Abstract
Background
Mammals, globally, are facing population declines. Protecting and breeding threatened populations inside predator-free havens and translocating them back to the wild is commonly viewed as a solution. These approaches can expose predator-naïve animals to predators they have never encountered and as a result, many conservation projects have failed due to the predation of individuals that lacked appropriate anti-predator responses. Hence, robust ways to measure anti-predator responses are urgently needed to help identify naïve populations at risk, to select appropriate animals for translocation, and to monitor managed populations for changes in anti-predator traits. Here, we undertake a systematic review that collates existing behavioural assays of anti-predator responses and identifies assay types and predator cues that provoke the greatest behavioural responses.
Methods
We retrieved articles from academic bibliographic databases and grey literature sources (such as government and conservation management reports), using a Boolean search string. Each article was screened against eligibility criteria determined using the PICO (Population–Intervention–Comparator–Outcome) framework. Using data extracted from each article, we mapped all known behavioural assays for quantifying anti-predator responses in mammals and examined the context in which each assay has been implemented (e.g., species tested, predator cue characteristics). Finally, with mixed effects modelling, we determined which of these assays and predator cue types elicit the greatest behavioural responses based on standardised difference in response between treatment and control groups.
Review findings
We reviewed 5168 articles, 211 of which were eligible, constituting 1016 studies on 126 mammal species, a quarter of which are threatened by invasive species. We identified six major types of behavioural assays: behavioural focals, capture probability, feeding station, flight initiation distance, giving-up density, and stimulus presentations. Across studies, there were five primary behaviours measured: activity, escape, exploration, foraging, and vigilance. These behaviours yielded similar effect sizes across studies. With regard to study design, however, studies that used natural olfactory cues tended to report larger effect sizes than those that used artificial cues. Effect sizes were larger in studies that analysed sexes individually, rather than combining males and females. Studies that used ‘blank’ control treatments (the absence of a stimulus) rather than a treatment with a control stimulus had higher effect sizes. Although many studies involved repeat measures of known individuals, only 15.4% of these used their data to calculate measures of individual repeatability.
Conclusions
Our review highlights important aspects of experimental design and reporting that should be considered. Where possible, studies of anti-predator behaviour should use appropriate control treatments, analyse males and females separately, and choose organic predator cues. Studies should also look to report the individual repeatability of behavioural traits, and to correctly identify measures of uncertainty (error bars). The review highlights robust methodology, reveals promising techniques on which to focus future assay development, and collates relevant information for conservation managers.
Similar content being viewed by others
Background
The need to quantify anti-predator responses
Mammal populations are experiencing alarming rates of extinction [1,2,3] due to anthropogenic impacts such as habitat loss and fragmentation, illegal hunting, and exotic predators [4]. Redressing this loss of biodiversity requires well-informed and well-tested management interventions. Many of these interventions will need to be underpinned by a mechanistic understanding of species’ behaviour.
How an animal responds to predators has substantial bearing on its ability to survive. Predation, particularly from introduced predators, has been a major driver of mammal declines and extinctions around the world [5,6,7,8,9]. This is especially true for individuals and populations that have had limited or no exposure to predators, such as many island populations [10, 11], individuals raised in captivity, and those moved to an environment with novel predators [12,13,14]. Improving our understanding of how animals behave in response to predatory stimuli should provide crucial insights for their conservation management and can improve our ability to retain antipredator traits in managed populations [12, 15, 16]. An animal’s response to predators may be either behavioural (e.g. spatial and temporal avoidance [17, 18], avoiding detection [19] and evasion [20]), or chemical [21] and physical defences [22]. Behavioural responses are likely to be more plastic and responsive at shorter time frames than physical responses and are therefore particularly important when considering the acute impacts of predators on the persistence of predator-naïve species. Such behavioural responses may be closely linked to animal personalities (or behavioural syndromes)—repeatable inter-individual differences in a set of behaviours [23], traditionally assessed along a bold-shy continuum [24, 25]. Personality is important to consider in the context of ecology as it contributes to inter- and intra-individual variations in behaviour, influencing both the ability of an individual to respond to environmental changes, as well as the efficiency with which natural selection can act [23].
Animal behaviour can be influenced by human interactions. For example, the coexistence of humans and wildlife in urban areas often selects for boldness in individuals [24, 25]. Conservation interventions can also indirectly affect behavioural responses in target species. Common strategies employed to prevent faunal extinctions include captive breeding [26], translocations (the deliberate movement of animals from one population or site for release in another [27]), and establishment of populations in predator-free havens (areas isolated from predators through a geographical or physical barrier, such as islands or fenced enclosures [28,29,30]). Such approaches have secured a number of populations of mammals, including African elephants [31, 32], European lynx [33], elk [34], giant pandas [35], and Tasmanian devils [36]. Despite their initial successes, these strategies are at risk of longer-term failure if they select for bold, predator-naïve behaviours. Such haven-adapted populations would be particularly vulnerable to acute population collapses from predator incursions and would face substantial challenges when reintroduced to areas containing predators.
Australia provides a compelling case study to illustrate the challenges of mammal conservation. More than one third of modern mammal extinctions have occurred in Australia, largely due to the introduction of feral cats and foxes [37]. In response, havens free of introduced predators are a key component of conserving much of the remaining mammal fauna [29, 30, 38]. Australia’s current network of havens provides habitats for at least 32 mammal species, and has secured at least 188 populations and sub-populations [29]. Evidence is emerging, however, that in the absence of feral and/or native predators, havened populations no longer exhibit anti-predator behaviours [13, 39,40,41,42,43,44]. This renders individuals in these populations fundamentally unfit for reintroduction back into where predators still persist. Because the success of many translocations has ultimately been compromised by predation [38, 45, 46], the future of mammal conservation in Australia, and more broadly, hinges on developing methods and strategies that can quantify and conserve antipredator behaviours in havened and translocated populations [42].
To undertake an adaptive management approach, we require monitoring and evaluation of anti-predator responses in mammalian species. Despite awareness that behavioural traits such as boldness or shyness can influence conservation outcomes, measuring such traits is rarely incorporated into monitoring and management [16, 47]. Anti-predator responses have only recently been identified as a potential barrier to the success of conservation projects [13, 40,41,42], and while an array of academic literature exists that details various methods for measuring these behaviours [15, 41, 42, 48,49,50,51,52,53], accessing the methodologies, comparing them for rigor, and identifying the most appropriate measure is labour intensive. Stakeholders, such as conservation and population managers, are likely to be seeking this information, but are also likely to be limited by the time and resources necessary to find it. Ultimately, we currently lack a robust framework for the universal monitoring and evaluation of anti-predator traits [54]. The first step to developing such a framework is to understand which behavioural assays have been used, which are the most effective (capture or provoke the greatest behavioural response), and whether the type of predator cue is important. In the absence of this crucial information, the adoption of inappropriate and poorly-performing behavioural metrics may prevail.
Identification and engagement of stakeholders
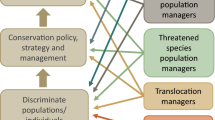
In addition to the review team, stakeholders relevant to this review have been identified as those who research or manage animal populations, for example, members of species recovery teams (Fig. 1). To ensure the information collected throughout this review is tailored toward the target audience, and thus of the most relevance for application, a variety of stakeholders from each of the categories in Fig. 1 were consulted during the development of the protocol. We invited 27 stakeholders to comment on the draft protocol, and after receiving 16 replies (ten from Australia and six from other countries), we incorporated their suggestions.
End-user stakeholder groups (right-hand boxes) consulted when designing a systematic review of methods that quantify anti-predator behaviour in mammals. Arrows indicate each groups’ broad interests in the various steps (left-hand boxes) required for improving conservation outcomes. Robust behavioural assays facilitate the accurate discrimination of individuals or populations based on certain desirable behavioural attributes. This information can then be used to directly inform conservation management, whereby effective conservation management strategies ultimately lead to improved conservation outcomes
Objective of the review
We present all available behavioural assays for measuring or quantifying anti-predator responses in mammals by collating information into an accessible format. Specifically, we: (1) reveal different methods, (2) describe the context within which each method was conducted, and (3) highlight methods or aspects that warrant further examination, thus guiding the future development of behavioural assays. Further, using a modelling approach, we then identify which types of behavioural assays and predator cues elicit the greatest responses in mammals (difference in effect size between the treatment and control conditions). A formal evidence synthesis is required to explore all potential methods and to avoid bias toward those published in academic journals, because much information may come from governmental reports and species recovery plans [16, 55]. The final review acts as a guide: it highlights existing methodologies and provides additional information to assess their relevance, allowing stakeholders to easily select the most appropriate and effective behavioural assay for their purpose.
Using the PICO (Population–Intervention–Comparator–Outcome) framework [56], we have broken our review into two questions that will define our search scope. We will first systematically map all known methodologies answering a primary question: what behavioural assays have been used to quantify anti-predator responses in mammals? The elements of this question are:
- Population:
-
Free-living, wild-caught, or captive mammals (global)
- Intervention:
-
(i) a behavioural assay that quantifies anti-predator responses to predator exposure
(ii) a behavioural assay that quantifies anti-predator responses to predator cues
Articles that conform to both the Population and Intervention criteria will be used to answer this primary question. A secondary question we seek to answer will be assessed quantitatively by modelling the data collected from each article, asking: which assay-types and predator cues elicit the greatest behavioural responses? This question utilises the same Population and Intervention criteria as the primary question but requires further assessment using Comparator and Outcome criteria to select studies for the systematic review. The additional elements of the secondary question are:
- Comparator:
-
Comparison between levels of predator exposure (e.g., before versus after exposure, exposure versus no exposure) or comparison between exposure to a predator cue versus a control.
- Outcome:
-
Difference in the behavioural response between the treatment (e.g., predator/predator cue exposure) and control conditions. Metrics of responses will differ between studies depending on assay type and will be compared using standardised effect sizes.
Articles that involve at least one Comparator element can then additionally be considered for the systematic review to investigate which Intervention elements (behavioural assays and predator cues) produce the greatest Outcome. The PICO elements of our two questions are illustrated in Fig. 2.
Methods
This systematic review follows a pre-defined protocol [57] that conforms to the CEE guidelines [56] and ROSES reporting standards (Additional file 1, ROSES checklist).
Deviations from the protocol
We were unable to use the entire search string to search EThOS: UK Theses and Dissertations, instead we searched using the search terms “anti-predator” and “antipredator”. The website https://opengrey.eu could not be searched as it has been removed. One eligibility criterion was added for the screening process; the article must contain primary results (e.g. a meta-analysis or book chapter that presents and cites findings from another study will not be included) as articles that present results from other studies do not contain enough methodological detail for this review, and we are confident that relevant original studies will be captured by our search strategy. We also clarified that to satisfy the population criteria, individuals must not have been physically or physiologically altered (e.g., had any substance administered or limb removed) as this may affect their response and bias our quantitative component. We intended to use the CEE Critical Appraisal tool to evaluate potential biases from our studies, however, found that many of the questions lacked relevance for studies of animal populations (as they were directed toward human subjects). We refined the criteria to better suit our review (Additional file 3).
