Abstract
Background
Groundwater quality is among the most important environmental issues as a result of heavy metals contamination from anthropogenic sources. Concentrations of heavy metals in hand-dug wells from Ejisu-Juaben Municipality were studied to understand the levels of heavy metals and their source of pollution.
Results
The results show that the average abundance of heavy metal concentration in the groundwater samples are in the order: Fe > Zn > Mn > Pb > Cu > Cd. The non-carcinogenic risk indicates that the groundwater is safe and therefore poses no health risks; however, the carcinogenic risk exceeds the acceptable limit of 10−6. Principal component analysis extracted two components, which explained 64.24 % of the total variance. Cd suggests that 63 % of the samples are highly polluted (Cd > 3), whereas HPI indicates that all the samples are above the critical limit (HPI > 100).
Conclusion
Our findings concluded that lithogenic and anthropogenic activities are the main source of contamination influencing the water quality.
Similar content being viewed by others
Background
Groundwater quality is important to human health, agriculture, aquaculture and industry (Vanloon and Duffy 2005). In the last decades, groundwater resource has become the potential source of domestic water supply in Ghana and the world at large (Hynds et al. 2014). Interestingly, many public health surveys and water quality analysis has shown that groundwater is not immune to contaminants such as waterborne pathogens, toxic elements (Asamoah and Amorin 2011).
Over the last few years, surface water and groundwater resources are among the most important environmental issues due to heavy metals contamination and human industrial activities (Vodela et al. 1997; Öztürk et al. 2009; Marcovecchio et al. 2007; Khodabakhshi et al. 2011; Ghasemi et al. 2011). Some of the heavy metals are essential for growth, development and health, whiles others are categorized as toxic species on living organisms (Underwood 1956). Heavy metal contamination has become a significant problem in several community and agricultural areas over the years due to the application of commercial agrochemicals on agricultural production (Vodela et al. 1997; Rattan et al. 2005). However, heavy metals originating from anthropogenic sources have been found in all components of the environment (Idris et al. 2007; Idris 2008; Ayni et al. 2011). In recent years more attention has been devoted to pollutants in the environment due to increase in anthropogenic contribution by heavy metals (Edmund et al. 2003; Marengo et al. 2006). Heavy metals can eventually dispersed and accumulated in the soil as well as surface and groundwater and may therefore impact adverse human health effect to living organisms (Rashed 2010; Chotpantarat et al. 2011; Chotpantarat and Sutthirat 2011; Taboada-Castro et al. 2012).
Groundwater quality, water type and sources of contaminants have been explored using Piper, Stiff plots and multivariate statistical techniques (Affum et al. 2015). Multivariate statistical techniques has extensively been used in literature to characterise and assess groundwater sources (Hussain et al. 2008; Shihab and AbdulBaqi 2010; Mahmood et al. 2011; Okogbue et al. 2012; Narany et al. 2014; Masoud 2014; Uddameri et al. 2014; Yadav et al. 2014; Asare-Donkor et al. 2015). However, the use of multivariate statistical technique and pollution evaluation index to assess the safety and quality of hand-dug well water in the Ejisu-Juaben Municipality is lacking.
Ejisu-Juaben Municipality is an agricultural region and have a high density of farmers that uses agrochemicals for producing various agricultural products, such as rice, cassava, chili and rubber trees (Anornu et al. 2009). The main chemically treated water is obtained from the Barekese Dam, which is produced by the Ghana Water Company Limited (GWCL) and distributed to individual households through pipes in the peri-urban communities of Ejisu. The demand for treated water by Ejisu-Juaben Municipality has increased over the years without any corresponding rise in quantity and quality. As a result of the above problems, individual households have resulted to the use of hand-dug wells as an alternative source of water supply, but the awareness of the health implications associated with the use of water from hand-dug wells have not been considered. This work is aiming to study the suitability and health risk associated with heavy metal contamination of groundwater and surface water for drinking purposes in the Ejisu-Juaben Municipality. The study will also employ multivariate statistical technique and pollution evaluation indices as complementary tool to identify the various possible sources of pollution that influence the water quality.
Methods
Study area
Geographically, Ejisu–Juaben Municipal Assembly lies within Latitudes 1°15′N and 1°45′N and Longitude 6°15′W and 7°00W. Ejisu-Juaben municipal area is one of the 27 administrative districts of Ashanti Region. Ejisu is the main capital of the municipality. The municipality has 4 main urban settlements namely: Ejisu, Juaben, Besease and Bonwire. The municipality can boast of about 88 communities. Ejisu stretches over an area of 637.2 km2 with population around 143,762. The municipality lies in the central part of the Ashanti Region and shares boundaries with six Districts in the Region. To its north east and west are Sekyere East and Afigya Kwabre, respectively, to the south; Bosomtwi and Asante Akim South, to its east; Asante Akim North and to the west; Kumasi metropolitan assembly. The mean monthly temperatures vary between 20 °C in August and 32 °C in March. The relative humidity ranges from 65 % in January to 85 % in August. The area usually experiences a wet semi-equatorial climate. Majority of the rainfall is from March to July and again from September to the later part of November, with a mean of 1200 mm which is ideal for minor season cropping as a result of climatic changes and seasonal drought. Groundwater is an important water resource for drinking, agriculture, and industrial uses in the study area. The area is characterized by rural setting and the major occupation of the people is agriculture. The main food crops grown are plantain, cassava, maize and cocoyam. One of the major cash crops grown includes cocoa, which is the driving force of the economy. Oil palm is also another cash crop that is widely grown in the district.
