Muscolo A, Cutrupi S, Nardi S. IAA detection in humic substances. Soil Biol Biochem. 1998;30:1199–201.
Pizzeghello D, Nicolini G, Nardi S. Hormone-like activity of humic substances in Fagus sylvaticae L. forests. N Phytol. 2001;151:647–57. https://doi.org/10.1046/j.0028-646x.2001.00223.x.
Pizzeghello D, Nicolini G, Nardi S. Hormone-like activities of humic substances in different forest ecosystems. N Phytol. 2002;155:393–402. https://doi.org/10.1046/j.1469-8137.2002.00475.x.
Mora V, Bacaicoa E, Zamarreno AM, Aguirre E, Garnica M, Fuentes M, Garcia-Mina JM. Action of humic acid on promotion of cucumber shoot growth involves nitrate-related changes associated with the root-to-shoot distribution of cytokinins, polyamines and mineral nutrients. J Plant Physiol. 2010;167:633–42. https://doi.org/10.1016/j.jplph.2009.11.018.
Mora V, Baigorri R, Bacaicoa E, Zamarreño AM, García-Mina JM. The humic acid-induced changes in the root concentration of nitric oxide, IAA and ethylene do not explain the changes in root architecture caused by humic acid in cucumber. Environ Exp Bot. 2012;76:24–32. https://doi.org/10.1016/j.envexpbot.2011.10.001.
Pizzeghello D, Francioso O, Ertani A, Muscolo A, Nardi S. Isopentenyladenosine and cytokinin-like activity of different humic substances. J Geochem Explor. 2013;129:70–5. https://doi.org/10.1016/j.gexplo.2012.10.007.
Zandonadi DB, Santos MP, Dobbss LB, Olivares FL, Canellas LP, Binzel ML, Okorokova-Façanha AL, Façanha AR. Nitric oxide mediates humic acids induced root development and plasma membrane H+-ATPase activation. Planta. 2010;231:1025–36. https://doi.org/10.1007/s00425-010-1106-0.
Zandonadi DB, Matos CRR, Castro RN, Spaccini R, Olivares FL, Canellas LP. Alkamides: a new class of plant growth regulators linked to humic acid bioactivity. Chem Biol Technol Agric. 2019;6:23. https://doi.org/10.1186/s40538-019-0161-4.
Jindo K, Canellas LP, Albacete A, Figueiredo dos Santos L, Frinhani RRL, Carvalho BD, Calenas NOA, Goron TL, Olivares FL. Interaction between humic substances and plant hormones for phosphorous acquisition. Agronomy. 2020;10:640. https://doi.org/10.3390/agronomy10050640.
Olaetxea M, Mora V, Bacaicoa E, Garnica M, Fuentes M, Casanova E, Zamarreño AM, Iriarte JC, Etayo D, Ederra I, Gonzalo R, Baigorri R, García-Mina JM. Abscisic acid regulation of root hydraulic conductivity and aquaporin gene expression is crucial to the plant shoot growth enhancement caused by rhizosphere humic acids. Plant Physiol. 2015;169:2587–96. https://doi.org/10.1104/pp.15.00596.
O’Donnell R. The auxin-like effects of humic preparations from leonardite. Soil Sci. 1973;116:106–12. https://doi.org/10.1097/00010694-197308000-00007.
Potters G, Tara SP, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK. Stress-induced morphogenic responses: growing out of trouble? Trend Plant Sci. 2007;12:98–105. https://doi.org/10.1016/j.tplants.2007.01.004.
Maggioni A, Varanini Z, Nardi S, Pinton R. Action of soil humic matter on plant roots: stimulation of ion uptake and effects on (Mg2++K+) ATPase activity. Sci Total Environ. 1987;62:355–63. https://doi.org/10.1016/0048-9697(87)90522-5.
Nardi S, Concheri G, Dell’Agnola G, Scrimin P. Nitrate uptake and ATPase activity in oat seedlings in the presence of two humic fractions. Soil Biol Biochem. 1991;23:833–6. https://doi.org/10.1016/0038-0717(91)90094-Z.
Pinton R, Varanini Z, Vizzotto G, Maggioni A. Soil humic substances affect transport properties of tonoplast vesicles isolated from oat roots. Plant Soil. 1992;142:203–10. https://doi.org/10.1007/BF00010966.
Pinton R, Cesco S, Santi S, Varanini Z. Soil humic substances stimulate proton release by intact oat seedling roots. J Plant Nut. 1997;20:857–69.
Pinton R, Cesco S, Iacoletti G, Astolf S, Varanini Z. Modulation of NO3- uptake by water-extractable humic substances: involvement of root plasma membrane H+-ATPase. Plant Soil. 1999;215:155–61. https://doi.org/10.1023/A:1004752531903.