Search for articles
Search terms and strings
To develop a search strategy, an initial scoping exercise was conducted using a test-list of 10 benchmark articles that assess anti-predator responses (Additional file 2), each selected by the review team as they cover a variety of different assays and predator scenarios. The titles, key words, and abstracts of each scoping article were mined, both manually, and using word clouds (R package wordcloud [58]; in the R environment [59]), to determine the most appropriate search terms [60]. An initial search string was then created using Boolean operators to combine the relevant terms based on the review team’s knowledge, and the terms identified from the scoping articles. Trial searches were conducted using the Web of Science: Core Collection. We systematically removed terms that appeared to broaden the search outside the scope of the review. To ensure the proposed strategy adequately returned relevant literature, the search output was scanned for relevant articles and each of the scoping benchmark articles. Unreturned articles were then closely inspected, and the search strategy was adjusted until it retrieved all 10 benchmark articles [56]. The comprehensiveness of the search strategy was then tested using a list of 5 independent articles (Additional file 2), all of which were retrieved by the final search strategy. The final search string (modified for each specific database language) was: TS = ((("antipredator response$" OR "anti-predator response$" OR "antipredator behavio$r" OR "anti-predator behavio$r" OR "escape behavio$r" OR "giving$up density" OR "FID" OR "GUD" OR "flight initiation distance") AND ("predator exposure" OR "prey naïveté" OR "naïve prey" OR "los$" OR "trait" OR "predator avoid*")) OR (("predator recognition" OR "predator exposure" OR "predation risk" OR "introduced predator$" OR "novel predator$" OR "predator odour") AND ("naïve prey" OR "prey naïveté" or "escape behavio$r" OR "giving$up density" OR "flight initiation distance" OR "FID" OR "GUD" OR "predator odour")) OR (("antipredator response$" OR "anti-predator response$" OR "antipredator behavio$r"OR "anti-predator behavio$r" OR "escape behavio$r") AND ("predator recognition" OR "predator exposure" OR "introduced predator$" OR "novel predator$")))
Search limitations
All searches were conducted in English which may exclude studies from regions that do not predominantly speak English. Searches were conducted between 7th and 21st December 2021.
Search sources
Academic literature
Using the above search string, we searched the following bibliographic databases from which to collect peer-reviewed journal articles: Web of Science (Core Collection, BIOSIS Citation Index, Zoological Record, CAB abstracts) and Scopus.
Grey literature
To reduce bias toward published literature, we also searched a variety of grey literature sources [54, 55]. We collated theses and dissertations from two bibliographic databases specific to grey literature: Proquest Dissertation (using the above search string) and EThOS: UK Theses and Dissertations (using the search terms “anti-predator” and “antipredator”). Conference proceedings were searched in the Web of Science database using the predetermined search string. The website trove.nla.gov.au was also searched, using the search terms “anti-predator” and “antipredator”. Specialist documents were searched for from within the following repositories, using the search terms “anti-predator” and “antipredator”: IUCN general publications (https://portals.iucn.org/library/dir/publications-list); IUCN Conservation Planning Specialist Group (http://www.cpsg.org/document-repository); Conservation Evidence (http://www.ConservationEvidence.com); WWF (https://www.worldwildlife.org/publications). A web-based search engine, Google (www.google.com), was used to supplement our search results. The first 50 links returned using each combination of the search terms “anti-predator/antipredator” and “behaviour/behavior”, were inspected and added to the article pool if not yet identified [61].
Article screening and study eligibility criteria
Screening process
Articles were uploaded into CADIMA software [62], where duplicates were removed and article screening took place. To remove bias, two screeners independently reviewed articles at title and abstract level simultaneously, followed by the full text versions, to decide which met the inclusion criteria. Each screener assessed an overlap of 50 articles at both the title/abstract stage, and at the full text stage (1.5% and 9.5% respectively). Reliability between screeners was assessed using Kappa calculations and were deemed reliable (inter-screener reliability scores of 1 and 0.8 at title/abstract and full text stages respectively). In instances where screeners did not agree on the inclusion/exclusion of an article, they discussed, and consulted a third member of the review team if a decision could not be reached. If reviewers found themselves assessing their own work, a third impartial member of the review team supervised the assessment of any conflicting articles. A full list of excluded articles, detailing reasoning for their exclusion can be found in Additional file 4.
Eligibility criteria
Each article was screened against eligibility criteria based on the PICO framework as outlined in Table 1.
Articles that satisfied the Population and Intervention eligibility criteria were used to pursue the primary question, and were then additionally assessed against the Comparator and Outcome eligibility criteria for inclusion in the secondary quantitative component where they addressed the effectiveness of the Intervention elements; either assay types or predator cue types. All articles considered for this analysis must have incorporated at least one of the Comparator elements and all of the Outcome elements listed in Table 1.
Study validity assessment
To assess the risk of bias in each study, we evaluated the risk of biases from 6 criteria as defined in the CEE Critical Appraisal tool v.0.3 [64]: confounding biases; post-intervention/exposure selection biases; misclassified comparison biases; detection biases; outcome reporting biases; and outcome assessment biases. Factors identified by the review team and stakeholders that may confound the effectiveness of a behavioural assay or predator cue were scored during the meta-data extraction (e.g. species, sex, population origin, control type).
Using our Critical Appraisal checklist (adapted from the CEE Critical Appraisal tool; Additional file 3), we assessed the risk of bias for each of the aforementioned criteria (low, medium or high) and rated the overall risk of bias (low, medium or high) for each study. There were many cases where multiple studies (from the same article) were part of one experiment, for example, where each study considered a different treatment, or a different behaviour measured. In these instances, one checklist was completed, unless the review team were unable to evaluate them as one because different studies posed different risks of bias. In this case, and in cases where studies were from the same article but from a different experiment, independent checklists were completed. To evaluate the consistency of critical appraisal decisions, two members of the review team assessed an overlap of 20 studies (9.5%) and reached the same conclusion for each of them. In the statistical analysis, studies were weighted according to their risk of bias as described below (data synthesis).
Data coding and extraction strategy
The variables detailed in Table 2 were extracted or scored from included studies where possible.
For the quantitative component, we extracted the mean response of each treatment, its corresponding variance (standard deviation, standard error or confidence intervals), and the sample size for each treatment. Where articles alternatively present the median, minimum, maximum and interquartile range, we extracted these values, and used them to estimate the mean and standard error (please see below). In articles where such values were presented graphically, we measured the values directly from the figures (with the axes as scale bars) using the software Image J [65]. For a worked through example of effect size extraction using Image J, please see Addition file 6. Data were recorded using a customised data sheet (Additional file 5). To ensure consistency in data extraction, 20 studies (9.5%) were cross checked by two review-team members. Both members came to the same conclusions for all data extracted, and a pairwise t-test (in the R environment [59]) revealed no difference in the values extracted using Image J between scorers. We contacted authors from 17 articles to follow up missing information; we received four replies, and excluded the remaining 13 articles. Additional information regarding the species tested (threat status, threats faced, distribution) were collected from the IUCN Red List [66]. Extracted data can be found in Additional file 6.
Potential effect modifiers/reasons for heterogeneity
The list of factors to be investigated by the review were compiled using the expertise of the review team, incorporating suggestions from stakeholders. We may have unintentionally excluded some useful data by only searching articles written in the English language. There may be a bias in the types of animals for which measures have been developed, for example, threatened or charismatic species. The type of predator cue used may substantially affect the outcome, as less effective cues may not be representative of an individuals’ response to a true predation event [64,63,64,67]. For the most robust quantification of behaviour, methodology should use repeat measures, incorporate measures of repeatability, and validate the assays, for example, by quantifying the fitness outcomes of various behavioural responses [68, 69]. With such a systematic review, we hope to highlight where biases may be occurring, and reveal areas where more robust methodology is needed to guide the development of behavioural assays.
Data synthesis and presentation
The results from this systematic review are presented both as a narrative synthesis to address the primary question and as a quantitative analysis to address the secondary question [56]. To answer the primary question, what behavioural assays have been used to quantify anti-predator responses in mammals, we collated two tables of findings, and discuss specific examples and descriptive statistics in the text.
The secondary question, which assay-types and predator cues elicit the greatest behavioural response, has been answered via extracting meta-data. To make studies comparable, we converted all observations of effect sizes and their variance/error margins to means and standard deviation. If articles only provided standard errors these were transformed into standard deviation using the following equation:
where \(\mathrm{SE}\) is standard error, and \(n\) is sample size. If articles only provided 95% confidence intervals, normal distribution was assumed, and these were transformed into standard deviation using the following equation:
where \(n\) is sample size, and \(\mathrm{UL}\) and \(\mathrm{LL}\) are the upper and lower confidence limits respectively. There was one instance where an article provided mean deviation. From these values we estimated standard deviation by multiplying the values by a factor of \(\surd \frac{2}{\pi }\). There was one article that provided the mean and the range. From these values, standard deviation was estimated by quartering the range (\(\frac{1}{4}(Max-Min)\)). If articles presented their results as box plots (providing the median, interquartile range, and minimum/maximum), we used the following equation [67] to estimate the mean:
where \(a\) is the minimum, \({q}_{1}\) and \({q}_{3}\) are the first and third quartiles respectively, \(m\) is the median, and \(b\) is the maximum. We estimated standard deviation following a method devised by Wan and colleagues [68]:
Both \(\varepsilon\) and η are functions of \(n\) as defined in [68] (Additional file 7). Using the treatment means, standard deviations and sample sizes extracted and estimated from each study, we then calculated a standardized measure of effect size for differences between means using Hedges’ g [63]:
where \({\mu }_{t}\) is the mean of the treatment group, \({\mu }_{c}\) is the mean of the control group and \({S}_{p}\) is the pooled standard deviation. The formula for pooled standard deviation is:
where \({n}_{t}\) and \({s}_{t}\) are the number of observations and standard deviation for the treatment group respectively, and \({n}_{c}\) and \({s}_{c}\) are the number of observations and standard deviation for the control group respectively. Hedges’ g was chosen over other effect size measures such as Cohen’s d, as it is suited to a range of sample sizes and because it facilitates comparisons across studies by weighting each measure based on the number of observations [69]. As we were interested in the magnitude of difference in effect sizes between the treatment and control conditions, rather than the direction of difference, we used absolute values of Hedges’ g in our analysis. Finally, to evaluate potential publication bias, we created and visually inspected a funnel plot (using the metafor package [70] in R [59]) which suggests an approximately symmetrical distribution of studies (Additional file 8).
We built two mixed effects models using R [59] to identify which predator cue types and behavioural assay types elicit the greatest difference in effect size (Hedges’ g), while controlling for potential confounding factors where possible. We included each article’s unique identifier as a random effect in both models to account for the non-independence of multiple effect sizes from each article. We also included genus as a random effect to account for potential bias towards taxa more heavily represented in the literature (e.g., Rattus). Studies were weighted based on their risk of bias determined through critical appraisal whereby studies with a low, moderate, or high risk were weighted with a value of 3, 2, or 1 respectively.
Model selection was used to determine factors that most influenced the response variables of each model. To select the best model, we used likelihood ratio tests to test the relative weight of each variable by comparing a model with that parameter removed to the full model [71]. We also compared AIC values of candidate models to ensure the final models were the most parsimonious (∆AIC < 2) [72]. As we did not have observations for all combinations of categories, we were unable to test for the following biologically relevant interaction effects from the assay type model: assay type and species type; behaviour measured and species size; assay type and predator type (aerial/terrestrial); source population (captive, wild, haven) and predator source (native, novel, historic, introduced); behaviour measured and sex. From the predator cue type model, we were unable to test for: cue type (direct/indirect) and cue structure (synthetic/organic); cue type and source population. In the predator cue type model, the cue properties variable (the appropriate combination of visual, auditory and olfactory) showed high collinearity and was excluded from the analysis.
Review findings
Review descriptive statistics
Our search returned 5228 articles (18 theses + 5210 peer reviewed articles; totalling 3629 after duplicates had been removed) that had publication dates between 1984 and 2022. Of these, 211 articles passed the screening process, resulting in 1016 studies (Fig. 3). Full lists of search results by source, eligible studies, and reasons for exclusion, are available in Additional file 9. Critical appraisal determined that all studies posed a low risk of bias. Critical appraisal checklists for each study can be found in CADIMA (please see data availability section).
Species assayed
We identified 126 species for whom anti-predator responses have been investigated. Australia contained the most assayed species (27 species), followed closely by species from North American and Asian continents (18 and 22 species respectively). Species from South America and Africa were underrepresented in the literature (Fig. 4a). Excluding domesticated species, the threat status of all 126 species has been assessed by the International Union for the Conservation of Nature (the IUCN). Most species were listed as Least Concern, with only 20% of assayed species listed as threatened (Vulnerable, Endangered, Critically Endangered), or Extinct in the Wild; Fig. 4b. According to the species’ Red List profiles, one quarter are threatened by “Invasive non-native/alien species” (Fig. 4c).