Sampling
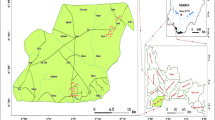
All solvents and reagents used were of high analytical grade supplied by BDH Chemical Ltd, UK. Double-distilled deionized water was used throughout the experiments. A total of nineteen (19) samples were obtained from the Ejisu-Juaben Municipality. The sampling stations are shown in Fig. 1. The sampling standard methods prescribed by APHA (2005) were followed carefully for the groundwater collection and analytical techniques. Samples were collected in 1.5 l high-density polyethylene (HDPE) containers. Prior to sampling, the bottles were rinsed with the water to be sampled and the samples were preserved by acidifying to pH <2 with HNO3 and kept at a temperature of 4 °C until analysis.
Heavy metal analysis
The preserved sample was taken out from the refrigerator and kept at room temperature until the attainment of thermal equilibrium. Prior to AAS analysis, water samples were digested with 5 mL of concentrated HNO3(aq). The mixture was heated on a hot plate and filtered using Whatman filter paper into a volumetric flask. Distilled water was added to mark to the top the volumetric flask. The heavy metals (Mn, Pb, Fe, Cd, Cu and Zn) was analysed using atomic Absorption Spectrophotometer (AAS) (VARIAN SpectrAA 220) with air acetylene flame.
Quality assurance and quality control
Replicate blanks and reference materials, NIST 1640(a) (USA: National Institute of Standards and Technology) were used for the method of validation and quality control. Replicate analysis of these reference materials showed good accuracy (relative standard deviation, RSD, ≤3 %) and recovery rates ranged from 94.5 to 105.8 %. The concentrations of heavy metal in the groundwater were reported in mg/L.
Pollution evaluation indices
Generally, pollution indices are estimated for a specific use of the water under consideration. The heavy metal pollution index (HPI), heavy metal evaluation index (HEI) and degree of contamination (Cd) were used to evaluate the drinking water quality. The HPI and HEI methods provide an overall quality of the water with regard to heavy metals using the ratios of monitored values of the desired number of parameters and the maximum admissible concentrations of the respective parameters. In the Cd method, the quality of water is evaluated by computation of the extent of contamination and computed as the sum of the contamination factors of each component exceeding the upper permissible limit. Therefore, the Cd summarizes the combined effects of a number of quality parameters regarded as unsafe to household water.
Heavy metal pollution index
The HPI method was developed by assigning a rating or weightage (W i ) for each chosen parameter and selecting the pollution parameter on which the index was to be based. The rating is an arbitrary value between 0 and 1 and its selection reflects the relative importance of individual quality considerations. W i is defined as inversely proportional to the recommended standard (S i ) for each parameter (Horton 1965; Reddy 1995; Mohan et al. 1996). In this study, the concentration limits (i.e., the highest permissible value for drinking water (S i ) and maximum desirable value (I i ) for each parameter) were taken from the WHO (2011) standard. The uppermost permissive value for drinking water (S i ) refers to the maximum allowable concentration in drinking water in absence of any alternate water source. The desirable maximum value (I i ) indicates the standard limits for the same parameters in drinking water. According to Mohan et al. (1996), HPI is determined according to Eq. (1):
where Qi and Wi are the sub-index and unit weight of the ith parameter, respectively, and n is the number of parameters considered. The sub-index (Qi) is calculated according to Eq. (2):
where M i , I i , and S i are the monitored heavy metal, ideal, and standard values of the ith parameter, respectively.
Heavy metal evaluation index
Heavy metal evaluation index HEI gives an overall quality of the water with respect to heavy metals (Edet and Offiong 2002) and is expressed using Eq. (3):
where Hc and Hmac are the monitored value and maximum admissible concentration (MAC) of the ith parameter, respectively.
Degree of contamination (Cd)
The contamination index (Cd) summarizes the combined effects of several quality parameters considered harmful to domestic water (Backman et al. 1997) and is calculated as follows:
where Cfi; CAi and CNi represent contamination factor, analytical value and upper permissible concentration of the ith component, respectively. N denotes the ‘normative value’ and CNi is taken as MAC.
Risk assessment
Risk assessment methods and parameters previously reported by Wongsasuluk et al. (2014) were used in the present study. Risk assessment is defined as the process of estimating the probability of occurrence of an event and the probable magnitude of adverse health effects on human exposures to environmental hazards over a specified time period (NRC 1983; Kolluru et al. 1996; Paustenbach 2002; Wongsasuluk et al. 2014). Risk assessment consists of hazard identification, exposure assessment, dose response and risk characterization (Lee et al. 2005). According to Lim et al. (2008), two toxicity risk indices reported are the slope factor (SF) for carcinogen risk characterization and the reference dose (RfD) for non-carcinogen characterization (Table 1).
Siriwong (2006) reported the estimations of the magnitude, frequency and duration of human exposure in the environment as average daily dose, for each water sample as:
All the parameters in Eq. (6) have been defined in Table 2.
