Abstract
Immune-checkpoint inhibitors (ICIs), different from traditional cancer treatment models, have shown unprecedented anti-tumor effects in the past decade, greatly improving the prognosis of many malignant tumors in clinical practice. At present, the most widely used ICIs in clinical immunotherapy for a variety of solid tumors are monoclonal antibodies against cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1) and their ligand PD-L1. However, tumor patients may induce immune-related adverse events (irAEs) while performing immunotherapy, and irAE is an obstacle to the prospect of ICI treatment. IrAE is a non-specific disease caused by immune system imbalance, which can occur in many tissues and organs. For example, skin, gastrointestinal tract, endocrine system and lung. Although the exact mechanism is not completely clear, related studies have shown that irAE may develop through many ways. Such as excessive activation of autoreactive T cells, excessive release of inflammatory cytokines, elevated levels of autoantibodies, and common antigens between tumors and normal tissues. Considering that the occurrence of severe IrAE not only causes irreversible damage to the patient’s body, but also terminates immunotherapy due to immune intolerance. Therefore, accurate identification and screening of sensitive markers of irAE are the main beneficiaries of ICI treatment. Additionally, irAEs usually require specific management, the most common of which are steroids and immunomodulatory therapies. This review aims to summarize the current biomarkers for predicting irAE in gastric cancer and their possible mechanisms.
Similar content being viewed by others
Introduction
In recent years, immune checkpoint inhibitors (ICIs) have achieved satisfactory results in various tumor types, greatly changing the treatment strategy of tumors and bringing more benefits to patients’ survival [1,2,3]. However, the survival benefit of cancer patients depends not only on the efficacy of ICI, but also on the occurrence of related adverse events caused by ICI, namely immune-related adverse events (irAE) [4, 5]. IrAEs are very common in ICI treatment and can occur in multiple tissues and organs [6, 7]. Among them, the most commonly affected organs for grade 3 or above irAE are the digestive system, endocrine system, and lungs, while others include the nervous system, kidneys, liver, and heart [8, 9]. Kawazoe et al. [10] found in a study evaluating the safety and efficacy of pembrolizumab combined with S-1 plus oxaliplatin as first-line treatment for advanced gastric/gastroesophageal junction cancer that the incidence of grade 3 or above irAE was 57.4%. The more common irAEs were thrombocytopenia (14.8%), neutropenia (13.0%), colitis (5.6%), and adrenal insufficiency (5.6%). Other irAEs included pneumonia, type 1 diabetes, and peripheral neuropathy. For low-grade irAEs that occur during ICI treatment, corresponding routine treatments (such as steroid hormones and immunosuppressants) can be given to relieve clinical symptoms, while high-grade irAEs may consider suspending or terminating immunotherapy [11, 12]. Previous hypotheses suggested that the mechanisms behind irAEs include overactivation of the immune system, excessive release of inflammatory cytokines, elevated levels of pre-existing autoantibodies, and the presence of shared antigens between tumors and normal tissues [9, 13, 14] (Fig. 1). However, given the relatively hidden clinical features of irAE, the immature mechanism of occurrence and lack of identification of sensitive markers that may occur irAE, which makes early prediction of patients susceptible to irAE particularly difficult [9]. Thus, understanding irAE and developing predictive biomarkers for their occurrence are crucial for achieving the maximum benefit–risk ratio for ICI-treated patients. Considering the unique clinical value, convenience and accuracy of biomarkers, we list the currently known predictive biomarkers for gastric cancer irAE in this review, providing targeted insights into irAEs as a reference for future studies.
The potential mechanism of irAE related to ICI treatment. A Blocking CTLA-4 can induce the activation of autoreactive T cells by inducing Treg depletion and functional defects, thereby stimulating B cells to increase the production of autoantibodies. B Blocking CTLA4 and/or PD1 may be related to the expansion and activation of pre-existing tissue-resident memory T cells. C Blocking PD-1 can induce the reactivation of depleted/disabled T cells, which leads to the overactivation of autoreactive T cells. Epitope diffusion can lead to the destruction of tolerance. D The death of tumor cells killed by T cells can induce an increase in the level of pro-inflammatory cytokines, which in turn leads to damage to normal tissues or organs. E ICl may directly damage tissues or organs by binding to CTLA-4 and/or PD1 expressed in normal tissues. DC, dendritic cells; MHC, major histocompatibility complex; Treg, regulatory T cells; TCR, T cell receptor
Potential biomarkers associated with therapeutic response to ICIs in GC
Currently, many biomarkers are used in clinical practice, including PD-L1 clinical prediction score (CPS), microsatellite instability (MSI) status, and other recognized markers such as tumor mutation burden (TMB) and gene expression score (GEP) [15,16,17,18]. However, although the above markers have certain predictive value for ICIs, their predictive ability is still not ideal, and there are contradictions in some studies. Therefore, finding reliable biomarkers is crucial for the initial identification of patients with tumors that may benefit from ICIs treatment.
Studies have shown that tissue-resident memory T cells (TRM) and tertiary lymphoid structures (TLS) are associated with good prognosis [19, 20]. Mori et al. [19] discovered through immunohistochemical detection CD103 + T cells and evaluated the relationship between CD103 + T cells and TLS that CD103 + T cells were located around TLSs, and patients with high CD103 showed rich TLS, and patients with high CD103 cells and rich TLSs had better prognosis. In addition, CD103 + CD8 + cells in GC showed better prognosis and were associated with TLS. For patients receiving ICI treatment, high CD103 and TLSs enrichment showed excellent anti-tumor immune response. Moreover, CD103 + CD8 + T cells in GC express higher levels of PD-1, granzyme B, and interferon-γ than CD103—CD8 + T cells. In a study on the distribution and proportion of TLSs and TRMs in lung adenocarcinoma, Zhao et al. [20] showed that the proportion of TRMs within TLSs was significantly higher than that outside TLSs, and was positively correlated with patient survival. In addition, CD103 + TRMs were closely related to TLS maturity, and higher maturity TLSs showed higher proportions of CD4 + CD103 + TRMs and CD8 + CD103 + TRMs. Subsequently, Nose et al. [21] also showed that in patients with ICI treatment, those with high levels of CD103 in peripheral blood CD8 + T cells had significantly higher progression-free survival than the low-level group, and the CD103 + CD8 + T cell population was mainly composed of central memory T cells, showing high Ki-67 expression and a small amount of cytotoxic particles.