Species for whom anti-predator behaviours have been assayed: a Number of species assayed per country that they are found in, b species extinction risk category according to the IUCN Red List (DD: Data Deficient, LC: Least Concern, NT: Near Threatened, VU: Vulnerable, EN: Endangered, CR: Critically Endangered, EW: Extinct in the Wild), and c proportion of species threatened by ‘Invasive or non/native species’
Range of behavioural assays
From the literature, we identified six major types of behavioural assays: stimulus presentations (71.3% of studies), feeding station (14.6%), behavioural focals (3.7%), giving-up density (7.4%), capture probability (2.0%), and flight initiation distance (1.0%). These assays measured five primary behaviours: activity, escape, exploration, foraging and vigilance (Table 3). Additional behaviours that did not fall into these categories, such as aggression or grooming, were rarely measured, and were grouped into an “other” category.
Behavioural focals were commonly used to compare anti-predator responses in populations with varying predator exposure or naïveté, without the use of a predator stimulus. Ross and colleagues [5], for example, used this method to compare anti-predator responses between two populations of greater bilbies (Macrotis lagotis): a naïve havened population and a population exposed to cats (an introduced meso-predator). This method facilitates observation of general behaviours, such as foraging and vigilance trade-offs, and can be used to discern varying personality phenotypes (e.g., bold or shy individuals) and to evaluate neophobia (aversion to novelty [285]).
Capture probability, which represents an animal’s exploratory behaviour, is an innovative way to assay behaviour using live animal traps. This method was predominantly used to assay behaviour in small rodents. For example, both Russell and colleagues [83] and Dickman and colleagues [86] evaluated anti-predator responses in rodents, including Australian bush rats (Rattus fuscipes), wood mice (Apodemus sylvaticus) and shrews (Sorex araneus), by setting an array of scented traps—treated with predator odours or not—giving the focal species a choice of trap to explore. This particular assay can discern whether individuals recognise the scent of predators, and behave differently when there is immediate evidence of predators.
Feeding stations can be used to reveal how animals forage; a behaviour that commonly trades off against anti-predator behaviours such as vigilance. Feeding may make an individual vulnerable, and how they allocate their time to vigilance versus other behaviours can reveal the risk they perceive. For example, Saxon-Mills and colleagues [51] compared the foraging, exploratory and activity behaviours of burrowing bettongs (Bettongia lesueur) from both naïve and cat exposed populations at predator scented food trays. Such methodology provides insight into an animal’s priorities, and can highlight contexts of interest—for example, an animal ignoring predator cues in the pursuit of food may be experiencing resource limitation.
Flight initiation distances are a particularly cost-effective method for quantifying anti-predator responses because they can be conducted with very little, or completely without specialized equipment. Cappa and colleagues [119], for example, used this method to identify the predation risk perceived by guanacos (Lama guanicoe) to the presence of human poachers.
Giving-up densities (GUD) are an effective tool for quantifying perceived risk. General foraging patterns and perceived risk between populations can be compared, or else can be paired with a predator stimulus to reveal predator awareness. GUD’s can also be used to establish landscapes of fear. Makin and colleagues [118], for example, used this method to evaluate the predation risk perceived by kudu (Tragelaphus strepsiceros), sable (Hippotragus niger) and warthogs (Phacochoerus africanus) before and after the release of a predator species, the African wild dog (Lycaon pictus). Using a slightly different approach, Jolly and colleagues [143] used giving-up densities to quantify the ability of Northern quolls (Dasyurus hallucatus) to detect and identify predators by measuring the food left from a choice of three GUD trays, each scented with a different predator odour (including a control).
Stimulus presentations represent a broad array of assays that facilitate investigation into a wide range of behaviours. For example, Aschemeier and colleagues [231] examined how woodchucks (Marmota monax) responded to conspecific and heterospecific alarm call playbacks. Shier and colleagues [175] compared the activity and vigilance behaviour of naïve and trained prairie dogs (Cynomys ludovicianus) confronted with live predators: ferrets, hawks, and snakes. Blumstein and colleagues [163] observed activity, foraging, vigilance, and escape behaviours of yellow-bellied marmots (Marmota flaviventris) presented with life-sized predator photographs.
Range of predator cues
Predator cues fell into two broad categories: direct cues, or contextual cues. Direct predator cues indicate that a predator is immediately present, whereas contextual cues imply that the predator may have recently been present, or is nearby. Within these categories, predator cues can target different sensory systems, for example, cues may include visual (e.g. Fig. 5), olfactory, and/or audible stimuli. We provide examples of these categories of stimuli, and list some of their applications in Table 4.
Repeatability and assay validation
Measuring the repeatability of behavioural assays allows observers to determine whether responses are consistent across time and/or across contexts. In the case of anti-predator responses, we would expect behavioural assays to be highly repeatable, and for individuals to respond consistently to potential predation scenarios. Of studies that had repeat observations of known individuals, 15.4% calculated the within individual repeatability of the behaviours measured. Another robust approach is to validate the methodology, for example, by determining how the quantified responses correlate to survival, or to successful predator escape. While this is not always feasible, across all studies, only 2.4% validated their behavioural assays.
Most effective behavioural assays
Across studies (n = 973), there was no one behavioural assay type that elicited greater effect sizes (Hedges’ g) than others (Table 5). Model predictions show that studies utilizing flight initiation distances as a metric for anti-predator responses had slightly higher Hedges’ g (Fig. 6a), but this difference was not statistically significant. Similarly, there was no one behaviour measured that lead to greater effect sizes (Table 5). Model estimates show that studies measuring activity had slightly higher Hedges’ g scores (Fig. 6b). Studies of small (< 5 kg) and medium (5–20 kg) species, had lower Hedges’ g compared to studies of large (> 20 kg) species (Table 5). Studies that analysed sex independently reported higher Hedges’ g than those that pooled responses from males and females (Table 5). In contrast, population source (captive, wild or haven), predator type (aerial or terrestrial), and predator source (historic, native, novel, or introduced) did not influence Hedges’ g scores, and each of these covariates was excluded during model selection.
Most effective predator cues
Of the studies that incorporated predator cues (n = 867), the properties examined here did not substantially explain effect sizes. Neither cue structure (organic or synthetic), cue movement (yes or no), cue type (direct or indirect), predator type (aerial or terrestrial) or population source (wild, captive, havened) had any effect and all were eliminated during model selection. Predator source (historic, introduced, novel, or native) was not excluded during model selection but did not have a significant effect (Table 6.) Control type, however, had a substantial effect on Hedges’ g: studies incorporating blank control types (i.e. lack of stimulus) had larger effect sizes than those that utilised control stimulus cues. As with the assay model above, studies that analysed males and females independently had higher effect sizes (Table 6).
Potential confounding factors
Anti-predator responses or responses to novel stimuli?
Creating realistic predator cues is a challenge. While studies aiming to quantify anti-predator responses may find differences among individual responses to predator cues, it is difficult to discern whether these responses accurately reflect how individuals would act in the face of real predators. Observed differences may instead be a response to a novel environment or stimulus (e.g. neophobia [285]), or may relate more to an animals’ personality (repeatable inter-individual differences in behaviour [23]) than their ability to evade predation. It is crucial to be able to distinguish between these scenarios, especially in the context of conservation translocations, where mistaking a curious response to a predator stimulus for predator recognition and avoidance abilities may result in high mortality if such naïve individuals are exposed to predators.
We found that studies that used blank controls compared to those that used non-predator stimulus controls had significantly larger effect sizes (p < 0.01; Fig 7a). To avoid the confounding effect of novel stimuli, it is essential to use appropriate control cues. For example, it could be appropriate to use a predator faeces as a stimulus, and herbivore faeces as a control, or a predator vocalisation stimulus and a song from a non-threatening bird as a control. Of those studies that incorporated predator cues, most used suitable controls, however, 21% used “blank” controls (i.e. the absence of any stimulus as a control). While this is suitable when comparing responses at different levels of predator exposure, it is not advisable when predator cues are used. This is because without an appropriate control, it cannot be discerned whether the behaviours measured are in response to the cue of a predator, or merely those associated with investigating a novel or unusual cue. Hence, to avoid incorrect over-inflation of effect sizes, studies using predator cues should select their control stimuli carefully.
Difference in effect sizes between studies that had non-predator stimulus control cues (stimulus), compared to those that had the absence of any stimuli as a control (blank) (a), and between studies that pool males and females, compared to those who analyse males and females independently (b). Error bars indicate mean +− standard errors of the mean
Single or pooled sexes
Owing to differences in reproductive strategies, males and females may employ different anti-predator responses. For example, males, who might regularly traverse unfamiliar territories in pursuit of mating opportunities, may freeze when confronted with a predator as they would be unfamiliar with refuges in the area. In contrast a female, who remains within her known territory, may flee in the face of a predator as she may be aware of refuges, and may aim to distract the predator away from vulnerable young. For instance, this difference has been observed in rats where males respond to fear with a “freeze” response, whereas females prefer the “dart” response [287]. Without differentiating between such strategies, it might appear that some individuals display inappropriate anti-predator responses (e.g., freezing when they are expected to flee). Failing to account for sexual dimorphism in behavioural responses may thus cause anti-predator abilities to be underestimated. Our results showed that effect sizes from studies that analysed males and females independently were approximately three times greater than those who analysed sexes in combination (Table 5). While it is not always possible to account for sex, for example, in studies of wild animals where sex is not known, this finding highlights the important effect that behavioural sexual dimorphism might have on studies of animal behaviour.
Organic and synthetic predator cues
Thirteen percent of studies reviewed here used artificial or synthetic predator cues, for example, plastic predator models, or synthesized volatile components of faeces (trimethylthiazoline, TMT; fox odour). We found no differences in effect sizes for organic or synthetic predator cues (Table 6). A selection of studies that we came across, however, reported that some synthetic compounds, such as TMT, were less effective at eliciting responses than organic alternatives [182, 185, 210, 212, 244]. While our findings did not support this, we only compared organic and synthetic cues broadly, and were unable to draw these comparisons among cues with different properties (olfactory, visual, auditory). We recommend that as a precaution, organic cues be selected over synthetic ones where possible, especially in the case of olfactory cues.
Review limitations
Our review reveals a lack of studies on species from South America and Africa (Fig. 4a). This finding could be explained by the fact that we only considered articles written in English. Despite a search strategy that also targeted grey-literature, the returned results were almost exclusively peer-reviewed articles (8% theses, 92% peer-reviewed journal articles, 0 articles from government or NGO reports). Further, of studies that reported means and error margins (rather than box plots), 14.9% did not specify what unit the error margin represented (i.e. standard deviation, standard error, or confidence intervals), which, despite attempts to contact corresponding authors, forced us to reject some studies from the quantitative analyses. During the article screening process, we may have unintentionally introduced biases by only performing consistency checks on a subset of the articles, however, given our high inter-rater reliability scores throughout, we are confident that any such biases would have minimal effect on our findings. Finally, our analysis was limited by our inability to examine interaction effects between variables, for example, the interaction between cue type and cue properties (olfactory, visual, auditory). Behavioural responses are complex, and without investigating fine scale details, such as the effectiveness of predator cues that target different sensory systems among taxa, our ability to comment on the most effective methodology (behavioural assays and predator cues) was hampered. Our review delivers a detailed global overview of methodology for quantifying anti-predator responses in mammals, but owing to limitations in our data set, was unable to provide taxa or sensory system specific insights.
Review conclusions
Implications for management
This review identifies six major types of behavioural assay (behavioural focals, capture probability, feeding station, flight initiation distance, giving-up density, and stimulus presentations) that measure five primary behaviours (activity, escape, exploration, foraging and vigilance). As quantified by differences in effect sizes, there was no behavioural assay, nor particular behaviour that was universally the best at capturing variation in anti-predator responses. Similarly, there were no predator cue types or properties found to be universally most effective.