The health risk was assessed in relation to its non-carcinogenic as well as carcinogenic effects based on the calculation of ADD estimates and defined toxicity according to the following relationships (USEPA IRIS 2011; Wongsasuluk et al. 2014). The non-carcinogenic was computed as:
The risk assessments of a mixture of chemicals, the individual HQs are summed to form hazard index (HI):
According Lim et al. (2008) an HI/HQ >1 means an unacceptable risk of non-carcinogenic effects on health, while HI/HQ <1 means an acceptable level of risk. Table 2 shows the principal exposure factors that have been taken into account to carry out the risk assessment calculations.
Chronic daily intake (CDI) in the present study was calculated using Eq. (4) modified from Muhammed et al. (2011); Wu et al. (2009) and De Miguel et al. (2007).
where, Ci, DI, and BW represent the concentration of heavy metal in the water samples (mg/L), average daily intake rate (2.2 L/day), and body weight (70 kg), respectively.
Statistical analysis
IBM Statistical Package for the Social Sciences (SPSS) ‘20 was used for the data analysis. Principal component analysis was used to identify the possible sources of heavy metals. Factor analysis was performed by varimax rotation (Howitt and Cramer 2005), which minimized the number of variables with a high loading on each component, thus facilitating the interpretation of PCA results. Cluster analysis was applied to identify groups of samples with similar heavy metal contents (Panda et al. 2006). CA was formulated according to the Ward-algorithmic method, and the rescaled linkage distance was employed for measuring the distance between clusters of similar metal contents. R-mode CA was used to determine the association of different water quality parameters and pollutant sources. Pearson’s correlation matrix was also used to identify the elements’ relationship.
Results and discussion
Descriptive statistics related to the heavy metal concentrations in the Ejisu-Juaben municipality are presented in Table 3. Coefficient of variation (CV) was the most important factor in describing the variability of groundwater properties. Data was ranked according to amount of variation as low variability (CV ≤ 15 %), moderate variability (CV 15–35 %), or high variability (CV, > 35 %) (Wilding 1985). The CV values in the groundwater ranged from 0 to 2 %. All the heavy metals in the study area were found to exhibit low variability. These low values of CV indicate a homogenous distribution of these metals in the corresponding sampling points and may increase the effect of non-point sources. The concentrations of these heavy metals are illustrated in the box plot (Fig. 2). In Fig. 2, iron has relatively large inter-quartile range than the other metals. The mean concentration of heavy metals in the groundwater samples follows the order: Fe > Zn > Mn > Pb > Cu > Cd.
The concentration of lead in groundwater ranged from 0.000 to 0.040 mg/L with a mean concentration of 0.010 mg/L (Table 3). About 26 % of the groundwater samples contained lead concentration above the levels permitted by WHO (2011). The main sources of lead contamination are industrial discharges from smelters, battery manufacturing units, run off from contaminated land areas, atmospheric fall out and sewage effluents. The levels of Pb from this study were lower than study reported by Kortatsi (2007), Wassa West district, Addo et al. (2013); Nassef et al. (2006), Sadat Industrial City. On the other hand the results in this study generally agree with those reported by Wongsasuluk et al. (2014), Ubon Ratchathani province, Thailand.
The concentration of iron in the groundwater ranged from 0.002 to 0.568 mg/L, with a mean of 0.166 mg/L. The maximum allowable limit of iron concentration in groundwater is 0.3 mg/L as per WHO (2011) classification. About 89 % of the samples were within the WHO permitted limit. Under reducing conditions, the solubility of Fe-bearing minerals increase in water, leading to the enrichment of dissolved iron in groundwater (Applin and Zhao 1989; White et al. 1991). Chronic consumption of water with iron overload may results in fatigue, weight loss, joint pains and ultimately heart disease, liver problems and diabetes (US-CDC 2011). Study done by Ansa-Asare et al. (2009) in Adidome district indicated that the levels of Fe were higher compared to this study. However, the present study generally agrees with those reported by Tay and Kortatsi (2007).
Cadmium concentration in the groundwater ranged from 0.000 to 0.002 mg/L with a mean concentration of 0.002 mg/L. The mean concentrations of cadmium were within the WHO guideline of 0.003 mg/L. Exposure to higher concentration of Cd may cause kidney damage as well as producing acute health effects (Momodu and Anyakora 2010). The mean concentration of Cd in this study was comparable to reports by Kortatsi (2007), Wassa West district; Asare-Donkor et al. (2015), Obuasi and Kuma (2004), Tarkwa.
In the study area, copper concentration ranged from 0.003 to 0.019 mg/L with a mean concentration of 0.010 mg/L. Zinc concentration in groundwater of the study area ranged from 0.000 to 0.047 mg/L with a mean concentration of 0.014 mg/L. Manganese concentration in the groundwater ranges from 0.005 to 0.020 mg/L with a mean value of 0.012 mg/L. The concentrations of manganese, copper and zinc in groundwater are within the maximum allowable limit as per WHO standard. Cadmium, manganese, copper and zinc are likely to be derived from the natural water–rock reaction processes since none of these metals exhibited concentration values outside the WHO (2011) limits. Study done by Nkansah et al. (2010) and Apau et al. (2014) indicated that the levels of Mn and Zn were comparable to this study. On the other hand, the levels of Zn and Mn from this study were higher than the study by Li et al. (2014), Henan-Liaocheng Irrigation Area; Zhang et al. (2013), Yellow River and Buschmann et al. (2008), Vietnam.
