Abstract
Background
Orthodontic debonding procedure produces inevitable enamel surface alterations, sequelae to which are enamel demineralization, sensitivity and retention of pigments. Several agents have been employed to counterbalance the same. The purpose of this study was (1) To evaluate the hypothesis that no significant difference exists in the remineralising potential of nano hydroxyapatite (NanoHAP) dentifrice and fluoridated dentifrice after orthodontic debonding, (2) To estimate the enamel topographic parameters following use of nano HAP dentifrice, post orthodontic debonding.
Methods
Sixty upper first bi-cuspids (30 subjects) planned for therapeutic extraction for the orthodontic treatment were bonded with a light cured adhesive. Envelope method of randomisation was followed in this prospective in-vivo study. In each subject, one of the first premolar brackets was debonded using a debonding plier and polished following standard protocols. Envelope method of randomisation was used to determine the side of the premolar to be debonded first. Patient was advised to use fluoridated (Group I) dentifrice for the first 15 days, then the first premolar was covered with a heavy-bodied putty cap, extracted and subjected to atomic force microscopy (AFM). Contralateral first premolar was then debonded and polished using similar protocol, and patient was advised to use nano hydroxyapatite dentifrice (Group II) for next 15 days. The premolar was then extracted and analyzed for surface roughness using AFM. The remineralizing potential of dentifrices was assessed by evaluating surface roughness parameters of the two groups and were compared using a two-sample t test.
Results
A significant difference was found amongst Group I (Fluoridated dentifrice) and Group II (NanoHAP dentifrice) (p > 0.001***) for enamel surface roughness variables which reflect remineralising potential of dentifrices. Group II showed significantly lesser value of surface roughness characteristics.
Conclusions
NanoHAP dentifrice was shown, after 15 days, to be superior to fluoridated dentifrice in remineralising enamel post orthodontic debonding.
Similar content being viewed by others
Introduction
Bonding is the fundamental step to a successful orthodontic treatment. Etching being the primary procedure in bonding, creates micro-morphological variation on the resistant enamel surface [1]. At the end of treatment, when orthodontic attachments are debonded, a porous enamel surface with micro-cracks, fractures and retained resin tags remain [2, 3]. Loss of enamel layer ranging up to 52–55 μm due to orthodontic debonding has been reported previously [4, 5]. These micro-damages result in an enamel surface which is rougher and is at further risk for demineralization [2, 3].
Residual resin removal using bur and polishing the enamel surface takes us a step closer to restoring to pre-treatment enamel surface conditions, however it does not fully restore the enamel surface [3, 6,7,8]. Fluorides [9], compounds of novamin [10], pro-arginine [11], amorphous calcium phosphates [12, 13], hydroxyapatite agents [14, 15] are occasionally prescribed after orthodontic treatment completion for utilising their remineralisation potential.
Fluoridated dentifrices with a fluoride concentration ranging from 1000 to 1450 ppm are commonly used as they are known to decrease demineralization by promoting formation of fluorapatite [16]. However, this acid resistant layer seems to prevent diffusion of remineralising ions into subsurface layers, thus complete remineralization process to depth of lesion does not take place [17]. Apart from professional chair-side application of high concentration fluoride, studies have also assessed potential of high concentration fluoride dentifrice in remineralising carious lesions and reducing the prevalence of white spot lesions post-orthodontic debonding [18,19,20]. Contrasting results have been displayed regarding the same and recent literature a low remineralizing potential of high fluoride preparations compared to regular fluoride [21]. Increased fluoride content also possesses increased risk of fluorosis. Alternatively, non-fluoridated dentifrices have been suggested as aforementioned.