Additionally, based on the whole and single-cell RNA-seq data of tumor-infiltrating immune cells, Yang et al. [22] found that tumor-infiltrating PD-1hiCD8 + T cells could serve as effective biomarker for ICI treatment response in multiple cancers, including GC. In clinical samples and animal models, they found that high-score PD-1hiCD8 + T cell subsets have better therapeutic response and longer survival time. Moreover, tumor-infiltrating PD-1hiCD8 + T cells showed better predictive performance when combined with tumor mutation burden (TMB), which can be used as an effective supplementary biomarker for TMB. Zhang et al. [23] established an EV-score based on four plasma EV proteins (ARG1/CD3/PD-L1/PD-L2) to predict immunotherapy results and dynamically monitor disease progression. They believe that high EV-score reflects stronger anti-tumor immune microenvironment characteristics, manifested as more activated CD8 + T/NK cells, higher Th1/Th2 ratio and higher IFN-γ/perforin/granzyme expression in peripheral blood. Among GC patients, those with an EV-score ≥ 1 can benefit more from ICIs, while those with EV-score < 1 could potentially benefit more from ICIs in combination with HER2 treatment.
According to reports, the TGFβ signaling plays a key role in cancer progression by forming the tumor structures and inhibiting the anti-tumor activity of immune cells [24]. Increasing evidence suggests that TGF-β is involved in regulating the composition and behavior of immune components in the tumor microenvironment (TME), thereby inducing tumor immune escape, especially ICI [25,26,27]. Related studies have confirmed that overactive TGF-β signaling is associated with ICI resistance, and the synergistic effect of TGF-β blockers and ICI can significantly reduce tumor immune tolerance [28,29,30]. For example, TGF-β/PD-L1 bispecific antibodies such as YM101, BiTP, and M7824 have shown strong anti-tumor activity in preclinical and clinical models. All of them showed high binding affinity to TGF-β/PD-L1 dual targets, and had better anti-tumor activity than single anti-PD-L1 or anti-TGF-β treatment [29,30,31]. Mechanistically, YM101 exerts anti-tumor effects by increasing the number of tumor-infiltrating lymphocytes and dendritic cells, increasing the proportion of M1/M2, and enhancing the production of cytokines in T cells [29]. BiTP demonstrates potent anti-tumor effects by reducing collagen deposition, enhancing CD8 + T cell penetration in the tumor microenvironment and increasing tumor T lymphocyte infiltration [30]. M7824 can activate dual anti-immunosuppressive functions through TME, induce anti-tumor activity by the innate and adaptive immune system, and block TGF-β1-induced tumor interstitialization and PD-L1-dependent immunosuppression potential [31,32,33]. In addition, TGFβ2 is highly expressed and is associated with the expression of genes drive epithelial–mesenchymal transition (EMT) in GC [34]. Meanwhile, TGFβ2 was associated with high levels of multiple immune cell infiltration and cytokine expression in GC microenvironment. The expression of PD-1, PD-L1 and CTLA-4 was significantly higher in tissues with high TGFβ2 expression, and the reactivity of immune checkpoint blockade (ICB) was significantly enhanced [35].
However, there is basically no specific signaling pathway in the process of anti-tumor immunity, such as Notch signaling pathway; PI3K–AKT signaling pathway; hedgehog signaling pathway; NF-κB signaling pathway; JAK–STAT signaling pathway; Wnt/β-catenin signaling pathway, etc [17, 36,37,38,39,40]. Almost all biological processes of cell proliferation and transformation involve these same signaling pathways. For example, Notch signaling is associated with anti-tumor immunity/immunotherapy [41, 42]. Co-mutations of NOTCH1-3 and homologous repair genes are associated with lasting clinical benefits [43]. Recent studies by Long et al. [44] have shown that NOTCH4 mutations have better clinical benefits in patients with gastric cancer, and NOTCH4 mutations are significantly associated with enhanced immunogenicity, including TMB, co-stimulatory molecule expression and activation of antigen processing mechanisms. In addition, Wnt/catenin pathway is also involved in tumor immunotherapy [45]. Blocking the Wnt/catenin pathway can increase the sensitivity of gastric cancer cells to PD-1 antibody.
Studies show that inflammatory biomarkers have a potential prognostic effect on ICI treatment in cancer patients [46, 47]. The GIPI nomogram established by Formica et al. [48] based on NLR, CRP and ALB showed a significant prognostic value for mGOJ/GC at the metastatic gastroesophageal junction receiving ICI. Similarly, Chen et al. [49] controlled nutritional status (CONUT) score based on total lymphocyte count (TL), total cholesterol level (T-CHOL) and serum albumin (ALB), providing a useful immunological prognostic biomarkers for cancer patients. Patients with high CONUT scores were associated with shorter PFS and OS of ICI or chemotherapy. In addition, patients with high CONUT score had lower PFS and OS in the case of PD-1/PDL1 positive expression.
With the shift towards individualized and precise therapy in the current treatment modes, circulating tumor DNA (ctDNA) has become a crucial biomarker for evaluating the therapeutic effect of ICI before or after treatment of solid tumors [50,51,52]. Compared to traditional marker screening, ctDNA has the following unique advantages [53, 54]. First, ctDNA detection is less invasive and only requires a minimal peripheral blood sample instead of a biopsy. Secondly, ctDNA mainly consists of genomic DNA fragments released from cell apoptosis, necrosis or active secretion. Thus, it reflects more comprehensive tumor information compared to tissue biopsy. Besides, tissue biopsy can only be further detected after tumor progression, while ctDNA can be monitored in real time. Kim et al. [55] evaluated serum ctDNA levels in ICI-treated metastatic GC and showed that decreased ctDNA was associated with improved prognosis.
Recently, research on ICI-induced irAE has also found a correlation between the occurrence of irAE and good treatment outcomes [56, 57]. Two hypotheses may explain this phenomenon [18, 58, 59]. One is that the immune response triggered in unrelated areas to the tumor may be non-specific immune or inflammatory, and the other may be the response to antigens that cross-react with tumor-associated antigens. Therefore, irAEs are considered potential clinical biomarker for predicting ICI response. Known gastric cancer ICIs related biomarkers are shown in Table 1.