Pollution evaluation indices of water
The results of pollution evaluation indices (HPI) are presented in Table 4. The HPI values ranged from 319.20 to 688.05, with a mean of 374.47. The HPI results showed that all the samples were above the critical limit of 100 proposed for drinking water by Prasad and Bose (2001). The degree of contamination (Cd) was used as reference of estimating the extent of metal pollution (Rubio et al. 2000). The Cd values in the groundwater ranged from 0.26 to 26.80, with a mean of 7.79 (Table 4). According to Edet and Offiong (2002) and Backman et al. (1997), Cd may be classified into three categories as follows: low (Cd < 1), medium (Cd = 1–3) and high (Cd > 3) (Edet and Offiong 2002; Backman et al. 1997). As per the above classification, 21 % of the samples were classified as low zone, 16 % as medium zone and 63 % as high zone. The Cd and HPI indices show that most of the samples were highly polluted. The HEI values ranged from 2.25 to 29.88 with a mean value of 10.08. The proposed HEI criteria are as follows: low (HEI < 10), medium (HEI = 10–20) and high (HEI > 20). The HEI results show that 58 % of samples are within the low zone, 32 % fall within the medium zone and 10 % fall within the high zone. Cd, HPI and HEI values show similar trends at various sampling points (Fig. 3).
Correlation study
Correlation analysis establish the relationships between heavy metal characteristics of water samples, which can reveal the sources and pathways of heavy metals that generated the observed water compositions (Azaza et al. 2011; Parizi and Samani 2013). A high correlation coefficient (near 1 or 1) means a good positive relationship between two variables and its value around zero means no relationship between them at a significant level of p < 0.05 (Varol and Davraz 2014). More precisely, it can be said that parameters showing r > 0.7 are considered strongly correlated whereas r between 0.5 and 0.7 shows moderate correlation (Manish et al. 2006). The correlation matrix of the heavy metals is given in Table 5. Mn shows a moderate negative correlation with Zn (r = −0.500, p < 0.05). Cu shows a moderate positive correlation with Zn (r = 0.580, p < 0.01). Pb shows a moderate positive correlation with Cd (r = 0.583, p < 0.01) and Fe (r = 0.459, p < 0.05). Fe does not show any significant correlation with other heavy metals, indicating that their distributions were not controlled by the same factor. The strong correlations between some of the heavy metals indicate the same input sources and similar geochemical behaviour (Lu et al. 2010; Saeedi et al. 2012). Therefore the associations of metals clearly indicate that the groundwater has assimilated various contaminants from the processes of chemical industries and landfill leachate or municipal sewage systems.
Pollution source identification
Principal component analysis was used to further explore the extent of metal pollution and source identification following standard procedures (Dragovic et al. 2008; Franco-Uría et al. 2009). Varimax rotation (Gotelli and Ellison 2004) was used to maximize the sum of the variance of the factor coefficients which better explained the possible groups or sources that influenced the water system. Corresponding components, variable loadings, and the variances are presented in Table 6. Components loadings are classified by Liu et al. (2003) as “strong”, “moderate”, and “weak” corresponding to absolute loading values of >0.75, 0.75–0.50, and 0.50–0.30, respectively. R-mode analysis extracted two components with eigenvalues >1, which explained 64.24 % of the total variance. Positive scores in PCA indicate that water samples are affected by the presence of the parameters that are significantly loaded on a specific component, whereas negative scores suggest that water quality is essentially unaffected by those parameters. The 36.8 % of total variance is contributed by PC1 with higher positive loadings for Cu and Zn and moderate negative loadings for Mn. The occurrence of higher Zn concentration may be attributed to the greatest frequency of nearby sources like hazardous waste sites and the emission of industrial effluents. Components in PC1 are derived from mixed sources due to chemical induction of landfill leachate or municipal sewage (Bhuiyan et al. 2010). PC2 with high positive contribution of Pb and Cu, accounts for 27.4 % of the total variance. Despite the natural occurrence of Pb in the environment, anthropogenic sources such as discharge of various industrial effluents and public sewage also play a major role in the higher Pb loadings in the study area.
R-mode hierarchical cluster analysis
The R-mode HCA was used to determine the relationship among the various heavy metals using Ward’s method (Squared Euclidean distance as measure of similarity). Cluster analysis (CA) grouped the heavy metals into clusters on the basis of similarities within a group and dissimilarities between different groups. Parameters belonging to the same cluster are likely to have originated from a common source. The R-mode CA performed on the samples produced two clusters based on spatial similarities and dissimilarities (Fig. 4). The presence of Fe in cluster 1 reveals lithogenic contribution source of contamination and were identified in the higher contamination level. The second cluster includes Cd, Zn, Pb, Cu and Mn, which were derived from anthropogenic input.
Human health risk assessment
Heavy metal pollution has being one of the important issues in environmental sciences. Ingestion of significant amounts of metal-containing drinking water will harm human health, resulting in several types of cancers (Wu et al. 2009; Yu et al. 2010). Using the measured data for the 6 heavy metals, the HQ were calculated using USEPA risk assessment models (US EPA 2011). The human health risk assessment of Cd, Fe, Cu, Pb, Zn and Mn showed HQ values to be <1 suggesting an acceptable level of non-carcinogenic adverse health risk (Table 7). The average HQ values of Pb in the water samples (0.4924) were highest, followed by Cd (0.3267), Mn (0.0101), Fe (0.0089), Cu (0.0029) and Zn (0.0007). The HI values of all the 6 metals (Cd, Fe, Cu, Pb, Zn, Mn) ranging from 0.0001 to 0.2022 were also <1, indicating acceptable risk for non-carcinogenic adverse health effect. The HQ values were found to be comparable with study by Muhammed et al. (2011), Kohistan region of Pakistan; Çelebi et al. (2014), Melenwatershed, Turkey and Sprang et al. (2009), European rivers. On the other hand, the levels of Cr were lower than the study by Keleperzis (2014), Thiva area of Greece.