Hydroxyapatite crystals make up the fundamental and major inorganic fragment of matured enamel. Synthetic forms of hydroxyapatites (HAP) have been used for restoring enamel surfaces. Application of nanotechnology as nanomaterials has surfaced well in the field of orthodontics from nanoparticle coated orthodontic attachments, resin adhesive with nanofillers like silver and nano apatites [22,23,24]. A more biomimetic form of hydroxyapatite having particle size scaled within nanometers known as nanohydroxyapatite have been successfully used to remineralise enamel surface [25]. Apart from being more biocompatible and bioactive, nano hydroxyapatite crystals have shown to have an appreciable affinity and adaptability towards enamel than a conventional apatites, reason being an increased surface area due to smaller particle size of nano hydroxyapatite crystals. Layers of nanoHAP deposited on the surface of enamel are extremely resistant to acidic solutions and therefore can protect the underlying enamel from future demineralisation [18, 26].
Perusing the enamel surface for surface roughness various methods such as use of stereo microscope, scanning electron microscope (SEM), contact profilometer or AFM have been used [27, 28]. However, the SEM results are not reliable, are subjective and doesn't give a quantitative evaluation. Alternatively, the atomic force microscope produces multiple conspicuous scans and is suitable to assess the nano irregularities on any surface [29].
The loss of enamel surface structure owing to orthodontic procedure is inexorable and sequelae that follow it are also unavoidable. Therefore, it is of clinical importance to evaluate remineralising effects of nano hydroxyapatite dentifrice post-debonding. There is no updated literature that tested and compared the biomimetic effects of nano-hydroxyapatite with that of fluoridated dentifrice after orthodontic debonding. Few in-vitro studies have reported on the role of NanoHAP in reducing surface roughness after orthodontic debonding [14]. However, till date no in-vivo study has been conducted which reaffirms the finding of the in-vitro studies.
The present study was conducted (1) To evaluate the hypothesis that no significant difference exists in the remineralising potential of NanoHAP dentifrice and fluoridated dentifrice after orthodontic debonding, (2) To estimate the enamel parameters following use of nano Hydroxyapatite dentifrice, post orthodontic debonding.
Methods
Ethical clearance was obtained by the Scientific review Board of Saveetha Institute of Medical and Technical Sciences (IHEC/SDC/ORTHO-1806/20/45). Patients requiring extraction of both maxillary first premolars for the purpose of orthodontic treatment were listed. 30 subjects who were willing to participate in this split mouth trial were enrolled. In total 60 premolars of 30 subjects undergoing orthodontic treatment were contained for the study. Each subject consented to participate in the study by signing a written consent form.
The inclusion was performed on the basis of visual assessment of the compactness of labial surface i.e., no surface micro-fractures/pits, enamel hypoplasia, caries, pigmented surfaces as in smokers, and any restorative procedures involving buccal aspect of bicuspids.
Enamel surfaces were rendered clean and buffed using a rubber-cup topped with pumice paste, in a low-speed. The surface was parched using compressed air (oil-free). The bicuspids were subjected to 37% phosphoric acid gel (D-tech etching gel, D-tech, India) for 10 s, the etchant was washed off completely and the surface was air-dried till a frosty white appearance was seen. Etched enamel surface was painted with a fine coat of primer (OrthoSolo, Ormco) and the metal bracket base (3 M, Unitek Gemini MBT Brackets) was coated with resin adhesive. The brackets were then placed, firmly pressed and excess composite flash was removed. Bonding was accomplished by light- curing bracket for 12 s, each edge cured for 3 s.
After 24 h (maturation period of composite) of bonding, one side of the premolar bracket was debonded using a debonding plier. Mesial and distal wings of the brackets were squeezed together to debond. The premolar to be debonded was determined by the Envelope method of randomisation for each patient. After debonding a 12-fluted tungsten carbide bur (Komet Adhesive Remover, Germany) was used in slow-speed with coolant for removal of remnant resin adhesive from enamel surface. After adhesive resin removal, the surface was polished using pumice slurry coated onto a rubber cup. Under direct illumination of chair-light, meticulous inspection of enamel surface was performed to ensure no resins remain. An experienced orthodontist performed all aforementioned procedures on all subjects and a new polishing inventory was used for every patient including the tungsten carbide bur and polishing cup. Post polishing treatment, fluoridated dentifrice (Amflor dentifrice, Group Pharmaceuticals Ltd, India) was conferred to the participating subject. Subject was given following instructions adhering to the manufacturer’s guidelines:
-
(a)
Subject was directed to use fluoridated paste regularly for 15 days.