Population managers seeking to quantify anti-predator responses can use this review to understand the primary assay types and behaviours that have been recorded in past research. Population managers can also find information to replicate existing assays for a particular species, with references to specific examples, in Additional file 9. Our review has also highlighted the importance of controlling for potentially confounding factors. We advise managers to take care in selecting appropriate control stimuli (where necessary), to consider using organic predator cues over synthetic ones, and to be alert to the possibility of sexual dimorphism in anti-predator behaviour.
Despite specifically targeting government and NGO sources during our search strategy, no studies were returned. This suggests that despite growing recognition of the impact of anti-predator behaviour on the persistence of mammals [288], measurement of anti-predator behaviours are not being widely incorporated into conservation monitoring and management. Given that behaviour is a key trait mediating impact from predation, we urge population managers to incorporate an understanding of these behaviours into future management strategies.
Implications for research
Information provided by anti-predator behavioural assays can be critically important for informing species conservation and management. To further this analysis, future work could aim to quantify how well different behavioural assays can predict vulnerability to predation in mammals. Future research might also be directed at categories that were underrepresented in this review, such as species from South America or Africa, and species highly threatened with extinction. Additionally, further investigations should focus on understanding the anti-predator responses of mammals impacted by invasive species, or species that largely exist in isolation from predators (e.g., in captive breeding programs, or in predator-free havens). An enhanced understanding of anti-predator responses would be most beneficial for the conservation management of such species.
Gaps in our data set limited our ability to investigate the most effective behavioural assays and predator cues by preventing us from examining interaction effects. It is likely that such effects would provide valuable insights into complex behavioural responses. Future research could investigate these intricacies within a particular group of mammals, or more broadly, in another taxonomic group. The search strategy developed here may be applied to other systematic reviews and used for such a purpose. As we used a broad search strategy independent of species information, and scored the satisfaction of each article against all criteria, parties interested in replicating this review for alternate taxonomic groups (e.g. fish, reptiles, amphibians, birds) could refer to Additional file 4 to extract articles from our search which satisfy the I, C, O criteria, but not our Population criteria, and re-assess this subset against a new Population criteria.
We encourage future studies of anti-predator responses to incorporate measures of individual repeatability, as it not only strengthens behavioural inferences that can be drawn, but also allows us to assess the robustness of the particular behavioural assay. Assays of repeatable behaviours can facilitate robust comparisons among individuals, and between populations, offering valuable opportunities to compare anti-predator abilities between naïve and predator-exposed populations. We further encourage studies of this nature to consider validating their methodology, increasing both the reliability of their results, and the robustness of their behavioural assays, which may then be adopted by future studies. Finally, we urge future studies of this nature to be detailed in their reporting of statistical analyses and findings (including units of error reported) to ensure accurate, thorough, and transparent communication of results and to facilitate subsequent meta-analysis.
Availability of data and materials
References
Ceballos G, Ehrlich PR. Mammal population losses and the extinction crisis. Science. 2002;296(5569):904–7.
Schipper J, Chanson JS, Chiozza F, Cox NA, Hoffmann M, Katariya V, et al. The status of the world’s land and marine mammals: diversity, threat, and knowledge. Science. 2008;322(5899):225.
Spooner FEB, Pearson RG, Freeman R. Rapid warming is associated with population decline among terrestrial birds and mammals globally. Glob Change Biol. 2018;24(10):4521–31.
Newbold T, Hudson LN, Hill SLL, Contu S, Lysenko I, Senior RA, et al. Global effects of land use on local terrestrial biodiversity. Nature. 2015;520(7545):45–50.
Ross AK, Letnic M, Blumstein DT, Moseby KE. Reversing the effects of evolutionary prey naiveté through controlled predator exposure. J Appl Ecol. 2019;56(7):1761–9.
Salo P, Korpimäki E, Banks PB, Nordström M, Dickman CR. Alien predators are more dangerous than native predators to prey populations. Proc Biol Sci. 2007;274(1615):1237–43.
Radford JQ, Woinarski JCZ, Legge S, Baseler M, Bentley J, Burbidge AA, et al. Degrees of population-level susceptibility of Australian terrestrial non-volant mammal species to predation by the introduced red fox (Vulpes vulpes) and feral cat (Felis catus). Wildl Res. 2018;45(7):645–57.
Murphy BP, Woolley L-A, Geyle HM, Legge SM, Palmer R, Dickman CR, et al. Introduced cats (Felis catus) eating a continental fauna: the number of mammals killed in Australia. Biol Conserv. 2019;237:28–40.
Clavero M, García-Berthou E. Invasive species are a leading cause of animal extinctions. Trends Ecol Evol. 2005;20(3):110.
Sax Dov F, Gaines Steven D, Brown James H. Species invasions exceed extinctions on islands worldwide: a comparative study of plants and birds. Am Nat. 2002;160(6):766–83.
Loehle C, Eschenbach W. Historical bird and terrestrial mammal extinction rates and causes. Divers Distrib. 2012;18(1):84–91.
Greggor AL, Price CJ, Shier DM. Examining the efficacy of anti-predator training for increasing survival in conservation translocations: a systematic review protocol. Environ Evid. 2019;8(1):11.
Jolly CJ, Phillips BL. Rapid evolution in predator-free conservation havens and its effects on endangered species recovery. Conserv Biol. 2021;35(1):383–5.
Tavecchia G, Viedma C, Martínez-Abraín A, Bartolomé M-A, Gómez JA, Oro D. Maximizing re-introduction success: assessing the immediate cost of release in a threatened waterfowl. Biol Conserv. 2009;142(12):3005–12.
West R, Letnic M, Blumstein DT, Moseby KE. Predator exposure improves anti-predator responses in a threatened mammal. J Appl Ecol. 2018;55(1):147–56.
Greggor AL, Blumstein DT, Wong BBM, Berger-Tal O. Using animal behavior in conservation management: a series of systematic reviews and maps. Environ Evid. 2019;8(1):23.
Grassel SM, Rachlow JL, Williams CJ. Spatial interactions between sympatric carnivores: asymmetric avoidance of an intraguild predator. Ecol Evol. 2015;5(14):2762–73.
Higdon SD, Diggins CA, Cherry MJ, Ford WM. Activity patterns and temporal predator avoidance of white-tailed deer (Odocoileus virginianus) during the fawning season. J Ethol. 2019;37(3):283–90.
Hébert M, Versace E, Vallortigara G. Inexperienced preys know when to flee or to freeze in front of a threat. Proc Natl Acad Sci PNAS. 2019;116(46):22918–20.
Stankowich T, Coss RG. Effects of risk assessment, predator behavior, and habitat on escape behavior in Columbian black-tailed deer. Behav Ecol. 2006;18(2):358–67.
Medill SA, Renard A, Larivière S. Ontogeny of antipredator behaviour in striped skunks (Mephitis mephitis). Ethol Ecol Evol. 2011;23(1):41–8.
Emlen DJ. The evolution of animal weapons. Ann Rev Ecol Evol Syst. 2008;39(1):387–413.
Roche DG, Careau V, Binning SA. Demystifying animal “personality” (or not): why individual variation matters to experimental biologists. J Exp Biol. 2016;219(24):3832–43.
Gaynor KM, Cherry MJ, Gilbert SL, Kohl MT, Larson CL, Newsome TM, et al. An applied ecology of fear framework: linking theory to conservation practice. Anim Conserv. 2021;24(3):308–21.
Martínez-Abraín A, Quevedo M, Serrano D. Translocation in relict shy-selected animal populations: program success versus prevention of wildlife-human conflict. Biol Conserv. 2022;268:109519.
Rummel L, Martínez-Abraín A, Mayol J, Ruiz-Olmo J, Mañas F, Jiménez J, et al. Use of wild–caught individuals as a key factor for success in vertebrate translocations. Anim Biodivers Conserv. 2016;39(2):207–91.
Langridge J, Sordello R, Reyjol Y. Outcomes of wildlife translocations in protected areas: what is the type and extent of existing evidence? A systematic map protocol. Environ Evid. 2020;9(1):16.
Hayward MW, Kerley GIH. Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes? Biol Conserv. 2009;142(1):1–13.
Legge S, Woinarski JCZ, Burbidge AA, Palmer R, Ringma J, Radford JQ, et al. Havens for threatened Australian mammals: the contributions of fenced areas and offshore islands to the protection of mammal species susceptible to introduced predators. Wildl Res. 2018;45(7):627–44.
Ringma J, Legge S, Woinarski J, Radford J, Wintle B, Bode M. Australia’s mammal fauna requires a strategic and enhanced network of predator-free havens. Nat Ecol Evol. 2018;2(3):410–1.
Evans K, Moore R, Harris S. The social and ecological integration of captive-raised adolescent male African Elephants (Loxodonta africana) into a wild population. PLoS ONE. 2013;8(2):e55933.
Pinter-Wollman N, Isbell LA, Hart LA. Assessing translocation outcome: comparing behavioral and physiological aspects of translocated and resident African elephants (Loxodonta africana). Biol Conserv. 2009;142(5):1116–24.
Müller J, Wölfl M, Wölfl S, Müller DWH, Hothorn T, Heurich M. Protected areas shape the spatial distribution of a European lynx population more than 20 years after reintroduction. Biol Conserv. 2014;177:210–7.
Muller LI, Murrow JL, Lupardus JL, Clark JD, Yarkovich JG, Stiver WH, et al. Genetic structure in Elk persists after translocation. J Wildl Manage. 2018;82(6):1124–34.
Wei F, Swaisgood R, Hu Y, Nie Y, Yan L, Zhang Z, et al. Progress in the ecology and conservation of giant pandas. Conserv Biol. 2015;29(6):1497–507.
Thalmann S, Peck S, Wise P, Potts JM, Clarke J, Richley J. Translocation of a top-order carnivore: tracking the initial survival, spatial movement, home-range establishment and habitat use of Tasmanian devils on Maria Island. Aust Mammal. 2016;38(1):68–79.
Woinarski JCZ, Burbidge AA, Harrison PL. Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. PNAS. 2015;112(15):4531–40.
Morris SD, Brook BW, Moseby KE, Johnson CN. Factors affecting success of conservation translocations of terrestrial vertebrates: a global systematic review. Glob Ecol Conserv. 2021;28:e01630.
Muralidhar A, Moore FL, Easton LJ, Jamieson IG, Seddon PJ, van Heezik Y. Know your enemy? Conservation management causes loss of antipredator behaviour to novel predators in New Zealand robins. Anim Behav. 2019;149:135–42.
Blumstein DT, Daniel JC. The loss of anti-predator behaviour following isolation on islands. Proc Biol Sci. 2005;272(1573):1663–8.
Blumstein DT, Daniel JC, Springett BP. A test of the multi-predator hypothesis: rapid loss of antipredator behavior after 130 years of isolation. Ethology. 2004;110(11):919–34.
Jolly CJ, Webb JK, Phillips BL. The perils of paradise: an endangered species conserved on an island loses antipredator behaviours within 13 generations. Biol Lett. 2018;14(6):20180222.
Cooper WE, Pyron RA, Garland T. Island tameness: living on islands reduces flight initiation distance. Proc Biol Sci. 2014;281(1777):1–7.
Natasha DH, Ben LP, Nicola JM, Julia CW, Marika AM, Colin GW, Adrian FW. Perverse outcomes from fencing fauna: Loss of antipredator traits in a havened mammal population. Biol Conserv. 2023; 281:110000, ISSN 0006-3207. https://doi.org/10.1016/j.biocon.2023.110000.
Moseby KE, Cameron A, Crisp HA. Can predator avoidance training improve reintroduction outcomes for the greater bilby in arid Australia? Anim Behav. 2012;83(4):1011–21.