The health risk associated with drinking water depends on the volume of water consumed and the weight of the individual. In this regard, health risk assessment associated with the exposure duration (ADD) was determined using the concentration of Mn, Fe, Cu, Pb, Zn and Cd in the drinking water and the results are presented in Table 8. The ADD values ranged from 6.12 × 10−5 to 2.00 × 10−4, 2.45 × 10−5 to 8.04 × 10−2, 3.67 × 10−5 to 2.00 × 10−4, 1.22 × 10−5 to 5.00 × 10−4, 2.45 × 10−5 to 6.00 × 10−4 and 1.22 × 10−5 to 2.45 × 10−5 mg/kg/day for Mn, Fe, Cu, Pb, Zn and Cd, respectively (Table 8). Thus, the health risk assessment indicates an alarming situation within this study area. It is recommended that the use of the water from these contaminated hand-dug wells for domestic purposes must be discontinued, or appropriate remediation technology must be applied to save the health of the human population in the study area.
The average Chronic daily intake (CDI) levels for carcinogenic risk of Mn, Fe, Cu, Pb, Zn and Cd were found to be 3.68 × 10−4, 1.51 × 10−2, 2.89 × 10−4, 4.38 × 10−4, 5.02 × 10−4 and 4.19 × 10−5, respectively (Table 9). The CDI indices were in the order: Fe > Zn > Pb > Mn > Zn > Cu > Cd. In general, a CDI value of 1.0 × 10−6 is the limit for acceptable health risk (USEPA 2011). The total CDI of all the groundwater samples exceeds this acceptable value, indicating an increased cancer risk for individuals because of their lifetime exposure to these carcinogens (Iqbal and Shah 2013). Higher CDI values may be due to run-off from agricultural fertilization and fungicides, which intended to increase the concentration of these heavy metals and in turn can affect the water quality and ecosystem biodiversity (Li and Zhang 2010). Nguyen et al. (2009) determined that about 5 in 1000 people could suffer from cancer in Vietnam. In Nanjing, China, a study on six surface waters found carcinogenic value of 2.05–3.28 × 10−4 higher than the acceptable limits (Wu et al. 2009).
Conclusions
Pollution evaluation indices, principal component analysis, cluster analysis and correlation matrix have been used to assess the intensity and sources of pollution in groundwater samples from the Ejisu-Juaben Municipality, Ghana. The study is summarized as follows:
-
1.
The mean values of the heavy metal contents in the groundwater follow the decreasing order: Fe > Zn > Mn > Pb > Cu > Cd. The mean values of Pb and Fe in the groundwater were generally high compared to the threshold limits allowable for drinking water.
-
2.
The human health risk assessment showed hazard quotient (HQ) and Hazard index (HI) values to be <1, suggesting an acceptable level of non-carcinogenic adverse risk, however, the cancer risk exceed the acceptable limit of 1.0 × 10−6, indicating that cancer risk may occur.
-
3.
Principal component analysis with the support of cluster analysis identified both natural source and anthropogenic activities as the main contributing factors of metal profusion in the groundwater.
-
4.
According to correlation analysis, highly strong correlations were observed among some of the heavy metal pairs, suggesting common sources, mutual dependence and identical behaviour during transport.
-
5.
The Cd, HPI, and HEI concentrations show that 63, 100, and 10 % as highly polluted due to the municipal sewage being the main sources in the study area. The Cd and HEI concentration indices values increased trend along the study area may be due to the agriculture runoff, discharge of industrial wastewater, and municipal sewage through the soil.