-
(b)
Paste had to be massaged on the enamel surface for a minimum of 2 min, twice a day for 15 days.
-
(c)
After application the patient was advised to rinse the paste with water.
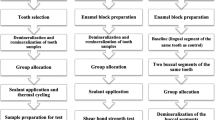
After 15 days the patient was recalled, the debonded premolar tooth was then subjected to extraction. After a week, the contralateral first premolar was then debonded and polished using the same protocol as aforementioned. NanoHAP dentifrice (Apagard Premio Toothpaste, Japan) was given to the patient and similar protocol for application was followed by the patient for the next 15 days. 15 days later, the patient was recalled and premolar was extracted. The study protocol has been briefly outlined in Fig. 1.
In order to circumvent enamel surface damage and surface alterations due to extraction forceps, the premolars were shielded with a heavy-bodied putty impression material before extraction (Fig. 2).
The extraction was done with the putty-shield. After extraction, the root surface was cleaned and distilled water was used for storage of samples till it was transported to the lab where AFM analysis was performed the very same day of extraction. All the premolars were subjected to AFM only once i.e., following respective intervention. A total of 60 premolars received indistinguishable debonding and polishing protocol, correlative pre-extraction preparation and a similar storage and transportation facility.
The Atomic Force Microscopy (AFM XE-100, Park System, Park Europe, Mannheim, Germany) was executed in contact mode to register the surface topography of enamel (Fig. 3). Atomic Force microscope is incorporated with a scanner which records in the range of 100 μm × 100 μm × 15 μm in x, y, and z axis in order. Scanning was done at 3 points and at each point 2D and 3D scans were obtained at a resolution of 256 × 256 pixels. Each scan measured 10 μm Average of three recorded readings was taken for performing statistical analysis (Fig. 4). Three roughness parameters were recorded in nanometers (nm) as mentioned:
-
Average roughness value (Ra): it is the average height of crests and troughs of valleys from the mean line. This signifies gross surface roughness.
-
Root mean square roughness (Rq): distribution of heights of crests and troughs in relation to the mean line.
-
Rz: it is the vertical length from height peak to lowest valley of five sampling lengths, with the evaluation length.
Statistics analysis
All the statistics were performed using SPSS software (IBM SPSS Statistics for Windows, Version 26.0, Armonk, NY: IBM Corp. Released 2019). Normality of data distribution was evaluated using Kolmogorov–Smirnov and Shapiro–Wilks test. Parametric tests were used to evaluate data for roughness. Inter-group comparison was performed using a two-sample t test. Significance level was fixed at 5% (α = 0.05).
Results
The Normality tests Kolmogorov–Smirnov and Shapiro–Wilks test results reveal that all variables (Ra, Rq, and Rz) follow normal distribution. Independent t test revealed that a statistically significant difference was found amongst the two groups for the values of Ra, Rq and Rz. The values were significantly lesser for Group II. (p < 0.001) (Table 1).
Value for Ra was significantly higher for Group I (90.47 ± 6.58 nm) compared to Group II (28.53 ± 6.12 nm). Similarly, mean values were higher for Rq and Rz in Group I being 101.16 nm and 716.55 nm respectively. Whereas, significantly lesser mean values were recorded for Group II. Details of mean, SD and significance have been mentioned in Table 1.
Discussion
Enamel, being crucial clinically brings along domineering distress in terms of bonding–debonding procedure. Increased enamel surface roughness after debonding serves as a niche for bacterial plaque, accumulation of the pigments, and decalcification, situations that are likely to cause esthetic problems [30]. Unavoidable modifications of enamel surface like loss of enamel, deteriorating surface characteristics, and discoloration post-orthodontic treatments have been reported in previous literature [31, 32].