As mentioned earlier, multiple studies have shown that the occurrence of irAE is associated with better clinical outcomes in ICI-treated various cancers [82, 83]. Hussaini et al. [84] conducted a study on the correlation between the incidence of irAE after using ICIs and the clinical prognosis of various solid tumors. They found that the occurrence of irAEs was positively correlated with ORR, PFS and OS, but not with tumor location and ICI type. Additionally, grade 3 or higher irAEs show a better ORR but worse OS. Similarly, Xu et al. [85] based on the correlation between irAE and ICI efficacy in patients with hepatocellular carcinoma (HCC) also showed that the occurrence of irAE was associated with clinical benefit. However, they believe that low-grade irAE is a better predictive marker for ICI treatment in HCC. Given that Hussaini et al. research was based on the evaluation of irAE and ICI treatment efficacy for multiple malignant tumors, while Xu et al. research object was only liver cancer patients, which may lead to differences in this result. Additionally, Xu et al.also found that patients with diarrhea/colitis, hyperthyroidism/hypothyroidism or rash had better prognosis [85]. Similarly, a previous meta-analysis also showed that the occurrence of endocrine, skin and gastrointestinal irAE was significantly associated with good prognosis in ICIs-treated patients, while other irAEs were not [86]. Considering the consistency of the results from multiple studies, the occurrence of irAE is significantly associated with excellent clinical prognosis. Therefore, can it be considered that the sensitive biomarkers predicting the occurrence of irAEs may also predict the therapeutic effect of ICI to some extent, and the biomarkers of the two are somewhat similar?
Potential biomarkers associated with irAEs in GC
IrAEs are mainly caused by non-specific activation of the immune system and can manifest as specific and non-specific symptoms [9]. Previous hypotheses mainly focused on the excessive release of inflammatory cytokines, the overactivation of the immune system, the amplification of pre-existing abnormal antibodies, and the existence of shared antigens between tumors and normal tissues [13, 14]. Non-specific symptoms typically include fever, cough and fatigue, while specific symptoms usually involve adverse reactions of specific organs or tissues to ICI treatment in different tissues or organs, such as colitis, hyperthyroidism and interstitial pneumonia [87]. However, most common clinical ICB strategies are a combination of immunotherapy with chemotherapy or targeted therapy, making it difficult to determine whether adverse events are caused by immunotherapy alone [9, 87]. Therefore, it is urgent to develop easy-to-detect biomarkers to identify irAEs. Various candidate biomarkers associated with irAEs have been reported, including gene expression profiles, C-reactive protein, human leukocyte antigen (HLA) and gut microbiome, which can predict irAE [88,89,90,91,92,93]. However, current irAE biomarkers for gastric cancer are relatively few and lack a comprehensive summary. Known irAE biomarkers are shown in Table 2.
EV-ICOS and EV-IDO1
Extracellular vesicles (EVs), nanoparticles (40–160 nm in diameter) containing various bioactive molecules, including proteins, lipids and nucleic acids, and are produced by almost all types of cells. EVs not only mediate signal transduction in cell communication, but also participate in physiological processes such as immune regulation and cancer progression [94]. Based on microarray screening and further validation, Jiang et al. [95] found that inducible T cell co-stimulatory factor (EV-ICOS) and indoleamine 2,3-dioxygenase 1 (EV-IDO1) can effectively predict and monitor irAE in ICI- treated GC and serve as biomarkers of irAE. Both the discovery and validation cohorts showed that the expression of EV-ICOS and EV-IDO1 in irAE patients was significantly lower than that those in non-IRAE patients after ICI treatment for a period of time. Furthermore, patients with high EV-ICOS and EV-IDO1 expression had longer time intervals from the start of treatment to irAE than those with low EV-ICOS and EV-IDO1 expression. Besides, EV-ICOS and EV-IDO1 levels were also positively correlated with CA72-4 levels. Taken together, these results suggest that GC patients with higher EVICOS or EV-IDO1 carry a lower risk of irAE with shorter intervals, representing a group of patients with better tolerance to ICI. However, EV-ICOS and EV-IDO1 were not associated with the efficacy of immunotherapy. Subsequently, they analyzed the tumor immune microenvironment (TIME) associated with EV-ICOS and EV-IDO1, and found that high expression of EV-ICOS and EV-IDO1 always showed higher immune cell infiltration and showed unique TIME.
HLA-DR15
ICI-induced secondary adrenal insufficiency is considered to be a ‘pituitary viral AE’ [96, 97]. Yano et al. [98] found that DR15, B52 and Cw12 were significantly higher in the study group than in the healthy control group by comparing the frequency of HLA alleles in pituitary irAE patients and healthy control group, which may be predisposing factor for pituitary irAE. Previous studies have shown that HLA-DR15 can participate in autoimmune diseases including ulcerative colitis, multiple sclerosis and Goodpaste’s syndrome through interleukin 17 (a cytokine produced by T helper cell 17) [99,100,101]. Previous studies have also shown that the combination of anti-PD-1 and anti-CTLA4 antibodies increases the risk of pituitary irAE [102]. Moreover, anti-PD-1 and anti-CTLA4 inhibitors up-regulated Th1 and Th17 pathways [103, 104]. This may suggest that HLA-DR15 can be used as a predictive marker of pituitary irAE.
Tertiary lymphatic structure (TLS)
Previous studies shown that TLSs are associated with anti-tumor immune responses and good prognosis in several cancers [105, 106]. TLS is an aggregation of various immune cells around B cells. It is similar to secondary lymphoid organs in structure and function, and plays a role in antigen preservation and activation of T cells [107, 108]. Mori et al. [19] found that high TLS showed excellent anti-tumor immune response and higher frequency of irAEs in GC patients with partial response (PR) to ICI. They believe that high TLS have stronger immune response and higher T cell activation, result in more irAE and better nivolumab efficacy. Previous have also reported that CD103 + T cells are memory T cells resident in tissues of interest as targets for immunotherapy, produce CXCL13, and are critical for the formation of TLS [107, 109]. Furthermore, various studies have shown that CD8 + T cells expressing CD103 are associated with good prognosis in some cancers, including GC, and about 70% of CD8 + TILs are resident memory T cells in GC [19, 110, 111].