Abbreviations
- CDI:
-
chronic daily intake
- AAS:
-
atomic absorption spectrophotometer
- RAGS:
-
Risk Assessment Guidance for Superfund
- RfD:
-
reference dose
- IR:
-
ingestion rate
- EF:
-
exposure frequency
- ED:
-
exposure duration
- BW:
-
average body weight
- AT:
-
is averaging time
- SA:
-
exposed skin area
- ET:
-
exposure time
- CF:
-
unit conversion factor
- HQ:
-
hazard quotient
- CR:
-
cancer risk
- PCA:
-
principal component analyses
- EPA:
-
environmental protection agency
- WHO:
-
World Health Organization
- GWCL:
-
Ghana Water Company Limited
- SPSS:
-
Statistical Package for the Social Sciences
- HCA:
-
hierarchical cluster analysis
- HPI:
-
pollution evaluation indices
- MAC:
-
maximum admissible concentration
- HEI:
-
Heavy Metal Evaluation Index
References
Addo MA, Darko EO, Gordon C, Nyarko BJB (2013) Water quality analysis and human health risk assessment of groundwater from open-wells in the vicinity of a cement factory at Akporkloe, Southeastern Ghana. e-J Sci Technol 8(4):15
Affum AO, Osae SD, Nyarko BJB, Afful S, Fianko JR, Akiti TT, Adomako D, Osafo Acquaah S, Dorleku M, Antoh E, Barnes F, Acheampong EA (2015) Total coliform, arsenic and Cadmium exposure through drinking water in the Western Region of Ghana. Environmental monitoring and assessment: application of multivariate statistical technique to groundwater quality. Environ Monit Assess 187(1):1–23
Anornu GK, Kortatsi BK, Zango MS (2009) Evaluation of groundwater resources potential in the Ejisu-Juaben District of Ghana. Afr J Environ Sci Technol 3(10):333–339
Ansa-Asare OD, Darko HF, Asante KA (2009) Groundwater quality assessment of Akatsi, Adidome and Ho districts in the Volta region of Ghana. Desalination 248:446–452
Apau J, Acheampong A, Bepule V (2014) Physicochemical and microbial parameters of water from hand-dug wells from Nyamebekyere, a surburb of Obuasi, Ghana. Int J Sci Technol 3(6):347–351
APHA (2005) Standard methods for water and wastewater, 21st edn. Am Publ Health Assoc, Washington, DC
Applin KR, Zhao N (1989) The kinetics of Fe(II) oxidation and well screen encrustation. Ground Water 27:168–174
Asamoah DN, Amorin R (2011) Assessment of the quality of bottled/sachet water in the Tarkwa-Nsuaem Municipality (TM) of Ghana. Res J Appl Sci Eng Technol 3:377–385
Asare-Donkor NK, Kwaansa-Ansah EE, Opoku F, Adimado AA (2015) Concentrations, hydrochemistry and risk evaluation of selected heavy metals along the Jimi River and its tributaries at Obuasi a mining enclave in Ghana. Environ Syst Res 4:12. doi:10.1186/s40068-015-0037-y
Ayni FE, Cherif S, Jrad A, Trabelsi-Ayadi M (2011) Impact of treated wastewater reuse on agriculture and aquifer recharge in a coastal area: Korba case study. Water Resour Manage 25:2251–2265
Azaza FH, Ketata M, Bouhlila R, Gueddari M, Riberio L (2011) Hydrogeochemical characteristics and assessment of drinking water quality in Zeuss-Koutine aquifer, southeastern Tunisia. Environ Monit Assess 174:283–298
Backman B, Bodis D, Lahermo P, Rapant S, Tarvainen T (1997) Application of a groundwater contamination index in Finland and Slovakia. Environ Geol 36:55–64
Bhuiyan MAH, Islam MA, Dampare SB, Parvez L, Suzuki S (2010) Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. J Hazard Mater 179(1–3):1065–1077
Buschmann J, Berg M, Stengel C, Winkel L, Sampson ML, Trang PTK, Viet PH (2008) Contamination of drinking water resources in the Mekong delta flood plains: arsenic and other trace metals pose serious health risks to population. Environ Int 34(6):756–764
Çelebi A, Sengörür B, Kløve BJ (2014) Human health risk assessment of dissolved metals in groundwater and surface waters in the Melen watershed, Turkey. J Environ Sci Health Part A Environ Sci 49:153–161
Chotpantarat S, Sutthirat C (2011) Different sorption approaches and leachate fluxes affecting on Mn2+? Transport through lateritic aquifer. Am J Environ Sci 7(1):65–72
Chotpantarat S, Ong SK, Sutthirat C, Osathaphan K (2011) Effect of pH on transport of Pb2+, Mn2+, Zn2+ and Ni2+ through lateritic soil: column experiments and transport modelling. J Environ Sci 23(4):640–648
De Miguel E, Iribarren I, Chacon E, Ordonez A, Charlesworth S (2007) Risk-based evaluation of the exposure of children to trace elements in playgrounds in Madrid (Spain). Chemosphere 66(3):505
Dragovıć S, Mihailovıć N, Gajıć B (2008) Heavy metals in soils: distribution, relationship with soil characteristics and radionuclide’s and multivariate assessment of contamination sources. Chemosphere 72(3):491–549
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin, (southeastern Nigeria). Geo J 57:295–304
Edmund WM, Shand P, Hart P, Ward RS (2003) The natural (baseline) quality of groundwater: a UK pilot study. Sci Total Environ 310:25–35
Franco-UríaA López-Mateo C, Roca E, Fernández-Marcos ML (2009) Source identification of heavy metals in pastureland by multivariate analysis in NW Spain. J Hazard Mater 165:1008–1015
Ghasemi M, Keshtkar AR, Dabbagh R, Jaber Safdari S (2011) Biosorption of uranium in a continuous flow packed bed column using Cystoseira indica biomass. Iran J Environ Health Sci Eng 8:65–74
Gotelli NJ, Ellison AM (2004) A primer of ecological statistics, 1st edn. Sinauer Associates, Sunder land, p 492
Horton RK (1965) An index systems for rating water quality. J Water Pollut Control Fed 37(3):300–306