Several remineralising agents have been used ranging from concentrated fluoride pastes and varnishes, CPP-ACP, pro-argin and nanoHAP serum. A study comparing remineralising effects of fluorides and CPP-ACP demonstrated no significant difference between two exists in terms of remineralising effect [12]. Arginine dentifrice has shown better remineralising potential compared to a fluoridated dentifrice [33]. Novamin has also shown effective remineralization [12] potential compared to a regular fluoridated compound [34]. Studies have also shown that synthetic hydroxyapatite dentifrices have a comparable remineralising potential than conventionally used fluoridated dentifrices [35]. However a biomimetic alternative to HAP, the NanoHAP is chemically and structurally similar to inorganic structure of enamel and has proved to be superior in remineralising the enamel surface than conventional HAP [36]. The nanoHAP particles display elevated surface energy and strong bonding towards the enamel surface [37]. Current study investigated remineralising potential by measuring the surface roughness value following use of NanoHAP dentifrice post-debonding and compared it to a control fluoridated group.
The results of the current study reported a significant difference in enamel surface roughness parameters amongst the two groups following application of both dentifrices for 15 days. As per the manufacturer’s instructions, NanoHAP formulation reaches its greatest effectiveness if applied daily for a minimum of 2–3 min up to a minimum for 10 days/month. A bi-weekly protocol was therefore adopted for this study.
The values for surface roughness were lower significantly in the NanoHAP group in comparison to the fluoridate group. Previous studies by Takikawa et al. [38] and Toko et al. [39] have claimed that NanoHAP restores the enamel surface to its original state. In another study by Scribante et al. [40] they assessed for visual remineralisation, bond strength and hardness of enamel post-orthodontic debonding. In their in-vitro experiment visual effects were recorded using an optical microscope. They concluded that application of the Nano hydroxyapatite solution induced a significant in vitro reduction of demineralised areas. Present study cannot claim so, as the AFM readings were taken only at a one-time point, but the NanoHAP group has shown better surface characteristics than fluoridated dentifrice.
Present study utilized AFM to analyze topographic characteristics of enamel. AFM was considered appropriate to obtain detailed three-dimensional surface characteristics in nanometers. Principal advantage of AFM over SEM is that AFM provides a quantitative information [41].Scanning electron microscopy can also be used to evaluate surface roughness but the SEM photomicrographs are subjective and not reliable as it does not provide quantitative evaluation of surfaces and therefore not suitable for comparative assessment enamel topography [42, 43].An alternative to SEM is AFM as this technology generates high resolution multiple mechanical scans in range of nanoscales. Additionally, AFM requires minimal sample preparation, generates 2D and 3D images simultaneously and allows for re-examination of samples as required [28, 44].
The present study is a novel in-vivo study where an attempt to estimate remineralising potential of NanoHAP dentifrice on surface roughness of enamel was made. Since previous literature has already reported an invariable loss of enamel surface post debonding, it was assumed that the values of baseline surface roughness parameters were high as recording the same was not feasible, study being an in-vivo trial. In-vitro setup being fully controlled does not recreate the natural oral environment, and the results can therefore not always be accurately taken for clinical consideration. The study was designed as a split-mouth trial in order to eliminate an inter-individual variability from the estimates of the treatment effect. However, to avoid any cross over effect the interventions were spaced out in time and only when the application of fluoridated dentifrice was completed after 15 days and the premolar was extracted, the contralateral premolar was debonded and intervention in form of NanoHAP dentifrice was introduced.
The hypothesis of the study was rejected, as a significant difference was found in the remineralising effects of NanoHAP dentifrice and fluoridated dentifrice after orthodontic debonding. Remineralising effects of NanoHAP dentifrice were found to be significantly superior to routine fluoridated dentifrice.
AFM acquires surface topographic images of only a smaller area, which could limit true representation of a wider area involved. To minimise this limiting factor, for each sample a minimum of three points were assessed for recording surface roughness and the average of the three readings were utilised for performing statistics. In future prospects, studies with larger sample size incorporating multiple interventions to assess and grade their remineralization potential can be carried out.
Conclusions
-
There is a significant difference in fluoridated dentifrice and NanoHAP dentifrice in reducing the enamel surface roughness.
-
The NanoHAP dentifrice is superior to fluoridated dentifrice in restoring the enamel surface post orthodontic debonding.