Moseby KE, Read JL, Paton DC, Copley P, Hill BM, Crisp HA. Predation determines the outcome of 10 reintroduction attempts in arid South Australia. Biol Conserv. 2011;144(12):2863–72.
Berger-Tal O, Blumstein DT, Carroll S, Fisher RN, Mesnick SL, Owen MA, et al. A systematic survey of the integration of animal behavior into conservation. Conserv Biol. 2016;30(4):744–53.
Harrison ND, Frick CH, Wayne AF. Repeatable measure of cage trap behaviour to quantify boldness and agitation in a macropod. Australian Mammalogy. 2022. https://doi.org/10.1071/AM22007.
Tay NE, Fleming PA, Warburton NM, Moseby KE. Predator exposure enhances the escape behaviour of a small marsupial, the burrowing bettong. Anim Behav. 2021;175:45–56.
Blumstein DT, Mari M, Daniel JC, Ardron JG, Griffin AS, Evans CS. Olfactory predator recognition: wallabies may have to learn to be wary. Anim Conserv. 2002;5(2):87–93.
Saxon-Mills EC, Moseby K, Blumstein DT, Letnic M. Prey naïveté and the anti-predator responses of a vulnerable marsupial prey to known and novel predators. Behav Ecol Sociobiol. 2018;72(9):151.
Steindler LA, Blumstein DT, West R, Moseby KE, Letnic M. Exposure to a novel predator induces visual predator recognition by naïve prey. Behav Ecol Sociobiol. 2020;74(8):102.
Bannister H, Brandle R, Moseby K. Antipredator behaviour of a native marsupial is relaxed when mammalian predators are excluded. Wildl Res. 2018;45(8):726–36.
Berger-Tal O, Greggor AL, Macura B, Adams CA, Blumenthal A, Bouskila A, et al. Systematic reviews and maps as tools for applying behavioral ecology to management and policy. Behav Ecol. 2019;30(1):1–8.
Rothstein HR, Sutton AJ, Borenstein M. Publication bias in meta-analysis: prevention, assessment and adjustments. Hoboken: Wiley; 2005.
Collaboration for Environmental Evidence. Guidelines for systematic review and evidence synthesis in environmental management. 2013. www.environmentalevidence.org/Documents/Guidelines/Guidelines4.2.pdf.
Harrison ND, Phillips BL, Hemmi JM, et al. Identifying the most effective behavioural assays and predator cues for quantifying anti-predator responses in mammals: a systematic review protocol. Environ Evid 2021;10:38. https://doi.org/10.1186/s13750-021-00253-9.
Fellows I. wordcloud: Word Clouds. R package version 2.6. 2018.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020.
Foo YZ, O'Dea RE, Koricheva J, Nakagawa S, Lagisz M. A practical guide to question formation, systematic searching and study screening for literature reviews in ecology and evolution. Methods Ecol Evol. 2021;n/a(n/a).
Smart JM, Burling D. Radiology and the internet: a systematic review of patient information resources. Clin Radiol. 2001;56(11):867–70.
Kohl C, McIntosh EJ, Unger S, Haddaway NR, Kecke S, Schiemann J, et al. Online tools supporting the conduct and reporting of systematic reviews and systematic maps: a case study on CADIMA and review of existing tools. Environ Evid. 2018;7(1):8.
Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107–28.
Konno K, Livoreil B, AS P. CEECAT: Collaboration for Environmental Evidence Critical Appraisal Tool Version 0.3 (prototype). 2021.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
IUCN. The IUCN Red List of Threatened Species. Version 2022-1. 2022. https://www.iucnredlist.org.
Bland M. Estimating mean and standard deviation from the sample size, three quartiles, minimum, and maximum. Int J Stat Med Res. 2014;4(1):57–64.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
Harrison F. Getting started with meta-analysis. Methods Ecol Evol. 2011;2(1):1–10.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48.
Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CED, et al. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ. 2018;6:e4794.
Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol. 2011;65(1):23–35.
Perrot-Sinal TS, Heale VR, Ossenkopp KP, Kavaliers M. Sexually dimorphic aspects of spontaneous activity in meadow voles (Microtus pennsylvanicus): effects of exposure to fox odor. Behav Neurosci. 1996;110(5):1126–32.
Uchida K, Shimamoto T, Yanagawa H, Koizumi I. Comparison of multiple behavioral traits between urban and rural squirrels. Urban Ecosyst. 2020;23(4):745–54.
Pickett KN, Hik DS, Newsome AE, Pech RP. The influence of predation risk on foraging behaviour of brushtail possums in Australian woodlands. Wildl Res. 2005;32(2):121–30.
Borowski Z, Owadowska E. Field vole (Microtus agrestis) seasonal spacing behavior: the effect of predation risk by mustelids. Naturwissenschaften. 2010;97(5):487–93.
Jayakody S, Sibbald AM, Gordon IJ, Lambin X. Red deer Cervus elephus vigilance behaviour differs with habitat and type of human disturbance. Wildl Biol. 2008;14(1):81–91.
Li CW, Monclus R, Maul TL, Jiang ZG, Blumstein DT. Quantifying human disturbance on antipredator behavior and flush initiation distance in yellow-bellied marmots. Appl Anim Behav Sci. 2011;129(2–4):146–52.
Crego RD, Jimenez JE, Rozzi R. Macro- and micro-habitat selection of small rodents and their predation risk perception under a novel invasive predator at the southern end of the Americas. Mammal Res. 2018;63(3):267–75.
Dickman CR. Predation and habitat shift in the house mouse, Mus domesticus. Ecology. 1992;73(1):313–22.
Fey K, Banks PB, Ylonen H, Korpimaki E. Behavioural responses of voles to simulated risk of predation by a native and an alien mustelid: an odour manipulation experiment. Wildl Res. 2010;37(4):273–82.
Orrock JL. When the ghost of predation has passed: do rodents from islands with and without fox predators exhibit aversion to fox cues? Ethology. 2010;116(4):338–45.
Russell BG, Banks PB. Do Australian small mammals respond to native and introduced predator odours? Austral Ecol. 2007;32(3):277–86.
Banks PB. Responses of Australian bush rats, Rattus fuscipes, to the odor of introduced Vulpes vulpes. J Mammal. 1998;79(4):1260–4.
Kovacs EK, Crowther MS, Webb JK, Dickman CR. Population and behavioural responses of native prey to alien predation. Oecologia. 2012;168(4):947–57.
Dickman CR, Doncaster CP. Responses of small mammals to red fox (Vulpes vulpes) odour. J Zool (Lond). 1984;204(4):521–31.
Baack JK, Switzer PV. Alarm calls affect foraging behavior in eastern chipmunks (Tamias striatus, Rodentia : Sciuridae). Ethology. 2000;106(12):1057–66.
Bedoya-Perez MA, Le A, McGregor IS, Crowther MS. Antipredator responses toward cat fur in wild brown rats tested in a semi-natural environment. Behav Ecol. 2021;32(5):835–44.
Clinchy M, Zanette LY, Roberts D, Suraci JP, Buesching CD, Newman C, et al. Fear of the human “super predator” far exceeds the fear of large carnivores in a model mesocarnivore. Behav Ecol. 2016;27(6):1826–32.
Garvey PM, Glen AS, Pech RP. Dominant predator odour triggers caution and eavesdropping behaviour in a mammalian mesopredator. Behav Ecol Sociobiol. 2016;70(4):481–92.
Leo V, Reading RP, Letnic M. Interference competition: odours of an apex predator and conspecifics influence resource acquisition by red foxes. Oecologia. 2015;179(4):1033–40.
Juliana JRS, Kotler BP, Wielebnowski N, Cox JG. Stress as an adaptation I: Stress hormones are correlated with optimal foraging behaviour of gerbils under the risk of predation. Evol Ecol Res. 2017;18(5):571–85.
Sundermann D, Scheumann M, Zimmermann E. Olfactory predator recognition in predator-naive gray mouse lemurs (Microcebus murinus). J Comp Psychol. 2008;122(2):146–55.
Tortosa FS, Barrio IC, Carthey AJR, Banks PB. No longer naive? Generalized responses of rabbits to marsupial predators in Australia. Behav Ecol Sociobiol. 2015;69(10):1649–55.
Adcock SJJ, Tucker CB. Naive domestic Bos taurus calves recognize the scent of a canine predator. Anim Behav. 2020;164:173–80.
Busch M, Burroni NE. Foraging activity of commensal Mus musculus in semi-captivity conditions. Effect of predator odours, previous experience and moonlight. Pest Manag Sci. 2015;71(12):1599–604.
Atkins R, Blumstein DT, Moseby KE, West R, Hyatt M, Letnic M. Deep evolutionary experience explains mammalian responses to predators. Behav Ecol Sociobiol. 2016;70(10):1755–63.
Freymiller GA, Whitford MD, Higham TE, Clark RW. Recent interactions with snakes enhance escape performance of desert kangaroo rats (Rodentia: Heteromyidae) during simulated attacks. Biol J Linnean Soc. 2017;122(3):651–60.
Anderson RB, Lawler SP. Behavioral changes in tadpoles after multigenerational exposure to an invasive intraguild predator. Behav Ecol. 2016;27(6):1790–6.
Burns-Cusato M, Glueck AC, Merchak AR, Palmer CL, Rieskamp JD, Duggan IS, et al. Threats from the past: Barbados green monkeys (Chlorocebus sabaeus) fear leopards after centuries of isolation. Behav Process. 2016;126:1–11.
Chamaille-Jammes S, Malcuit H, Le Saout S, Martin JL. Innate threat-sensitive foraging: black-tailed deer remain more fearful of wolf than of the less dangerous black bear even after 100 years of wolf absence. Oecologia. 2014;174(4):1151–8.
Christensen JW, Rundgren M. Predator odour per se does not frighten domestic horses. Appl Anim Behav Science. 2008;112(1–2):136–45.
Carthey AJR, Banks PB. Naïve, bold, or just hungry? An invasive exotic prey species recognises but does not respond to its predators. Biol Invasions. 2018;20(12):3417–29.
Borowski Z. Influence of predator odour on the feeding behaviour of the root vole (Microtus oeconomus Pallas, 1776). Can J Zool-Rev Can Zool. 1998;76(9):1791–4.
Bytheway JP, Banks PB. Overcoming prey naivete: free-living marsupials develop recognition and effective behavioral responses to alien predators in Australia. Glob Change Biol. 2019;25(5):1685–95.
Carlsen M, Lodal J, Leirs H, Jensen TS. The effect of predation risk on body weight in the field vole, Microtus agrestis. Oikos. 1999;87(2):277–85.
Jolly CJ, Smart AS, Moreen J, Webb JK, Gillespie GR, Phillips B. Trophic cascade driven by behavioral fine-tuning as naive prey rapidly adjust to a novel predator. Ecology. 2021;102(7):11.
Arnould C, Signoret JP. Sheep food repellents: efficacy of various products, habituation, and social facilitation. J Chem Ecol. 1993;19(2):225–36.
Epple G, Mason JR, Nolte DL, Campbell DL. Effects of predator odors on feeding in the mountain beaver (Aplodontia rufa). J Mammal. 1993;74(3):715–22.
Hayes RA, Nahrung HF, Wilson JC. The response of native Australian rodents to predator odours varies seasonally: a by-product of life history variation? Anim Behav. 2006;71(6):1307–14.
Jessop TS, Ariefiandy A, Purwandana D, Benu YJ, Hyatt M, Letnic M. Little to fear: largest lizard predator induces weak defense responses in ungulate prey. Behav Ecol. 2019;30(3):624–36.
Rosell F. Effectiveness of predator odors as gray squirrel repellents. Can J Zool. 2001;79(9):1719–23.
Stryjek R, Mioduszewska B, Spaltabaka-Gędek E, Juszczak GR. Wild Norway rats do not avoid predator scents when collecting food in a familiar habitat: a field study. Sci Rep. 2018;8(1):9475.