P-CRP and CA19-9
The theory that high C-reactive protein (CRP) levels are closely related to poor prognosis is well known [112, 113]. It has been reported that P-CRP (another platelet-associated inflammatory marker) is a useful prognostic indicator for various cancers, including GC [112,113,114,115]. Inflammatory cytokines, including interleukin-6 (IL-6), are mediators of tumor-associated inflammation that can lead to elevated CRP [116, 117]. Given that serum CA19-9 often increase in cancer patients, it is also used as a tumor marker for GC patients. Matsunaga et al. [118] found that CA19-9 and P-CRP were effective predictors of irAE, and the predictive ability of the combination of P-CRP and CA19-9 was much higher than that of P-CRP or CA19-9 alone.
NLR and PLR
Neutrophil-to-lymphocyte (NLR) and platelet-to-lymphocyte (PLR) ratios, as conventional inflammatory markers, often represent poor prognosis [119, 120]. Tumor microenvironment is composed of inflammatory cells, which play an important role in tumorigenesis and tumor growth [121]. Pavan et al. reported that NLR and PLR are also important predictors for the development of irAE in advanced non-small cell lung cancer [122]. Subsequently, Takada et al. [123] evaluated NLR and PLR as early predictive markers of irAE in GC and found that 120% of NLR before treatment was significantly correlated with increased risk, while PLR did not show significant correlation. In addition, their results suggested that time-dependent changes in NLR elevation can be useful markers for predicting the development of severe irAE after immunotherapy. However, Fan et al. [124] found that PLR < 135 was associated with a higher incidence of irAE.
ALB and type I hypersensitivity
Research shown that the incidence of irAE in patients with autoimmune diseases is higher than those without autoimmune diseases [125, 126]. Shimozaki et al. [127] found that type I hypersensitivity was associated with the occurrence of irAE and was a risk factor for irAE by analyzing the data of patients with and without irAE. Type I hypersensitivity is an allergic reaction caused by IgE binding to chemokines such as histamine, prostaglandins, and leukotrienes released by mast cells, and allergen-specific CD4 + T cells are involved in type T hypersensitivity [128]. In addition, when PD-1 is blocked by ICB, allergen-specific CD4 + T cells may be activated to produce cytokines such as IFN-γ, TNF-α, and IL-5, leading to irAE [129]. Previous studies have also reported that poor nutritional status and poor performance status (PS) according to the European Cooperative Oncology Group are associated with lower immunotherapy efficacy [130, 131]. Shimozaki et al.also found that patients with good PS showed a tendency to irAE, but did not show a significant correlation, and serum albumin level ≥ 3.6 g/dl was a risk factor for irAE [127].
Management of irAE
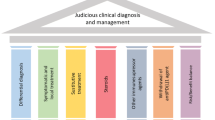
Although the clinical application of ICIs against CTLA-4 and PD-1 has shown significant anti-tumor response and improved the treatment of various cancers, irAE often occurs [4, 5]. IrAE is considered to be impaired self-tolerance caused by loss of T cell suppression, involving all organ systems. Among them, skin, gastrointestinal tract, liver, endocrine system and lungs being the most common [8, 9]. The main treatment for irAE is corticosteroids or other immunosuppressive agents such as infliximab, and most irAE can be controlled through appropriate management, but may be fatal in some cases [132]. Additionally, the clinical features of irAE are relatively hidden, and the imaging findings are not obvious [9]. So early diagnosis and management of irAE is a challenge for doctors (Fig. 2).
Gastrointestinal adverse events: diarrhea/colitis
Diarrhea or colitis is mainly manifested as abdominal pain, diarrhea, mucous bloody stools and fever, and usually begins 6–8 weeks after ICIs treatment [11]. Studies shown that the incidence of gastrointestinal irAEs is significantly higher in anti-CTLA-4 monotherapy than that of anti-PD-1/PD-L1, with the incidence of about 35%. In addition, the highest incidence of diarrhea/colitis occurs when two ICIs are combined [11, 133, 134]. NCCN guidelines [11] recommend that patients with mild diarrhea continue to receive immunotherapy and symptomatic management, while closely monitoring patients to prevent colitis symptoms. For patients with moderate diarrhea/colitis, corticosteroids are usually the preferred treatment, and over 50% of patients’ symptoms are relieved with corticosteroids. However, when corticosteroids are unable to control symptoms and diarrhea/colitis may persist or worsen, consider using infliximab (a monoclonal anti-tumor necrosis factor alpha (TNF-a)) for the treatment of various autoimmune diseases, including Crohn’s disease, ulcerative colitis, rheumatoid arthritis, etc.). For patients with grade 3 or above or severe life-threatening diarrhea/colitis, inpatient care should be considered, along with corticosteroids or immunosuppressants, and suspension or termination of immunotherapy if necessary.
Hepatitis
Immune-related hepatitis is mainly characterized by liver dysfunction (elevated alanine transaminase (ALT) or aspartate transaminase (AST), with or without elevated bilirubin). It usually occurs 6–14 weeks after ICIs treatment. When ALT/AST reaches 3–5 times the upper limit of normal, it is recommended to continue or reduce ICI treatment, and regularly monitor liver function. If there is no improvement or deterioration, steroids should be used. When ALT/AST reaches more than 5 times the upper limit of normal and steroid treatment is not significantly relieved, consider suspending or terminating ICI [12, 135].
Skin diseases
The most common manifestation of skin toxicity is rash, usually occurs in the first few weeks of ICI treatment, and is more common in late-stage melanoma, with an incidence of about 24.3%. When skin toxicity occurs, it is recommended that patients contact to avoid chemicals and use sun protection and other measures. In addition, for mild-to-moderate symptoms, topical use of steroids, about 4 weeks can be subsided. However, in severe cases, prednisolone or methylprednisolone can be used for treatment [136].
Pulmonary
The incidence of pneumonia is relatively low in ICI treatment, about 2–5%. The main manifestations were dyspnea or cough. But when two ICI combinations are used, the incidence of pneumonia can reach 5–10%. ICI can be continued for asymptomatic pneumonia, but if pneumonia-related symptoms occur, consider interrupting ICI and starting steroid therapy [12].