-
Routine use of NanoHAP dentifrice bi-weekly, twice a day following orthodontic treatment will help reduce post-debonding roughness and sensitivity.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- NanoHAP:
-
Nano hydroxyapatite
- AFM:
-
Atomic force microscope
- Nm:
-
nanometer
References
Goes MF, Sinhoreti MA, Consani S, Silva MA. Morphological effect of the type, concentration and etching time of acid solutions on enamel and dentin surfaces. Braz Dent J. 1998;9:3–10.
Mohebi S, Shafiee H-A, Ameli N. Evaluation of enamel surface roughness after orthodontic bracket debonding with atomic force microscopy. Am J Orthod Dentofac Orthop. 2017;151:521–7. https://doi.org/10.1016/j.ajodo.2016.08.025.
Palmer JA, Mang T, Tabbaa S, Al-Jewair T. Analysis of enamel surface roughness after different adhesive removal techniques for orthodontic bracket debonding. Lasers Dent Sci. 2018;2:95–101. https://doi.org/10.1007/s41547-018-0024-5.
Arhun N, Arman A. Effects of orthodontic mechanics on tooth enamel: a review. Semin Orthod. 2007;13:281–91. https://doi.org/10.1053/j.sodo.2007.08.009.
van Waes H, Matter T, Krejci I. Three-dimensional measurement of enamel loss caused by bonding and debonding of orthodontic brackets. Am J Orthod Dentofac Orthop. 1997;112:666–9. https://doi.org/10.1016/s0889-5406(97)70232-4.
Kumar P, Garg R, Dixit P, Khosla T, Gupta P, Kalra H. enamel surface roughness after debonding: a comparative study using three different burs. J Contemp Dent Pract. 2018;19:521–26. https://doi.org/10.5005/jp-journals-10024-2293.
Danny DT, Sugareddy SR. Comparison of the enamel surface roughness before bonding and after debonding by diamond, tungsten carbide and fiber reinforced composite burs under AFM an in-vitro study. IP Indian J Orthod Dentofac Res. 2020;4:151–5. .
Karan S, Kircelli BH, Tasdelen B. Enamel surface roughness after debonding. Angle Orthod. 2010;80:1081–8. https://doi.org/10.2319/012610-55.1.
Boyd RL. Comparison of three self-applied topical fluoride preparations for control of decalcification. Angle Orthod. 1993;63:25–30. https://doi.org/10.1043/0003-3219(1993)063%3c0025:COTSTF%3e2.0.CO;2.
Mohapatra S, Pradeep Kumar R, Meignana Arumugham I, Sri Sakthi D, Jayashri P. Assessment of microhardness of enamel carious like lesions after treatment with nova min, bio min and remin pro containing toothpastes: an in vitro study. Indian J Public Health Res Dev. 2019;10:375. https://doi.org/10.5958/0976-5506.2019.02832.8.
Arantes DC, Limeira FIR, Yamauti M, Moreira AN, Abreu LG, de Magalhães CS. Comparison of clinical efficacy of pro-argin and NovaMin toothpastes in relieving dentin hypersensitivity: a systematic review and meta-analysis. Oral Health Prev Dent. 2019;17:403–12. https://doi.org/10.3290/j.ohpd.a43272.
Singh S, Singh SP, Goyal A, Utreja AK, Jena AK. Effects of various remineralizing agents on the outcome of post-orthodontic white spot lesions (WSLs): a clinical trial. Prog Orthod. 2016;17:25. https://doi.org/10.1186/s40510-016-0138-9.
Wu G, Liu X, Hou Y. Analysis of the effect of CPP-ACP tooth mousse on enamel remineralization by circularly polarized images. Angle Orthod. 2010;80:933–8. https://doi.org/10.2319/110509-624.1.
Ajami S, Pakshir HR, Babanouri N. Impact of nanohydroxyapatite on enamel surface roughness and color change after orthodontic debonding. Prog Orthod. 2016;17:11. https://doi.org/10.1186/s40510-016-0124-2.