Sullivan TP, Crump DR, Sullivan DS. Use of predator odors as repellents to reduce feeding damage by herbivores-III. Montane and meadow voles (Microtus montanus and Microtus pennsylvanicus). J Chem Ecol. 1988;14(1):363–77.
Sullivan TP, Jin ZH, Li HL, Wang SC. Control of vole populations in young pine plantations in northeast China. For Chron. 1991;67(1):43–7.
Swihart RK. Modifying scent-marking behavior to reduce woodchuck damage to fruit trees. Ecol Appl. 1991;1(1):98–103.
Andersen GE, Johnson CN, Jones ME. Sympatric predator odour reveals a competitive relationship in size-structured mammalian carnivores. Behav Ecol Sociobiol. 2016;70(11):1831–41.
Makin DF, Chamaillé-Jammes S, Shrader AM. Changes in feeding behavior and patch use by herbivores in response to the introduction of a new predator. J Mammal. 2018;99(2):341–50.
Cappa F, Campos V, Giannoni S, Andino N. The effects of poaching and habitat structure on anti-predator behavioral strategies: a guanaco population in a high cold desert as case study. PLoS ONE. 2017;12(8):11.
Engelhardt SC, Weladji RB. Effects of levels of human exposure on flight initiation distance and distance to refuge in foraging eastern gray squirrels (Sciurus carolinensis). Can J Zool-Rev Can Zool. 2011;89(9):823–30.
Guo C, Zhou S, Krzton A, Xiang Z. Variation in escape response of Himalayan marmots (Marmota himalayana) under different human disturbances. Ethology. 2021. https://doi.org/10.1111/eth.13248.
Kittendorf A, Dantzer B. Urban fox squirrels exhibit tolerance to humans but respond to stimuli from natural predators. Ethology. 2021;127(9):697–709.
Saltz D, Berger-Tal O, Motro U, Shkedy Y, Raanan N. Conservation implications of habituation in Nubian ibex in response to ecotourism. Anim Conserv. 2019;22(3):220–7.
Roure EC. Flexibility in antipredator behaviour of Tasmanian macropods to altered devil abundance: The University of Tasmania; 2019.
Bleicher SS, Brown JS, Embar K, Kotler BP. Novel predator recognition by Allenby’s gerbil (Gerbillus andersoni allenbyi): do gerbils learn to respond to a snake that can “see” in the dark? Isr J Ecol Evol. 2016;62(3–4):178–85.
Bleicher SS, Kotler BP, Shalev O, Dixon A, Embar K, Brown JS. Divergent behavior amid convergent evolution: a case of four desert rodents learning to respond to known and novel vipers. PLoS ONE. 2018;13(8):17.
Bytheway JP, Carthey AJR, Banks PB. Risk vs. reward: how predators and prey respond to aging olfactory cues. Behav Ecol Sociobiol. 2013;67(5):715–25.
Carthey AJR, Banks PB. Naivete is not forever: responses of a vulnerable native rodent to its long term alien predators. Oikos. 2016;125(7):918–26.
Cremona T, Crowther MS, Webb JK. Variation of prey responses to cues from a mesopredator and an apex predator. Austral Ecol. 2014;39(7):749–54.
Kelleher V, Hunnick L, Sheriff MJ. Risk-induced foraging behavior in a free-living small mammal depends on the interactive effects of habitat, refuge availability, and predator type. Front Ecol Evol. 2021;9:9.
Kirmani SN, Banks PB, McArthur C. Integrating the costs of plant toxins and predation risk in foraging decisions of a mammalian herbivore. Oecologia. 2010;164(2):349–56.
Mella VSA, Banks PB, McArthur C. Negotiating multiple cues of predation risk in a landscape of fear: what scares free-ranging brushtail possums? J Zool. 2014;294(1):22–30.
Orrock JL, Danielson BJ, Brinkerhoff RJ. Rodent foraging is affected by indirect, but not by direct, cues of predation risk. Behav Ecol. 2004;15(3):433–7.
Welch RJ, Periquet S, Petelle MB, le Roux A. Hunter or hunted? Perceptions of risk and reward in a small mesopredator. J Mammal. 2017;98(6):1531–7.
Bengsen AJ, Leung LKP, Lapidge SJ, Gordon IJ. Artificial illumination reduces bait-take by small rainforest mammals. Appl Anim Behav Sci. 2010;127(1–2):66–72.
Bleicher SS, Kotler BP, Downs CJ, Brown JS. Intercontinental test of constraint-breaking adaptations: testing behavioural plasticity in the face of a predator with novel hunting strategies. J Anim Ecol. 2020;89(8):1837–50.
Bleicher SS, Kotler BP, Downs CJ, Brown JS. Playing to their evolutionary strengths; heteromyid rodents provide opposite snake evasion strategies in the face of known and novel snakes. J Arid Environ. 2020;173:104025.
Bleicher SS, Kotler BP, Embar K. Ninja owl Gerbils over-anticipate an unexpected flying predator. Behav Process. 2020;178:104161.
Esparza-Carlos JP, Íñiguez-Dávalos LI, Laundré J. Microhabitat and presence of top predators affect prey apprehension in a subtropical mountain forest. J Mammal. 2018;99(3):596–607.
Felts J, Schmidt KA. Multitasking and eavesdropping in cotton rats foraging under predation risk. Behav Ecol. 2010;21(5):1080–6.
Heale VR, Vanderwolf CH. Toluene and weasel (2-propylthietane) odors suppress feeding in the rat. J Chem Ecol. 1994;20(11):2953–8.
Herman CS, Valone TJ. The effect of mammalian predator scent on the foraging behavior of Dipodomys merriami. Oikos. 2000;91(1):139–45.
Jolly CJ, Webb JK, Gillespie GR, Phillips BL. Training fails to elicit behavioral change in a marsupial suffering evolutionary loss of antipredator behaviors. J Mammal. 2020;101(4):1108–16.
Jones M, Dayan T. Foraging behavior and microhabitat use by spiny mice, Acomys cahirinus and A. russatus, in the presence of Blanford’s fox (Vulpes cana) odor. J Chem Ecol. 2000;26(2):455–69.
Kotler BP. Patch use by gerbils in a risky environment: manipulating food and safety to test four models. Oikos. 1997;78(2):274–82.
Letnic M, Dworjanyn SA. Does a top predator reduce the predatory impact of an invasive mesopredator on an endangered rodent? Ecography. 2011;34(5):827–35.
Nowak K, le Roux A, Richards SA, Scheijen CPJ, Hill RA. Human observers impact habituated samango monkeys’ perceived landscape of fear. Behav Ecol. 2014;25(5):1199–204.
Orrock JL, Danielson BJ. Temperature and cloud cover, but not predator urine, affect winter foraging of mice. Ethology. 2009;115(7):641–8.
Orrock JL, Danielson BJ. Rodents balancing a variety of risks: invasive fire ants and indirect and direct indicators of predation risk. Oecologia. 2004;140(4):662–7.
Pfister JA, Müller-Schwarze D, Balph DF. Effects of predator fecal odors on feed selection by sheep and cattle. J Chem Ecol. 1990;16(2):573–83.
Powell F, Banks PB. Do house mice modify their foraging behaviour in response to predator odours and habitat? Anim Behav. 2004;67(4):753–9.
Shrader AM, Brown JS, Kerley GIH, Kotler BP. Do free-ranging domestic goats show “landscapes of fear”? Patch use in response to habitat features and predator cues. J Arid Environ. 2008;72(10):1811–9.
Sievert T, Haapakoski M, Palme R, Voipio H, Ylönen H. Secondhand horror: effects of direct and indirect predator cues on behavior and reproduction of the bank vole. Ecosphere. 2019;10(6):e02765.
Strauss A, Solmsdorff KY, Pech R, Jacob J. Rats on the run: removal of alien terrestrial predators affects bush rat behaviour. Behav Ecol Sociobiol. 2008;62(10):1551–8.
Thomsen SK, Green DJ. Cascading effects of predation risk determine how marine predators become terrestrial prey on an oceanic island. Ecology. 2016;97(12):3530–7.
Thorson JM, Morgan RA, Brown JS, Norman JE. Direct and indirect cues of predatory risk and patch use by fox squirrels and thirteen-lined ground squirrels. Behav Ecol. 1998;9(2):151–7.
Wasko DK, Bonilla F, Sasa M. Behavioral responses to snake cues by three species of Neotropical rodents. J Zool. 2014;292(2):142–50.
Barreto GR, Macdonald DW. The response of water voles, Arvicola terrestris, to the odours of predators. Anim Behav. 1999;57(5):1107–12.
Kotler BP, Brown JS, Bouskila A. Apprehension and time allocation in gerbils: the effects of predatory risk and energetic state. Ecology. 2004;85(4):917–22.
Baruzzi C, Lovari S, Fattorini N. Catch me if you can: antipredatory behaviour of chamois to the wolf. Ethol Ecol Evol. 2017;29(6):589–98.
Berton F, Vogel E, Belzung C. Modulation of mice anxiety in response to cat odor as a consequence of predators diet. Physiol Behav. 1998;65(2):247–54.
Blumstein DT, Daniel JC, Griffin AS, Evans CS. Insular tammar wallabies (Macropus eugenii) respond to visual but not acoustic cues from predators. Behav Ecol. 2000;11(5):528–35.
Blumstein DT, Ferando E, Stankowich T. A test of the multipredator hypothesis: yellow-bellied marmots respond fearfully to the sight of novel and extinct predators. Anim Behav. 2009;78(4):873–8.
Dalerum F, Belton L. African ungulates recognize a locally extinct native predator. Behav Ecol. 2015;26(1):215–22.
de Faria CM, Sa FD, Costa DDL, da Silva MM, da Silva BC, Young RJ, et al. Captive-born collared peccary (Pecari tajacu, Tayassuidae) fails to discriminate between predator and non-predator models. Acta Ethol. 2018;21(3):175–84.
de Faria CM, Sa FD, Costa DDL, da Silva MM, da Silva BC, Young RJ, et al. Captive-born collared peccaries learning about their predators: lessons learnt but not remembered. Behav Process. 2020;171:8.
Durant SM. Living with the enemy: avoidance of hyenas and lions by cheetahs in the Serengeti. Behav Ecol. 2000;11(6):624–32.
Ericsson G, Neumann W, Dettki H. Moose anti-predator behaviour towards baying dogs in a wolf-free area. Eur J Wildl Res. 2015;61(4):575–82.
Fardell LL, Bedoya-Perez MA, Dickman CR, Crowther MS, Pavey CR, Narayan EJ. Are physiological and behavioural responses to stressors displayed concordantly by wild urban rodents? Sci Nat. 2021;108(1):15.
Jones ME, Smith GC, Jones SM. Is anti-predator behaviour in Tasmanian eastern quolls (Dasyurus viverrinus) effective against introduced predators? Anim Conserv. 2004;7(2):155–60.
Kappel P, Hohenbrink S, Radespiel U. Experimental evidence for olfactory predator recognition in wild mouse lemurs. Am J Primatol. 2011;73(9):928–38.
Li C, Yang X, Ding Y, Zhang L, Fang H, Tang S, et al. Do Pere David’s deer lose memories of their ancestral predators? PLoS ONE. 2011;6(8):e23623-6.
Ramp D, Russell BG, Croft DB. Predator scent induces differing responses in two sympatric macropodids. Aust J Zool. 2005;53(2):73–8.
Searcy YM, Caine NG. Hawk calls elicit alarm and defensive reactions in captive Geoffroy’s marmosets (Callithfix geoffroyi). Folia Primatol. 2003;74(3):115–25.
Shier DM, Owings DH. Effects of predator training on behavior and post-release survival of captive prairie dogs (Cynomys ludovicianus). Biol Conserv. 2006;132(1):126–35.
Steindler L, Letnic M. Not so naive: endangered mammal responds to olfactory cues of an introduced predator after less than 150 years of coexistence. Behav Ecol Sociobiol. 2021;75(1):10.