Howitt D, Cramer D (2005) Introduction to SPSS in psychology: with supplement for releases 10, 11, 12 and 13. Pearson, Harlow
Hussain M, Ahmed SY, Abderrahman W (2008) Cluster analysis and quality assessment of logged water at an irrigation project, eastern Saudi Arabia. J Environ Manage 86:297–307
Hynds PD, Thomas MK, Pintar KDM (2014) Contamination of groundwater systems in the US and Canada by Enteric Pathogens, 1990–2013: a review and pooled-analysis. PLoS One 9(5):e93301. doi:10.1371/journal.pone.0093301
Idris AM (2008) Combining multivariate analysis and geochemical approaches for assessing heavy metal. Microchem J 90:159–163
Idris AM, Al-Tayeb MAH, Potgieter-Vermaak Sanja S, Van Greken R, Potgieter JH (2007) Assessment of heavy metals pollution in Sudanese harbours along the Red Sea Coast. Microchem J 87:104–112
Iqbal J, Shah MH (2013) Health risk assessment of metals in surface water from freshwater source lakes, Pakistan. Human Ecol Risk Assess 19:1530–1543
Keleperzis E (2014) Investigating the sources and potential health risks of environmental contaminants in the soils and drinking waters from the rural clusters in Thiva area (Greece). Ecotoxicol Environ Saf 100:258–265
Khodabakhshi A, Amin MM, Mozaffari M (2011) Synthesis of magnetic nanoparticles and evaluation efficiency for arsenic removal from simulated industrial waste water. Iran J Environ Health Sci Eng 8:189–200
Kolluru RV, Bartell SM, Pitblado RM, Stricoff RS (1996) Risk assessment and management handbook. McGraw-Hill, New York
Kortatsi BK (2007) Groundwater quality in the Wassa West district of the western region of Ghana. West Afr J Appl Ecol 11:25–40
Kuma SJ (2004) Is groundwater in the Tarkwa gold mining district of Ghana potable? Environ Geol 45(3):391–400
Lee J-S, Chon H-T, Kim K-W (2005) Human risk assessment of As, Cd, Cu and Zn in the abandoned metal mine site. Environ Geochem Health 27:185–191
Li S, Zhang Q (2010) Spatial characterization of dissolved trace elements and heavy metals in the upper Han River (China) using multivariate statistical techniques. J Hazard Mater 176:579–588
Li J, Li FD, Liu Q et al (2014) Impacts of Yellow River irrigation practice on trace metals in surface water: a case study of the Henan-Liaocheng Irrigation Area, China. Human Ecol Risk Assess 20:1042–1057
Lim HS, Lee JS, Chon HT, Sager M (2008) Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J Geochem Explor 96:223–230
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a black foot disease area in Taiwan. Sci Total Environ 313(1–3):77–89
Lu XW, Wang LJ, Li LY, Lei K, Huang L, Kang D (2010) Multivariate statistical analysis of heavy metals in street dust of Baoji, NW China. J Hazard Mater 173:744–749
Mahmood A, Muqbool W, Mumtaz MW, Ahmad F (2011) Application of multivariate statistical techniques for the characterization of groundwater quality of Lahore, Gujranwala and Sialkot (Pakistan). Pak J Anal Environ Chem 12(1–2):102–112
Manish K, Ramanathan A, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ Geol 50:1025–1039
Marcovecchio JE, Botte SE, Freije RH (2007) Heavy metals, major metals, trace elements. In: Nollet LM (ed) Handbook of water analysis, 2nd edn. CRC Press, London, pp 275–311
Marengo E, Gennaro MC, Robotti E, Rossanigo P, Rinaudo C, Roz-Gastaldi M (2006) Investigation of anthropic effects connected with metal ions concentration, organic matter and grain size in Bormida river sediments. Anal Chim Acta 560:172–183
Masoud AA (2014) Groundwater quality assessment of shallow aquifers west of the Nile Delta (Egypt) using multivariate statistical and geostatistical technique. J Afr Earth Sci 95:123–137
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metal in drinking water and development of heavy metal pollution index. J Environ Sci Health A 31:283–289
Momodu MA, Anyakora CA (2010) Heavy metal contamination of groundwater: the Surulere case study. Res J Environ Earth Sci 2:39–43
Muhammed S, Shari MT, Khan S (2011) Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem J 98:334–343
Narany ST, Ramli MF, Aris AZ, Sulaiman WNA, Fakharian K (2014) Spatiotemporal variation of groundwater quality using integrated multivariate statistical and geostatistical approaches in Amol-Babol plain, Iran. Environ Monit Assess 186:5797–5815
Nassef M, Hannigan R, Sayed KAE, Tahawy MSE (2006) Determination of some heavy metals in the environment of Sadat Industrial City. In: Proceedings of the 2nd Environmental Physics Conference, Alexandria, Egypt
Nguyen VA, Bang S, Viet PH, Kim KW (2009) Contamination of groundwater and risk assessment for arsenic exposure in Ha Nam province, Vietnam. Environ Int 35(3):466–472
Nkansah MA, Boadi NO, Badu M (2010) Assessment of the quality of water from Hand-Dug Wells in Ghana. Environ Health Insights 4:7–12
NRC (1983) Risk assessment in the federal government: managing the process. National Academy Press, Washington
Okogbue CO, Omonona OV, Aghamelu OP (2012) Qualitative assessment of ground water from Egbe-Mopa basement complex area, North-Central Nigeria. Environ Earth Sci 67:1069–1083
Öztürk M, Özözen G, Minareci O, Minareci E (2009) Determination of heavy metals in fish, water and sediments of Avsar Dam Lake in Turkey. Iran J Environ Health Sci Eng 6:73–80
Panda UC, Sundaray SK, Rath P, Nayak BB, Bhatta D (2006) Application of factor and cluster analysis for characterization of river and estuarine water systems—a case study: Mahanadi River (India). J Hydrol 331:434–445