Alencar CM, de MeloAlencar C, de Paula BLF, Ortiz MIG, Magno MB, Silva CM, et al. Clinical efficacy of nano-hydroxyapatite in dentin hypersensitivity: a systematic review and meta-analysis. J Dent. 2019;82:11–21. https://doi.org/10.1016/j.jdent.2018.12.014.
Rošin-Grget K. The cariostatic mechanisms of fluoride. Acta Med Acad. 2013;42:179–88. https://doi.org/10.5644/ama2006-124.85.
Roveri N, Battistella E, Bianchi CL, Foltran I, Foresti E, Iafisco M, et al. Surface enamel remineralization: biomimetic apatite nanocrystals and fluoride ions different effects. J Nanomater. 2009. https://doi.org/10.1155/2009/746383.
Bossù M, Saccucci M, Salucci A, Di Giorgio G, Bruni E, Uccelletti D, et al. Enamel remineralization and repair results of Biomimetic Hydroxyapatite toothpaste on deciduous teeth: an effective option to fluoride toothpaste. J Nanobiotechnol. 2019. https://doi.org/10.1186/s12951-019-0454-6.
Ferreira RS, Ricomini-Filho AP, Tabchoury CP, Vale GC. Effect of high-fluoride dentifrice and bracket bonding composite material on enamel demineralization in situ. Clin Oral Investig. 2020;24:3105–12. https://doi.org/10.1007/s00784-019-03182-7.
Sonesson M, Twetman S, Bondemark L. Effectiveness of high-fluoride toothpaste on enamel demineralization during orthodontic treatment-a multicenter randomized controlled trial. Eur J Orthod. 2014;36:678–82. https://doi.org/10.1093/ejo/cjt096.
Talwar M, Borzabadi-Farahani A, Lynch E, Borsboom P, Ruben J. Remineralization of demineralized enamel and dentine using 3 dentifrices—an invitro study. Dent J. 2019;7:91. https://doi.org/10.3390/dj7030091.
Borzabadi-Farahani A, Borzabadi E, Lynch E. Nanoparticles in orthodontics, a review of antimicrobial and anti-caries applications. Acta Odontol Scand. 2014;72:413–7. https://doi.org/10.3109/00016357.2013.859728.
De Stefani A, Bruno G, Preo G, Gracco A. Application of nanotechnology in orthodontic materials: a state-of-the-art review. Dent J. 2020. https://doi.org/10.3390/dj8040126.
Eslamian L, Borzabadi-Farahani A, Karimi S, Saadat S, Badiee MR. Evaluation of the shear bond strength and antibacterial activity of orthodontic adhesive containing silver nanoparticle, an in-vitro study. Nanomaterials (Basel). 2020. https://doi.org/10.3390/nano10081466.
Pepla E, Besharat LK, Palaia G, Tenore G, Migliau G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Ann Stomatol. 2014;5:108–14.
Lelli M, Putignano A, Marchetti M, Foltran I, Mangani F, Procaccini M, et al. Remineralization and repair of enamel surface by biomimetic Zn-carbonate hydroxyapatite containing toothpaste: a comparative in vivo study. Front Physiol. 2014. https://doi.org/10.3389/fphys.2014.00333.
Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986;56:930–3. https://doi.org/10.1103/physrevlett.56.930.
Kakaboura A, Fragouli M, Rahiotis C, Silikas N. Evaluation of surface characteristics of dental composites using profilometry, scanning electron, atomic force microscopy and gloss-meter. J Mater Sci Mater Med. 2007;18:155–63. https://doi.org/10.1007/s10856-006-0675-8.
Binnig G, Gerber C, Stoll E, Albrecht TR, Quate CF. Atomic resolution with atomic force microscope. Surf Sci. 1987;189–190:1–6. https://doi.org/10.1016/s0039-6028(87)80407-7.
6 Oral microbiological changes, long-term enamel alterations due to decalcification, and caries prophylactic aspects. In: Orthodontic Materials. 2001. https://doi.org/10.1055/b-0034-43094.
Berger SB, Palialol ARM, Cavalli V, Giannini M. Surface roughness and staining susceptibility of composite resins after finishing and polishing. J Esthet Restor Dent. 2011;23:34–43. https://doi.org/10.1111/j.1708-8240.2010.00376.x.