Steindler LA, Blumstein DT, West R, Moseby KE, Letnic M. Discrimination of introduced predators by ontogenetically naïve prey scales with duration of shared evolutionary history. Anim Behav. 2018;137:133–9.
Adamec R, Kent P, Anisman H, Shallow T, Merali Z. Neural plasticity, neuropeptides and anxiety in animals - Implications for understanding and treating affective disorder following traumatic stress in humans. Neurosci Biobehav Rev. 1998;23(2):301–18.
Barrio IC, Bueno CG, Banks PB, Tortosa FS. Prey naivete in an introduced prey species: the wild rabbit in Australia. Behav Ecol. 2010;21(5):986–91.
Best IN, Shaner PJL, Lo HY, Pei KJC, Kuo CC. Bigger doesn’t mean bolder: behavioral variation of four wild rodent species to novelty and predation risk following a fast-slow continuum. Front Zool. 2020;17(1):27.
Bian J, Wu Y, Liu J. Breeding behavior under temporal risk of predation in male root voles (Microtus oeconomus). J Mammal. 2005;86(5):953–60.
Blanchard DC, Markham C, Yang M, Hubbard D, Madarang E, Blanchard RJ. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav Neurosci. 2003;117(2):360–8.
Bowen MT, Keats K, Kendig MD, Cakic V, Callaghan PD, McGregor IS. Aggregation in quads but not pairs of rats exposed to cat odor or bright light. Behav Process. 2012;90(3):331–6.
Brachetta V, Schleich CE, Zenuto RR. Source odor, intensity, and exposure pattern affect antipredatory responses in the Subterranean Rodent Ctenomys talarum. Ethology. 2016;122(12):923–36.
Burwash MD, Tobin ME, Woolhouse AD, Sullivan TP. Laboratory evaluation of predator odors for eliciting an avoidance response in roof rats (Rattus rattus). J Chem Ecol. 1998;24(1):49–66.
Butler RK, Oliver EM, Sharko AC, Parilla-Carrero J, Kaigler KF, Fadel JR, et al. Activation of corticotropin releasing factor-containing neurons in the rat central amygdala and bed nucleus of the stria terminalis following exposure to two different anxiogenic stressors. Behav Brain Res. 2016;304:92–101.
Butler RK, Sharko AC, Oliver EM, Brito-Vargas P, Kaigler KF, Fadel JR, et al. Activation of phenotypically-distinct neuronal subpopulations of the rat amygdala following exposure to predator odor. Neuroscience. 2011;175:133–44.
Cox TE, Murray PJ, Hall GP, Li X. Manipulating resource use by goats with predator fecal odors. Wildl Soc Bull. 2012;36(4):802–6.
de Oliveira Crisanto K, de Andrade WMG, de Azevedo Silva KD, Lima RH, de Oliveira Costa MSM, de Souza Cavalcante J, et al. The differential mice response to cat and snake odor. Physiol Behav. 2015;152:272–9.
Dell’Omo G, Fiore M, Alleva E. Strain differences in mouse response to odours of predators. Behav Process. 1994;32(2):105–15.
Dent CL, Isles AR, Humby T. Measuring risk-taking in mice: balancing the risk between seeking reward and danger. Eur J Neurosci. 2014;39(4):520–30.
Endres T, Apfelbach R, Fendt M. Behavioral changes induced in rats by exposure to trimethylthiazoline, a component of fox odor. Behav Neurosci. 2005;119(4):1004–10.
Endres T, Fendt M. Aversion- Vs fear-inducing properties of 2,4,5-trimethyl-3-thiazoline, a component of fox odor, in comparison with those of butyric acid. J Exp Biol. 2009;212(15):2324–7.
Fendt M. Exposure to urine of canids and felids, but not of herbivores, induces defensive behavior in laboratory rats. J Chem Ecol. 2006;32(12):2617–27.
Fendt M, Brosch M, Wernecke KEA, Willadsen M, Wohr M. Predator odour but not TMT induces 22-kHz ultrasonic vocalizations in rats that lead to defensive behaviours in conspecifics upon replay. Sci Rep. 2018;8:11.
Ferland CL, Reichel CM, McGinty JF. Effects of oxytocin on methamphetamine-seeking exacerbated by predator odor pre-exposure in rats. Psychopharmacology. 2016;233(6):1015–24.
Gomes MGS, Tractenberg SG, Orso R, Viola TW, Grassi-Oliveira R. Sex differences in risk behavior parameters in adolescent mice: relationship with brain-derived neurotrophic factor in the medial prefrontal cortex. Neurosci Lett. 2022;766:136339.
Guo H, Hegab IM, Tan Y, Yao B, Wang C, Cai Z, et al. Exposure to eagle owl feces induces anti-predator behavior, physiology, and hypothalamic gene responses in a subterranean rodent, the plateau zokor (Eospalax baileyi). Behav Ecol Sociobiol. 2020;74(12):145.
Hacquemand R, Choffat N, Jacquot L, Brand G. Comparison between low doses of TMT and cat odor exposure in anxiety- and fear-related behaviors in mice. Behav Brain Res. 2013;238(1):227–31.
Hacquemand R, Jacquot L, Brand G. Postnatal exposure to predator odor (TMT) enhances spatial learning in mice adulthood. Behav Brain Res. 2012;229(1):113–7.
Hebb ALO, Zacharko RM, Gauthier M, Trudel F, Laforest S, Drolet G. Brief exposure to predator odor and resultant anxiety enhances mesocorticolimbic activity and enkephalin expression in CD-1 mice. Eur J Neurosci. 2004;20(9):2415–29.
Hegab IM, Jin Y, Ye M, Wang A, Yin B, Yang S, et al. Defensive responses of Brandt’s voles (Lasiopodomys brandtii) to stored cat feces. Physiol Behav. 2014;123:193–9.
Hegab IM, Wang A, Yin B, Yang S, Wanhong W. Behavioral and neuroendocrine response of Brandt’s voles, Lasiopodomys brandtii, to odors of different species. Eur J Wildl Res. 2014;60(2):331–40.
Heise-Pavlov S, Bradley A. When ancestry haunts-can evolutionary links to ancestors affect vulnerability of Australian prey to introduced predators? A preliminary study. Aust Mammal. 2021;44:98–108.
Homiack D, O’Cinneide E, Hajmurad S, Barrileaux B, Stanley M, Kreutz MR, et al. Predator odor evokes sex-independent stress responses in male and female Wistar rats and reduces phosphorylation of cyclic-adenosine monophosphate response element binding protein in the male, but not the female hippocampus. Hippocampus. 2017;27(9):1016–29.
Hwa LS, Neira S, Pina MM, Pati D, Calloway R, Kash TL. Predator odor increases avoidance and glutamatergic synaptic transmission in the prelimbic cortex via corticotropin-releasing factor receptor 1 signaling. Neuropsychopharmacology. 2019;44(4):766–75.
Keil MF, Briassoulis G, Nesterova M, Miraftab N, Gokarn N, Wu TJ, et al. Threat bias in mice with inactivating mutations of prkar1a. Neuroscience. 2013;241:206–14.
Kemble ED, Bolwahnn BL. Immediate and long-term effects of novel odors on risk assessment in mice. Physiol Behav. 1997;61(4):543–9.
Liang Z, King J, Zhang N. Neuroplasticity to a single-episode traumatic stress revealed by resting-state fMRI in awake rats. Neuroimage. 2014;103:485–91.
Maestas-Olguin CR, Parish MM, Pentkowski NS. Coyote urine, but not 2-phenylethylamine, induces a complete profile of unconditioned anti-predator defensive behaviors. Physiol Behav. 2021;229:113210.
May MD, Bowen MT, McGregor IS, Timberlake W. Rubbings deposited by cats elicit defensive behavior in rats. Physiol Behav. 2012;107(5):711–8.
McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all “predator odours” are equal: cat odour but not 2, 4, 5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res. 2002;129(1–2):1–16.
McPhee ME, Segal A, Johnston RE. Hamsters use predator odors as indirect cues of predation risk. Ethology. 2010;116(6):517–23.
Monclus R, Rodel HG, Palme R, Holst DV, Miguel JD. Non-invasive measurement of the physiological stress response of wild rabbits to the odour of a predator. Chemoecology. 2006;16(1):25–9.
Muñoz-Abellán C, Armario A, Nadal R. Do odors from different cats induce equivalent unconditioned and conditioned responses in rats? Physiol Behav. 2010;99(3):388–94.
Muñoz-Abellán C, Daviu N, Rabasa C, Nadal R, Armario A. Cat odor causes long-lasting contextual fear conditioning and increased pituitary-adrenal activation, without modifying anxiety. Horm Behav. 2009;56(4):465–71.
Nasca C, Orlando R, Marchiafava M, Boldrini P, Battaglia G, Scaccianoce S, et al. Exposure to predator odor and resulting anxiety enhances the expression of the α2δ subunit of voltage-sensitive calcium channels in the amygdala. J Neurochem. 2013;125(5):649–56.
Perrot-Sinal TS, Ossenkopp KP, Kavaliers M. Brief predator odour exposure activates the HPA axis independent of locomotor changes. Neuroreport. 1999;10(4):775–80.
Saridoğan GE, Aykaç A, Cabadak H, Cerit C, Çalişkan M, Gören MZ. D-Cycloserine acts via increasing the GluN1 protein expressions in the frontal cortex and decreases the avoidance and risk assessment behaviors in a rat traumatic stress model. Behav Brain Res. 2015;293:227–33.
Schmidt KA, Lee E, Ostfeld RS, Sieving K. Eastern chipmunks increase their perception of predation risk in response to titmouse alarm calls. Behav Ecol. 2008;19(4):759–63.
Sievert T, Laska M. Behavioral responses of CD-1 Mice to six predator odor components. Chem Senses. 2016;41(5):399–406.
Siviy SM. Effects of pre-pubertal social experiences on the responsiveness of juvenile rats to predator odors. Neurosci Biobehav Rev. 2008;32(7):1249–58.
Siviy SM, Harrison KA. Effects of Neonatal handling on play behavior and fear towards a predator odor in juvenile rats (Rattus norvegicus). J Comp Psychol. 2008;122(1):1–8.
Spencer EE, Crowther MS, Dickman CR. Risky business: do native rodents use habitat and odor cues to manage predation risk in Australian deserts? PLoS ONE. 2014;9(2):e90566.
Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008;151(4):937–47.
Stockman SL, McCarthy MM. Predator odor exposure of rat pups has opposite effects on play by juvenile males and females. Pharmacol Biochem Behav. 2017;152:20–9.
Wallace KJ, Rosen JB. Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behav Neurosci. 2000;114(5):912–22.
Wang ME, Fraize NP, Yin L, Yuan RK, Petsagourakis D, Wann EG, et al. Differential roles of the dorsal and ventral hippocampus in predator odor contextual fear conditioning. Hippocampus. 2013;23(6):451–66.
Wu R, Wu X, Li S, Li G, Jiang Z, Zhong H, et al. Predator odor exposure increases social contact in adolescents and parental behavior in adulthood in Brandt’s voles. Behav Process. 2021;186:104372.
Ward JF, MacDonald DW, Doncaster CP. Responses of foraging hedgehogs to badger odour. Anim Behav. 1997;53(4):709–20.
Aschemeier LM, Maher CR. Eavesdropping of woodchucks (Marmota monax) and eastern chipmunks (Tamias striatus) on heterospecific alarm calls. J Mammal. 2011;92(3):493–9.
Jedrzejewski W, Rychlik L, Jedrzejewska B. Responses of bank voles to odours of seven species of predators: experimental data and their relevance to natural predator-vole relationships. Oikos. 1993;68(2):251–7.
Wang Z. The effects of fitch odor on behavior of root voles. J Northeast Forestry Univ. 2001;29(4):34–7.
Middleton AD, Kauffman MJ, McWhirter DE, Jimenez MD, Cook RC, Cook JG, et al. Linking anti-predator behaviour to prey demography reveals limited risk effects of an actively hunting large carnivore. Ecol Lett. 2013;16(8):1023–30.