Varanini Z, Pinton R, De Biasi MG, Astolf S, Maggioni A. Low molecular weight humic substances stimulated H+-ATPase activity of plasma membrane vesicles isolated from oat (Avena sativa L.) roots. Plant Soil. 1993;153:61–9.
Canellas LP, Olivares FL, Okorokova-Façanha AL, Façanha AR. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant Physiol. 2002;130:1951–7. https://doi.org/10.1104/pp.007088.
Quaggiotti S, Ruperti B, Pizzeghello D, Francioso O, Tugnoli V, Nardi S. Effect of low molecular size humic substances on nitrate uptake and expression of genes involved in nitrate transport in maize (Zea mays L.). J Exp Bot. 2004;55:803–13.
Lamar RT. Possible role for electron shuttling capacity in elicitation of pb activity of humic substances on plant growth enhancement. In: Geelen D, Xu L, editors. The chemical biology of plant biostimulants. New York: Wiley; 2020. p. 97–121.
Nebbioso A, Piccolo A. Basis of a humeomics science: chemical fractionation and molecular characterization of humic biosuprastructures. Biomacromolecules. 2011;11:1187–99. https://doi.org/10.1021/bm101488e.
Piccolo A. The supramolecular structure of humic substances. a novel understanding of humus chemistry and implications in soil science. Advan Agron. 2002;75:57–134. https://doi.org/10.1016/S0065-2113(02)75003-7.
Canellas LP, Dobbss LB, Oliveira AL, Chagas JG, Aguiar NO, Rumjanek VM, Novotny EH, Olivares FL, Spaccini R, Piccolo A. Chemical properties of humic matter as related to induction of plant lateral roots. Eur J Soil Sci. 2012;63:315–24. https://doi.org/10.1111/j.1365-2389.2012.01439.x.
Aguiar NO, Olivares FL, Novotny EH, Dobbss LB, Balmori DM, Santos-Júnior LG, Chagas JG, Façanha AR, Canellas LP. Bioactivity of humic acids isolated from vermicomposts at different maturation stages. Plant Soil. 2013;362:161–74. https://doi.org/10.1007/s11104-012-1277-5.
Silva AC, Canellas LP, Olivares FL, Dobbss LB, Aguiar NO, Frade DAR, Rezende C, Peres LEP. Promoção do crescimento radicular de plântulas de tomateiro por substâncias húmicas isoladas de turfeiras. Rev Bras Ci Solo. 2011;35:1609–17. https://doi.org/10.1590/S0100-06832011000500015.
Canellas LP, Olivares FL, Canellas NOA, Mazzei P, Piccolo A. Humic acids increase the maize seedlings exudation yield. Chem Biol Technol Agric. 2019;6:3.
Yakhin OI, Lubyanov A, Yakhin IA, Brown PH. Biostimulants in plant science: a global perspective. Front Plant Sci. 2016;7:2049. https://doi.org/10.3389/fpls.2016.02049.
du Jardin P, Xu L. Geelen D Agricultural functions and action mechanisms of plant biostimulants (PBs): an introduction. In: Xu L, Geelen D, editors. The chemical biology of plant biostimulants. New York: Wiley; 2020. p. 3–31.
Prigogine I As leis do caos. Editora Unesp, São Paulo, 1993. 1993. 110.
Piccolo A, Spaccini R, Drosos M, Vinci G, Cozzolino V. The molecular composition of humus carbon: recalcitrance and reactivity in soils. In: García C, Nannipieri P, Hernandez T, editors. The future of soil carbon. Cambridge: Academic Press; 2018. p. 87–124.
Piccolo A, Spaccini R, Savy D, Drosos M, Cozzolino V. The soil humeome: chemical structure, functions and technological perspectives. In: Vaz S, editor. Sustainable agrochemistry. Switzerland: Springer; 2019. p. 183–222.
Acknowledgements
Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) Cientista do Nosso Estado programme. RMS is a doctoral fellow of CAPES. JGB was supported by Ministry of Science and Technology of Brazil (CNPq, process number 309477/2021-2).
Funding
This work was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) Cientista do Nosso Estado programme. RMS is a doctoral fellow of CAPES. JGB was supported by Ministry of Science and Technology of Brazil (CNPq, process number 309477/2021–2).
Author information
Authors and Affiliations
Contributions
LPC, JGB and FLO conceived the concept. LPC write the first version of this manuscript. RMS did the search literature and data analysis. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This manuscript is an original paper and has not been published in other journals. The authors agreed to keep the copyright rule.
Consent for publication
The authors agreed to the publication of the manuscript in this journal.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Canellas, L.P., da Silva, R.M., Busato, J.G. et al. Humic substances and plant abiotic stress adaptation. Chem. Biol. Technol. Agric. 11, 66 (2024). https://doi.org/10.1186/s40538-024-00575-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-024-00575-z