Endocrine
Endocrine toxicity is usually manifested as thyroid dysfunction. The onset time is usually about 8 weeks. For asymptomatic thyroid dysfunction, usually without intervention, only regular monitoring. For hypothyroidism with symptoms, it is recommended to take levothyroxine; hyperthyroidism patients recommended the use of βreceptor blocker treatment; if the symptoms are obvious, steroid or anti-thyroid medications should be considered. For severe patients, prednisolone is recommended [137, 138].
Based on the survival benefits of current ICI for a variety of advanced cancer patients and potential irAE. Ryan et al. proposed the following methods to reduce irAE in the future. First, the preventive use of drugs in high-risk populations to prevent their occurrence requires the search for reliable irAE biomarkers. Second, wait-and-see and use these immunosuppressive drugs or alter the dose and timing of ICI antibodies to maintain immune benefit while reducing immune-related toxicity. Third, alternative ICI with less toxicity and no irAE or lower irAE was developed [14].
Conclusion
IrAE is considered to be related to the toxicity of ICI antibody mechanism. Therefore, while ensuring the therapeutic effect of ICI, the occurrence of irAE should be avoided or reduced as much as possible. To the best of our knowledge, this is the first comprehensive summary of ICI efficacy and irAE biomarkers in gastric cancer patients. Unfortunately, there are currently relatively few studies on biomarkers associated with irAE in gastric cancer, so only a small number of marker studies have been shown. Given that ICI has certain prospects for current cancer treatment, it may be accompanied by more irAE. Therefore, developing better biomarkers is critical for the management of irAE.
Availability of data and materials
All data and materials in our study are available upon reasonable request.
References
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–46.
Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51.
Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15(4):235–47.
Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–85.
Jing Y, Yang J, Johnson DB, Moslehi JJ, Han L. Harnessing big data to characterize immune-related adverse events. Nat Rev Clin Oncol. 2022;19(4):269–80.
Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6(6):865–71.
von Itzstein MS, Khan S, Gerber DE. Investigational biomarkers for checkpoint inhibitor immune-related adverse event prediction and diagnosis. Clin Chem. 2020;66(6):779–93.
Friedlander P, Wood K, Wassmann K, Christenfeld AM, Bhardwaj N, Oh WK. A whole-blood RNA transcript-based gene signature is associated with the development of CTLA-4 blockade-related diarrhea in patients with advanced melanoma treated with the checkpoint inhibitor tremelimumab. J Immunother Cancer. 2018;6(1):90.
Jia XH, Geng LY, Jiang PP, et al. The biomarkers related to immune related adverse events caused by immune checkpoint inhibitors. J Exp Clin Cancer Res. 2020;39(1):284.
Kawazoe A, Yamaguchi K, Yasui H, et al. Safety and efficacy of pembrolizumab in combination with S-1 plus oxaliplatin as a first-line treatment in patients with advanced gastric/gastroesophageal junction cancer: cohort 1 data from the KEYNOTE-659 phase IIb study. Eur J Cancer. 2020;129:97–106.
Thompson JA, Schneider BJ, Brahmer J, et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1. 2020. J Natl Compr Canc Netw. 2020;18(3):230–41.
Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60.
Ke W, Zhang L, Dai Y. The role of IL-6 in immunotherapy of non-small cell lung cancer (NSCLC) with immune-related adverse events (irAEs). Thorac Cancer. 2020;11(4):835–9.
Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov. 2022;21(7):495–508.
Zhao JJ, Yap DWT, Chan YH, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol. 2022;40(4):392–402.
Kwon M, An M, Klempner SJ, et al. Determinants of response and intrinsic resistance to PD-1 blockade in microsatellite instability-high gastric cancer. Cancer Discov. 2021;11(9):2168–85.
Wu X, Gu Z, Chen Y, et al. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J. 2019;17:661–74.
Park R, Lopes L, Saeed A. Anti-PD-1/L1-associated immune-related adverse events as harbinger of favorable clinical outcome: systematic review and meta-analysis. Clin Transl Oncol. 2021;23(1):100–9.
Mori T, Tanaka H, Suzuki S, et al. Tertiary lymphoid structures show infiltration of effective tumor-resident T cells in gastric cancer. Cancer Sci. 2021;112(5):1746–57.
Zhao H, Wang H, Zhao Y, Sun Q, Ren X. Tumor-resident T cells, associated with tertiary lymphoid structure maturity, improve survival in patients with stage III lung adenocarcinoma. Front Immunol. 2022;13:877689.
Nose Y, Saito T, Yamamoto K, et al. The tissue-resident marker CD103 on peripheral blood T cells predicts responses to anti-PD-1 therapy in gastric cancer. Cancer Immunol Immunother. 2022. https://doi.org/10.1007/s00262-022-03240-2.
Yang Z, Deng Y, Cheng J, Wei S, Luo H, Liu L. Tumor-infiltrating PD-1hiCD8+-T-cell signature as an effective biomarker for immune checkpoint inhibitor therapy response across multiple cancers. Front Oncol. 2021;11:695006.
Zhang C, Chong X, Jiang F, et al. Plasma extracellular vesicle derived protein profile predicting and monitoring immunotherapeutic outcomes of gastric cancer. J Extracell Vesicles. 2022;11(4):e12209.
Holmgaard RB, Schaer DA, Li Y, et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer. 2018;6(1):47.
Villar VH, Subotički T, Đikić D, Mitrović-Ajtić O, Simon F, Santibanez JF. Transforming growth factor-β1 in cancer immunology: opportunities for immunotherapy. Adv Exp Med Biol. 2023;1408:309–28.
Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18(1):9–34.
Caja L, Dituri F, Mancarella S, et al. TGF-β and the tissue microenvironment: relevance in fibrosis and cancer. Int J Mol Sci. 2018;19(5):1294.
Yi M, Li T, Niu M, Wu Y, Zhao Z, Wu K. TGF-β: A novel predictor and target for anti-PD-1/PD-L1 therapy. Front Immunol. 2022;13:1061394.
Yi M, Zhang J, Li A, et al. The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1. J Hematol Oncol. 2021;14(1):27.
Yi M, Wu Y, Niu M, et al. Anti-TGF-β/PD-L1 bispecific antibody promotes T cell infiltration and exhibits enhanced antitumor activity in triple-negative breast cancer. J Immunother Cancer. 2022;10(12):e005543.
Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med. 2018;10(424):eaan5488.
Lind H, Gameiro SR, Jochems C, et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J Immunother Cancer. 2020;8(1):e000433.
Lan Y, Yeung TL, Huang H, et al. Colocalized targeting of TGF-β and PD-L1 by bintrafusp alfa elicits distinct antitumor responses. J Immunother Cancer. 2022;10(7):e004122.
Yang B, Bai J, Shi R, et al. TGFB2 serves as a link between epithelial-mesenchymal transition and tumor mutation burden in gastric cancer. Int Immunopharmacol. 2020;84:106532.
Han B, Fang T, Wang Y, Zhang Y, Xue Y. TGFβ2 is a prognostic biomarker for gastric cancer and is associated with methylation and immunotherapy responses. Front Genet. 2022;13:808041.
Katoh M. Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin Sci. 2019;133(8):953–70.
Wang Z, Wang X, Xu Y, et al. Mutations of PI3K-AKT-mTOR pathway as predictors for immune cell infiltration and immunotherapy efficacy in dMMR/MSI-H gastric adenocarcinoma. BMC Med. 2022;20(1):133.
Zhou C, Guo L, Cai Q, et al. Circulating neutrophils activated by cancer cells and M2 macrophages promote gastric cancer progression during PD-1 antibody-based immunotherapy. Front Mol Biosci. 2023;10:1081762.
Cui Y, Li Q, Li W, et al. NOTCH3 is a prognostic factor and is correlated with immune tolerance in gastric cancer. Front Oncol. 2021;10:574937.
Yamada K, Hori Y, Inoue S, et al. E7386, a selective inhibitor of the interaction between β-Catenin and CBP, exerts antitumor activity in tumor models with activated canonical Wnt signaling. Cancer Res. 2021;81(4):1052–62.
Roderick JE, Gonzalez-Perez G, Kuksin CA, et al. Therapeutic targeting of NOTCH signaling ameliorates immune-mediated bone marrow failure of aplastic anemia. J Exp Med. 2013;210(7):1311–29.
Minter LM, Turley DM, Das P, et al. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat Immunol. 2005;6(7):680–8.
Mazzotta M, Filetti M, Occhipinti M, et al. Efficacy of immunotherapy in lung cancer with co-occurring mutations in NOTCH and homologous repair genes. J Immunother Cancer. 2020;8(2):e000946.
Long J, Wang D, Yang X, et al. Identification of NOTCH4 mutation as a response biomarker for immune checkpoint inhibitor therapy. BMC Med. 2021;19(1):154.
Muto S, Enta A, Maruya Y, et al. Wnt/β-catenin signaling and resistance to immune checkpoint inhibitors: from non-small-cell lung cancer to other cancers. Biomedicines. 2023;11(1):190.
Namikawa T, Yokota K, Tanioka N, et al. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today. 2020;50(11):1486–95.
Zhang S, Qiu C, Yu H, Xu Y, Xu X. Prognostic value of neutrophil to lymphocyte ratio in gastric cancer patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Front Oncol. 2023;13:1070019.
Formica V, Morelli C, Patrikidou A, et al. Gastric inflammatory prognostic index (GIPI) in patients with metastatic gastro-esophageal junction/gastric cancer treated with pd-1/pd-l1 immune checkpoint inhibitors. Target Oncol. 2020;15(3):327–36.
Chen L, Sun H, Zhao R, et al. Controlling nutritional status (CONUT) predicts survival in gastric cancer patients with immune checkpoint inhibitor (PD-1/PD-L1) outcomes. Front Pharmacol. 2022;13:836958.
Zeng Z, Yang B, Liao Z. Biomarkers in immunotherapy-based precision treatments of digestive system tumors. Front Oncol. 2021;11:650481.
Jin Y, Chen DL, Wang F, et al. The predicting role of circulating tumor DNA landscape in gastric cancer patients treated with immune checkpoint inhibitors. Mol Cancer. 2020;19(1):154.
Catenacci DV, Kang YK, Uronis HE, et al. Circulating tumor DNA as a predictive biomarker for clinical outcomes with margetuximab and pembrolizumab in pretreated her2-positive gastric/ gastroesophageal adenocarcinoma. Oncology. 2023;37(4):176–83.
Yang M, Forbes ME, Bitting RL, et al. Incorporating blood-based liquid biopsy information into cancer staging: time for a TNMB system? Ann Oncol. 2018;29(2):311–23.
Hofman P, Heeke S, Alix-Panabières C, Pantel K. Liquid biopsy in the era of immuno-oncology: is it ready for prime-time use for cancer patients? Ann Oncol. 2019;30(9):1448–59.
Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58.
Kono Y, Choda Y, Nakagawa M, et al. Association between immune-related adverse events and the prognosis of patients with advanced gastric cancer treated with nivolumab. Target Oncol. 2021;16(2):237–48.
Suematsu H, Kano K, Yamada T, et al. Prognostic impact of immune-related adverse events in gastric cancer patients treated with nivolumab. Anticancer Res. 2022;42(3):1535–40.
Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther. 2017;6:73–82.
Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology. 2019;58(Suppl 7):59–67.
Zhang C, Liu T, Wang J, Zhang J. Development and verification of an immune-related gene prognostic index for gastric cancer. Sci Rep. 2022;12(1):15693.
Hu L, Zhang P, Sun W, Zhou L, Chu Q, Chen Y. PDPN is a prognostic biomarker and correlated with immune infiltrating in gastric cancer. Medicine. 2020;99(19):e19957.
Katsurahara K, Shiozaki A, Kosuga T, et al. ANO9 regulates PD-L2 expression and binding ability to PD-1 in gastric cancer. Cancer Sci. 2021;112(3):1026–37.
Huang T, Liang Y, Zhang H, et al. CSMD1 mutations are associated with increased mutational burden, favorable prognosis, and anti-tumor immunity in gastric cancer. Genes. 2021;12(11):1715.
Cheng L, Wang Y, Qiu L, et al. mTOR pathway gene mutations predict response to immune checkpoint inhibitors in multiple cancers. J Transl Med. 2022;20(1):247.