Anson JR, Dickman CR. Behavioral responses of native prey to disparate predators: naivet, and predator recognition. Oecologia. 2013;171(2):367–77.
Gigliotti LC, Slotow R, Sholto-Douglas C, de Vos C, Jachowski DS. Short-term predation risk and habitat complexity influence cheetah antipredator behavioursk. Anim Behav. 2021;178:175–84.
Mella VSA, Cooper CE, Davies S. Behavioural responses of free-ranging western grey kangaroos (Macropus fuliginosus) to olfactory cues of historical and recently introduced predators. Austral Ecol. 2014;39(1):115–21.
Weterings MJA, Zaccaroni M, van der Koore N, Zijlstra LM, Kuipers HJ, van Langevelde F, et al. Strong reactive movement response of the medium-sized European hare to elevated predation risk in short vegetation. Anim Behav. 2016;115:107–14.
Yorzinski JL. Predator recognition in the absence of selection. In: GurskyDoyen S, Supriatna J, editors. New York: Springer; 2010. 181-97 p.
Hacquemand R, Jacquot L, Brand G. Comparative fear-related behaviors to predator odors (TMT and natural fox feces) before and after intranasal ZnSO4 treatment in mice. Front Behav Neurosci. 2010;4(DEC):188.
Hacquemand R, Pourie G, Jacquot L, Brand G. Postnatal exposure to synthetic predator odor (TMT) induces quantitative modification in fear-related behaviors during adulthood without change in corticosterone levels. Behav Brain Res. 2010;215(1):58–62.
Kabitzke PA, Wiedenmayer CP. Effects of the stimulus and chamber size on unlearned fear across development. Behav Process. 2011;86(2):257–62.
Rosen JB, West EA, Donley MP. Not all rat strains are equal: differential unconditioned fear responses to the synthetic fox odor 2,4,5-trimethylthiazoline in three outbred rat strains. Behav Neurosci. 2006;120(2):290–7.
Staples LG, McGregor IS. Defensive responses of Wistar and Sprague-Dawley rats to cat odour and TMT. Behav Brain Res. 2006;172(2):351–4.
Taylor R, Coetsee AL, Doyle RE, Sutherland DR, Parrott ML. Sniffing out danger: Rapid antipredator training of an endangered marsupial. Aust Mammal. 2021.
Vaughan G, Kompanijec K, Malik S, Bechard AR. Childhood trauma and post-trauma environment affect fear memory and alcohol use differently in male and female mice. Drug Alcohol Depend. 2021;219:108471.
Yin B, Fan H, Li S, Hegab I, Lu G, Wei W. Behavioral response of Norway rats (Rattus norvegicus) to odors of different mammalian species. J Pest Sci. 2011;84(3):265–72.
Baklová A, Baranyiová E, Šimánková H. Antipredator behaviour of domestic guinea pigs (Cavia porcellus). Acta Vet Brno. 2016;85:293–301.
Ayon RE, Putman BJ, Clark RW. Recent encounters with rattlesnakes enhance ground squirrel responsiveness to predator cues. Behav Ecol Sociobiol. 2017;71(10):10.
Deppe AM. Brown Mouse Lemurs (Microcebus rufus) may lack opportunities to learn about predator calls. Folia Primatol. 2020;91(5):452–62.
Hollen LI, Manser MB. Persistence of alarm-call behaviour in the absence of predators: a comparison between wild and captive-born meerkats (Suricata suricatta). Ethology. 2007;113(11):1038–47.
Meno W, Coss RG, Perry S. Development of snake-directed antipredator behavior by wild white-faced capuchin monkeys: I. Snake-species discrimination. Am J Primatol. 2013;75(3):281–91.
Salandre JA, Beil R, Loehr JA, Sundell J. Foraging decisions of North American beaver (Castor canadensis) are shaped by energy constraints and predation risk. Mammal Res. 2017;62(3):229–39.
Adduci LB, León VA, Schlötelburg A, Busch M, Fraschina J. Avoidance behaviour in laboratory house mice (Musmusculus) and Norway rats (Rattus norvegicus) towards predator odours. PLoS ONE. 2021;16(1):e0245441.
Apfelbach R, Soini HA, Vasilieva NY, Novotny MV. Behavioral responses of predator-naïve dwarf hamsters (Phodopus campbelli) to odor cues of the European ferret fed with different prey species. Physiol Behav. 2015;146:57–66.
Du Y, Huang Y, Zhang H, Li D, Yang B, Wei M, et al. Innate predator recognition in giant pandas. Zool Sci. 2012;29(2):67–70.
Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119(1):280–92.
McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24(17):4134–44.
Sotnikov SV, Markt PO, Umriukhin AE, Landgraf R. Genetic predisposition to anxiety-related behavior predicts predator odor response. Behav Brain Res. 2011;225(1):230–4.
Bramley GN, Waas JR. Laboratory and field evaluation of predator odors as repellents for kiore (Rattus exulans) and ship rats (R. rattus). J Chem Ecol. 2001;27(5):1029–47.
Vlautin CT, Ferkin MH. The influence of predator and conspecific odor on sex differences in path choice in meadow voles. Behaviour. 2012;149(2):133–52.
Zhang JX, Cao C, Gao H, Yang ZS, Sun L, Zhang ZB, et al. Effects of weasel odor on behavior and physiology of two hamster species. Physiol Behav. 2003;79(4–5):549–52.
Blumstein DT, Cooley L, Winternitz J, Daniel JC. Do yellow-bellied marmots respond to predator vocalizations? Behav Ecol Sociobiol. 2008;62(3):457–68.
Blumstein DT, Holland BD, Daniel JC. Predator discrimination and “personality” in captive Vancouver Island marmots (Marmota vancouverensis). Anim Conserv. 2006;9(3):274–82.
Mpemba H, Fan Y, MacLeod KJ, Wen D, Jiang G. THE EFFECT OF NOVEL AND FAMILIAR PREDATOR CUES ON PREY VIGILANCE AND FORAGING BEHAVIORS IN THE GREATER KHINGAN MOUNTAINS, INNER MONGOLIA, CHINA. Appl Ecol Environ Res. 2019;17(4):8219–34.
Randler C. Anti-predator response of Eurasian red squirrels (Sciurus vulgaris) to predator calls of tawny owls (Strix aluco). Mamm Biol. 2006;71(5):315–8.
Suraci JP, Roberts DJ, Clinchy M, Zanette LY. Fearlessness towards extirpated large carnivores may exacerbate the impacts of naive mesocarnivores. Behav Ecol. 2017;28(2):439–47.
Bleicher SS, Ylönen H, Käpylä T, Haapakoski M. Olfactory cues and the value of information: voles interpret cues based on recent predator encounters. Behav Ecol Sociobiol. 2018;72(12):187.
Monclus R, Rodel HG, Von Holst D, De Miguel J. Behavioural and physiological responses of naive European rabbits to predator odour. Anim Behav. 2005;70:753–61.
Nolte DL, Farley JP, Campbell DL, Epple GM, Mason JR. Potential repellents to prevent mountain beaver damage. Crop Prot. 1993;12(8):624–6.
Stanford CB. The influence of chimpanzee predation on group size and anti-predator behaviour in red colobus monkeys. Anim Behav. 1995;49(3):577–87.
van der Meer E, Fritz H, Pays O. Anti-predator behaviour of kudu and impala in response to mimicked African wild dog presence: do age and sex matter? Behaviour. 2015;152(9):1209–28.
Adams JL, Camelio KW, Orique MJ, Blumstein DT. Does information of predators influence general wariness? Behav Ecol Sociobiol. 2006;60(5):742–7.
Blumstein DT, Daniel JC, Schnell MR, Ardron JG, Evans CS. Antipredator behaviour of red-necked pademelons: a factor contributing to species survival? Anim Conserv. 2002;5:325–31.
Deppe AM, Kushnick G. Olfactory predator recognition in the brown mouse lemur (Microcebus rufus) in Ranomafana National Park, Madagascar. Am J Primatol. 2020;82(10):12.
Gil-da-Costa R, Palleroni A, Hauser MD, Touchton J, Kelley JP. Rapid acquisition of an alarm response by a neotropical primate to a newly introduced avian predator. Proc R Soc B-Biol Sci. 2003;270(1515):605–10.
Griffin AS, Evans CS. The role of differential reinforcement in predator avoidance learning. Behav Process. 2003;61(1–2):87–94.
Muller CA, Manser MB. The information banded mongooses extract from heterospecific alarms. Anim Behav. 2008;75:897–904.
Partan SR, Fulmer AG, Gounard MAM, Redmond JE. Multimodal alarm behavior in urban and rural gray squirrels studied by means of observation and a mechanical robot. Curr Zool. 2010;56(3):313–26.
Seiler M, Schwitzer C, Gamba M, Holderied MW. Interspecific semantic alarm call recognition in the solitary Sahamalaza Sportive Lemur, Lepilemur sahamalazensis. PLoS ONE. 2013;8(6):12.
Asok A, Ayers LW, Awoyemi B, Schulkin J, Rosen JB. Immediate early gene and neuropeptide expression following exposure to the predator odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT). Behav Brain Res. 2013;248:85–93.
Monclús R, Rödel HG, Von Holst D. Fox odour increases vigilance in European rabbits: a study under semi-natural conditions. Ethology. 2006;112(12):1186–93.
Casar C. Anti-predator behaviour of black-fronted titi monkeys (Callicebus nigrifrons): University of St Andrews; 2012.
Seiler M, Holderied M, Schwitzer C. Effects of Habitat Alteration on Resting Behaviour of the Sahamalaza Sportive Lemur in North West Madagascar. In: Marsh LK, Chapman CA, editors. Berlin: Springer; 2013. 259–71 p.
Greggor AL, Thornton A, Clayton NS. Neophobia is not only avoidance: improving neophobia tests by combining cognition and ecology. Curr Opin Behav Sci. 2015;6:82–9.
Mella VSA, Banks PB, McArthur C. Negotiating multiple cues of predation risk in a landscape of fear: what scares free-ranging brushtail possums? J Zool (Lond). 2014;294(1):22–30.
Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. Sexually divergent expression of active and passive conditioned fear responses in rats. eLife. 2015;4:e11352.
Blumstein DT, Letnic M, Moseby KE. In situ predator conditioning of naive prey prior to reintroduction. Philos Trans R Soc B: Biol Sci. 2019;374(1781):20180058.
Acknowledgements
The authors thank Terena Solomons for training NDH in searching bibliographic databases and the stakeholders for their feedback on earlier drafts of the review protocol.
Funding
The source of funding for this study was the Hermon Slade Foundation (HSF21054), who played no role in the development of this study.
Author information
Authors and Affiliations
Contributions
NDH and RS screened articles, extracted data, and performed critical appraisal. NDH wrote the review and conducted the analyses. All authors conceived the study and contributed substantially to the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study does not report on any studies conducted on humans, and exclusively reports on studies conducted on animals by other researchers. The authors relied on the responsibility of the relevant researchers to have sought the approval of an appropriate ethics committee. Studies that failed to report ethics approval were excluded from the final review.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
ROSES checklist.
Additional file 2.
List of benchmark and test scoping articles.
Additional file 3.
CEE critical appraisal tool sheet.
Additional file 4.
Exclusion list (reasons for exclusions).
Additional file 5.
Customised data collection sheet.
Additional file 6.
Guide to using Image J for data extraction.
Additional file 7.
Definition of equation functions.
Additional file 8.
Funnel plot to assess publication bias.
Additional file 9.
Data extraction results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Harrison, N.D., Steven, R., Phillips, B.L. et al. Identifying the most effective behavioural assays and predator cues for quantifying anti-predator responses in mammals: a systematic review. Environ Evid 12, 5 (2023). https://doi.org/10.1186/s13750-023-00299-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13750-023-00299-x