Aykent F, Yondem I, Ozyesil AG, Gunal SK, Avunduk MC, Ozkan S. Effect of different finishing techniques for restorative materials on surface roughness and bacterial adhesion. Br Dent J. 2011;210:417. https://doi.org/10.1038/sj.bdj.2011.335.
Xu P, Deng M, Zhou X-D, Li J, Cheng L, Xu X. Effect of arginine dentifrice on remineralization of initial enamel carious lesions. Hua Xi Kou Qiang Yi Xue Za Zhi. 2014;32:32–5.
Vahid Golpayegani M, Sohrabi A, Biria M, Ansari G. Remineralization effect of topical NovaMin versus sodium fluoride (.%) on caries-like lesions in permanent teeth. J Dent. 2012;9:68–75.
Hornby K, Evans M, Long M, Joiner A, Laucello M, Salvaderi A. Enamel benefits of a new hydroxyapatite containing fluoride toothpaste. Int Dent J. 2009;59:325–31. https://doi.org/10.1002/idj.2009.59.6s1.325.
Zhang Y, Wang F. Use of Nanoparticles as Building Blocks for Bioapplications. Top Appl Phys. 2007. https://doi.org/10.1007/978-0-387-39938-6_15.
Tschoppe P, Zandim DL, Martus P, Kielbassa AM. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J Dent. 2011;39:430–7. https://doi.org/10.1016/j.jdent.2011.03.008.
Yamamoto E, Kato N. Restoration of tooth enamel using a flexible hydroxyapatite sheet coated with tricalcium phosphate. Bioceram Dev Appl. 2013. https://doi.org/10.4172/2090-5025.s1-006.
Jancin B. Bleach baths for reducing S. Aureus in atopy underused. Skin Allergy News. 2007. https://doi.org/10.1016/s0037-6337(07)70718-0.
Scribante A, Dermenaki Farahani MR, Marino G, Matera C, Rodriguez Y, Baena R, Lanteri V, et al. Biomimetic effect of nano-hydroxyapatite in demineralized enamel before orthodontic bonding of brackets and attachments: visual adhesion strength and hardness in in vitro tests. Biomed Res Int. 2020;2020:6747498. https://doi.org/10.1155/2020/6747498.
Hashimoto Y, Hashimoto Y, Nishiura A, Matsumoto N. Atomic force microscopy observation of enamel surfaces treated with self-etching primer. Dent Mater J. 2013;32:181–8. https://doi.org/10.4012/dmj.2012-227.
Piacentini C, Sfondrini G. A scanning electron microscopy comparison of enamel polishing methods after air-rotor stripping. Am J Orthod Dentofac Orthop. 1996;109:57–63. https://doi.org/10.1016/s0889-5406(96)70163-4.
Eliades T. Enamel surface roughness following debonding using two resin grinding methods. Eur J Orthod. 2004;26:333–8. https://doi.org/10.1093/ejo/26.3.333.
Tholt B, Miranda-Júnior WG, Prioli R, Thompson J, Oda M. Surface Roughness evaluation of different polishing techniques on dental porcelains by atomic force microscopy. J Dent Maxillofac Res. 2018. https://doi.org/10.31038/jdmr.2018124.
Acknowledgements
The author(s) would like to acknowledge the Department of Chemistry, IIT-Madras for helping out with AFM sample loading and surface analysis.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
PV designed the study protocol supervised by SMP. Clinical procedures were performed by SMP. Data interpretation and analysis was done by PV. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance was obtained by the Scientific review Board of Saveetha Institute of Medical and Technical Sciences (IHEC/SDC/ORTHO-1806/20/45).
Consent for publication
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Verma, P., Muthuswamy Pandian, S. Bionic effects of nano hydroxyapatite dentifrice on demineralised surface of enamel post orthodontic debonding: in-vivo split mouth study. Prog Orthod. 22, 39 (2021). https://doi.org/10.1186/s40510-021-00381-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40510-021-00381-5