Zhang F, Li X, Chen H, et al. Mutation of MUC16 is associated with tumor mutational burden and lymph node metastasis in patients with gastric cancer. Front Med. 2022;9:836892.
Ohmura H, Yamaguchi K, Hanamura F, et al. OX40 and LAG3 are associated with better prognosis in advanced gastric cancer patients treated with anti-programmed death-1 antibody. Br J Cancer. 2020;122(10):1507–17.
Tan KT, Yeh CN, Chang YC, et al. PRKDC: new biomarker and drug target for checkpoint blockade immunotherapy. J Immunother Cancer. 2020;8(1):e000485.
Li L, Li M, Wang X. Cancer type-dependent correlations between TP53 mutations and antitumor immunity. DNA Repair. 2020;88:102785.
Lu Y, Li D, Cao Y, et al. A genomic signature reflecting fibroblast infiltration into gastric cancer is associated with prognosis and treatment outcomes of immune checkpoint inhibitors. Front Cell Dev Biol. 2022;10:862294.
Chen Z, Chen C, Li L, Zhang T, Wang X. Pan-cancer analysis reveals that E1A binding protein p300 mutations increase genome instability and antitumor immunity. Front Cell Dev Biol. 2021;9:729927.
Song J, Yang R, Wei R, Du Y, He P, Liu X. Pan-cancer analysis reveals RIPK2 predicts prognosis and promotes immune therapy resistance via triggering cytotoxic T lymphocytes dysfunction. Mol Med. 2022;28(1):47.
Chen DL, Sheng H, Zhang DS, et al. The circular RNA circDLG1 promotes gastric cancer progression and anti-PD-1 resistance through the regulation of CXCL12 by sponging miR-141-3p. Mol Cancer. 2021;20(1):166.
Rong X, Lv J, Liu Y, et al. PET/CT imaging of activated cancer-associated fibroblasts predict response to PD-1 blockade in gastric cancer patients. Front Oncol. 2022;11:802257.
Xue S, Ma M, Bei S, et al. Identification and validation of the immune regulator CXCR4 as a novel promising target for gastric cancer. Front Immunol. 2021;12:702615.
Qin X, Chen Y, Ma S, Shen L, Ju S. Immune-related gene TM4SF18 could promote the metastasis of gastric cancer cells and predict the prognosis of gastric cancer patients. Mol Oncol. 2022;16(22):4043–59.
Iwasaki A, Shinozaki-Ushiku A, Kunita A, et al. Human leukocyte antigen class I deficiency in gastric carcinoma: an adaptive immune evasion strategy most common in microsatellite instable tumors. Am J Surg Pathol. 2021;45(9):1213–20.
Hu Q, Nonaka K, Wakiyama H, et al. Cytolytic activity score as a biomarker for antitumor immunity and clinical outcome in patients with gastric cancer. Cancer Med. 2021;10(9):3129–38.
Kumagai S, Koyama S, Itahashi K, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40(2):201-218.e9.
Fan Y, Ying H, Wu X, et al. The mutational pattern of homologous recombination (HR)-associated genes and its relevance to the immunotherapeutic response in gastric cancer. Cancer Biol Med. 2020;17(4):1002–13.
Zhang P, Gu Y, Fang H, et al. Intratumoral IL-1R1 expression delineates a distinctive molecular subset with therapeutic resistance in patients with gastric cancer. J Immunother Cancer. 2022;10(2):e004047.
Zhang MJ, Chen DS, Li S, Chen L, Qi YX, Zhang CJ. Helicobacter pylori infection as a potential favorable factor for immune checkpoint inhibitor therapy for gastric cancer. Invest New Drugs. 2021;39(5):1436–8.
Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306.
Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6(12):1952–6.
Hussaini S, Chehade R, Boldt RG, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—a systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134.
Xu S, Lai R, Zhao Q, Zhao P, Zhao R, Guo Z. Correlation between immune-related adverse events and prognosis in hepatocellular carcinoma patients treated with immune checkpoint inhibitors. Front Immunol. 2021;12:794099.
Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020;18(1):87.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–68.
Wang J, Li Q, Yuan J, et al. CDK4/6 inhibitor-SHR6390 exerts potent antitumor activity in esophageal squamous cell carcinoma by inhibiting phosphorylated Rb and inducing G1 cell cycle arrest. J Transl Med. 2017;15(1):127.
Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391.
Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–79.
Abolhassani AR, Schuler G, Kirchberger MC, Heinzerling L. C-reactive protein as an early marker of immune-related adverse events. J Cancer Res Clin Oncol. 2019;145(10):2625–31.
Hasan Ali O, Berner F, Bomze D, et al. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur J Cancer. 2019;107:8–14.
Andrews MC, Duong CPM, Gopalakrishnan V, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med. 2021;27(8):1432–41.
Zhang H, Lu J, Liu J, Zhang G, Lu A. Advances in the discovery of exosome inhibitors in cancer. J Enzyme Inhib Med Chem. 2020;35(1):1322–30. https://doi.org/10.1080/14756366.2020.1754814.
Jiang F, Zhang Z, Chong X, et al. Extracellular vesicle-derived protein file from peripheral blood predicts immune-related adverse events in gastric cancer patients receiving immunotherapy. Cancers. 2022;14(17):4167.
Johnson J, Goldner W, Abdallah D, Qiu F, Ganti AK, Kotwal A. Hypophysitis and secondary adrenal insufficiency from immune checkpoint inhibitors: diagnostic challenges and link with survival. J Natl Compr Canc Netw. 2023;21(3):281–7.
Iglesias P, Peiró I, Biagetti B, et al. Immunotherapy-induced isolated ACTH deficiency in cancer therapy. Endocr Relat Cancer. 2021;28(12):783–92.
Yano S, Ashida K, Sakamoto R, et al. Human leucocyte antigen DR15, a possible predictive marker for immune checkpoint inhibitor-induced secondary adrenal insufficiency. Eur J Cancer. 2020;130:198–203.
International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2, Sawcer S, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476(7359):214–9.
Sugimura K, Asakura H, Mizuki N, et al. Analysis of genes within the HLA region affecting susceptibility to ulcerative colitis. Hum Immunol. 1993;36(2):112–8.
Phelps RG, Rees AJ. The HLA complex in Goodpasture’s disease: a model for analyzing susceptibility to autoimmunity. Kidney Int. 1999;56(5):1638–53.
Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173–82.
Bamias G, Delladetsima I, Perdiki M, et al. Immunological characteristics of colitis associated with anti-ctla-4 antibody therapy. Cancer Invest. 2017;35(7):443–55.
Dulos J, Carven GJ, van Boxtel SJ, et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35(2):169–78.
Germain C, Gnjatic S, Tamzalit F, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189(7):832–44.
Nielsen JS, Sahota RA, Milne K, et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18(12):3281–92.
Dieu-Nosjean MC, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautès-Fridman C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev. 2016;271(1):260–75.
Pimenta EM, Barnes BJ. Role of tertiary lymphoid structures (TLS) in anti-tumor immunity: potential tumor-induced cytokines/chemokines that regulate TLS formation in epithelial-derived cancers. Cancers. 2014;6(2):969–97.
Workel HH, Lubbers JM, Arnold R, et al. A transcriptionally distinct CXCL13+CD103+CD8+ T-cell population is associated with b-cell recruitment and neoantigen load in human cancer. Cancer Immunol Res. 2019;7(5):784–96.
Djenidi F, Adam J, Goubar A, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194(7):3475–86.
Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014;20(2):434–44.
Migita K, Matsumoto S, Wakatsuki K, et al. Postoperative serum C-reactive protein level predicts long-term outcomes in stage I gastric cancer. J Surg Res. 2019;242:323–31.
Kim EY, Yim HW, Park CH, Song KY. C-reactive protein can be an early predictor of postoperative complications after gastrectomy for gastric cancer. Surg Endosc. 2017;31(1):445–54.
Shishido Y, Saito H, Shimizu S, et al. Prognostic significance of platelet×C-reactive protein multiplier in patients with esophageal squamous cell carcinoma. Surg Today. 2020;50(2):185–92.
Ide S, Toiyama Y, Okugawa Y, et al. High platelet×C-reactive protein level multiplier is a negative prognostic marker in rectal cancer treated by neoadjuvant chemoradiotherapy. Int J Clin Oncol. 2021;26(4):708–16.
Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: the role of interleukin 6 (IL-6). BJU Int. 2014;113(6):986–92.
Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14(6):e218–28.
Matsunaga T, Saito H, Kuroda H, et al. CA19-9 in combination with P-CRP as a predictive marker of immune-related adverse events in patients with recurrent or unresectable advanced gastric cancer treated with nivolumab. BMC Cancer. 2022;22(1):418.
Zhang J, Zhang L, Duan S, Li Z, Li G, Yu H. Single and combined use of the platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, and systemic immune-inflammation index in gastric cancer diagnosis. Front Oncol. 2023;13:1143154.
Tomás TC, Eiriz I, Vitorino M, et al. Neutrophile-to-lymphocyte, lymphocyte-to-monocyte, and platelet-to-lymphocyte ratios as prognostic and response biomarkers for resectable locally advanced gastric cancer. World J Gastrointest Oncol. 2022;14(7):1307–23.
Yamamoto T, Kawada K, Obama K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci. 2021;22(15):8002.
Pavan A, Calvetti L, Dal Maso A, et al. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist. 2019;24(8):1128–36.
Takada S, Murooka H, Tahatsu K, et al. Identifying early predictive markers for immune-related adverse events in nivolumab-treated patients with renal cell carcinoma and gastric cancer. Asian Pac J Cancer Prev. 2022;23(2):695–701.
Fan X, Wang D, Zhang W, et al. Inflammatory markers predict survival in patients with advanced gastric and colorectal cancers receiving anti-PD-1 therapy. Front Cell Dev Biol. 2021;9:638312.
Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol. 2018;14(10):569–79.
Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med. 2018;168(2):121–30.
Shimozaki K, Sukawa Y, Sato Y, et al. Analysis of risk factors for immune-related adverse events in various solid tumors using real-world data. Future Oncol. 2021;17(20):2593–603.
Wambre E, James EA, Kwok WW. Characterization of CD4+ T cell subsets in allergy. Curr Opin Immunol. 2012;24(6):700–6.
Rosskopf S, Jahn-Schmid B, Schmetterer KG, Zlabinger GJ, Steinberger P. PD-1 has a unique capacity to inhibit allergen-specific human CD4+ T cell responses. Sci Rep. 2018;8(1):13543.
Shoji F, Takeoka H, Kozuma Y, et al. Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer. 2019;136:45–51.
Khaki AR, Li A, Diamantopoulos LN, et al. Impact of performance status on treatment outcomes: a real-world study of advanced urothelial cancer treated with immune checkpoint inhibitors. Cancer. 2020;126(6):1208–16.
Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073–126.
Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42(4):406–17.
Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28(4):264–8.
De Martin E, Michot JM, Rosmorduc O, Guettier C, Samuel D. Liver toxicity as a limiting factor to the increasing use of immune checkpoint inhibitors. JHEP Rep. 2020;2(6):100170.
Muntyanu A, Netchiporouk E, Gerstein W, Gniadecki R, Litvinov IV. Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: a dermatology perspective on management. J Cutan Med Surg. 2021;25(1):59–76.
Wright JJ, Powers AC, Johnson DB. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. 2021;17(7):389–99.
Iwama S, Kobayashi T, Yasuda Y, Arima H. Immune checkpoint inhibitor-related thyroid dysfunction. Best Pract Res Clin Endocrinol Metab. 2022;36(3):101660.
Acknowledgements
Not applicable.
Funding
This work was supported by the Cultivating Outstanding Talents Project of Hebei Provincial Government Fund (No. 2019012); Hebei public health committee county-level public hospitals suitable health technology promotion and storage project (No. 2019024); Hebei University Science and Technology Research Project (No. ZD2019139).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: QZ; (II) administrative support: QZ; (III) Provision of study materials or patients: PD, PL; (IV) Collection and assembly of data: PD, LM; (V) data analysis and interpretation: PD; (VI) manuscript writing: all authors; (VII) final approval of manuscript: all authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ding, P., Liu, P., Meng, L. et al. Mechanisms and biomarkers of immune-related adverse events in gastric cancer. Eur J Med Res 28, 492 (2023). https://doi.org/10.1186/s40001-023-01365-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01365-3