Parizi HS, Samani N (2013) Geochemical evolution and quality assessment of water resources in the Sarcheshmeh copper mine area (Iran) using multivariate statistical techniques. Environ Earth Sci 69:1699–1718
Paustenbach DJ (2002) Human and ecological risk assessment: theory and practice. John Wiley and Sons, New York
Prasad B, Bose JM (2001) Evaluation of the heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environ Geol 41:183–188
Rashed MN (2010) Monitoring of contaminated toxic and heavy metals from mine tailings through age accumulation in soil and some wild plants at Southeast Egypt. J Hazard Mater 178(1–3):739–746
Rattan RK, Datta SP, Chhonkar PK, Suribabu K, Singh AK (2005) Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater: a case study. Agric Ecosyst Environ 109:310–322
Reddy SJ (1995) Encyclopaedia of environmental pollution and control. Environ Media Karlia 1:342
Rubio B, Nombela MA, Vilas F (2000) Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): an assessment of metal pollution. Mar Pollut Bull 40(11):968–980
Saeedi M, Li LY, Salmanzadeh M (2012) Heavy metals and polycyclic aromatic hydrocarbons: pollution and ecological risk assessment in street dust of Tehran. J Hazard Mater 227–228:9–17
Shihab AS, AbdulBaqi YT (2010) Multivariate analysis of groundwater quality of Makhmoor Plain North Iraq. Damascus Univ J 26(1):19–26
Siriwong W (2006) Organophosphate pesticide residues in aquatic ecosystem and health risk assessment of local agriculture community. Dissertation, Chulalongkorn University, Thailand
Sprang PAV, Verdonck FAM, Assche FV, Regoli L, Schamphelaere KACD (2009) Environmental risk assessment of zinc in European freshwaters: a critical appraisal. Sci Total Environ 407(20):5373–5391
Taboada-Castro M, Diéguez-Villar A, Rodríguez Luz, Blanco M, Teresa Taboada-Castro M (2012) Agricultural impact of dissolved trace elements in runoff water from an experimental catchment with hand-use changes. Commun Soil Sci Plant Anal 43:81–87
Tay C, Kortatsi B (2007) Groundwater quality studies. A case study of Densu Basin, Ghana. West Afr J Appl Ecol 12:81–99
Uddameri V, Honnungar V, Hernandez EA (2014) Assessment of groundwater quality in central and southern Gulf Coast aquifer, TX using principal component analysis. Environ Earth Sci 71:2653–2671
Underwood EJ (1956) Trace elements in humans and animals nutrition, 3rd edn. Academic Press, New York
United States Centre for Disease Control (2011) Iron overload and Hemochromalosis. Centre for Disease control. March 28
USEPA IRIS (2011) (US Environmental Protection Agency)’s Integrated Risk Information System. Environmental Protection Agency Region I, Washington DC 20460. US EPA, 2012. http://www.epa.gov/iris/
Vanloon GW, Duffy SJ (2005) The hydrosphere. In: environmental chemistry: a gold perspective, 2nd edn. Oxford University Press, New York
Varol S, Davraz A (2014) Evaluation of the groundwater quality with WQI (Water Quality Index) and multivariate analysis: a case study of the Tefenni plain (Burdur/Turkey). Environ Earth Sci. doi:10.1007/s12665-014-3531-z
Vodela JK, Renden JA, Lenz SD, Henney WHM, Kemppainen BW (1997) Drinking water contaminants (Arsenic, cadmium, lead, benzene, and trichloroethylene). Interaction of contaminants with nutritional status on general performance and immune function in broiler chickens. Pollut Sci 76:1474–1492
White AF, Benson SM, Yee AW, Woolenberg HA, Flexser S (1991) Groundwater contamination at the Kesterson reservoir, California-geochemical parameters influencing selenium mobility. Water Resour Res 27:1085–1098
Wilding LP (1985) Spatial variability: its documentation, accommodation and implication to soil surveys. In: Nielsen DR, Bouma J (eds) Soil spatial variability. Pudoc, Wageningen, pp 166–194
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ Geochem Health 36:169–182
World Health Organization (2011) Guidelines for drinking-water quality, 4th edn. Switzerland, Geneva
Wu B, Zhao DY, Jia HY, Zhang Y, Zhang XX, Cheng SP (2009) Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing section, China. Bull Environ Contam Toxicol 82:405–409
Yadav CI, Devi NL, Mohan D, Shihua Q, Singh S (2014) Assessment of groundwater quality with special reference to Arsenic in Nawalparasi District, Nepal using multivariate statistical technique. Environ Earth Sci 72:259–273
Yu FC, Fang GH, Ru XW (2010) Eutrophication, health risk assessment and spatial analysis of water quality in Gucheng Lake, China. Environ Earth Sci 59:1741–1748
Zhang Y, Li FD, Ouyang Z, Zhao GS, Li J, Liu Q (2013) Distribution and health risk assessment of heavy metals of groundwater in the irrigation district of the lower reaches of Yellow River. Environ Sci 34:121–128
Authors’ contributions
All authors read and approved the final manuscript.
Acknowledgements
The authors are very grateful to the National Council for tertiary Education (NTCE), Ghana for a research grant under the Teaching and Learning Innovation Fund (TALIF-KNUSTR/3/005/2005).
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Boateng, T.K., Opoku, F., Acquaah, S.O. et al. Pollution evaluation, sources and risk assessment of heavy metals in hand-dug wells from Ejisu-Juaben Municipality, Ghana. Environ Syst Res 4, 18 (2015). https://doi.org/10.1186/s40068-015-0045-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40068-015-0045-y