Abstract
Background
This study was conducted to analyze the genetic characteristics of 41 β-lactam-resistant Escherichia coli isolates, which are one of the common causes of environmental mastitis, isolated from the bulk tank milk of 290 dairy farms in five factories operated by three dairy companies in Korea.
Results
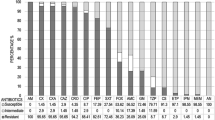
Analysis of the phenotypic and genotypic characteristics of β-lactam-resistant E. coli isolates revealed differences between factories even within the same company. Isolates from factory A1 and C1 showed high resistance to cephalothin (76.9 and 100%, respectively), which is a first-generation cephalosporins, whereas resistance to tetracycline was showed by only the isolates from factories B1 (60.0%), C2 (66.7%), and C3 (100%). Although all the 41 β-lactam-resistant E. coli isolates were positive for blaOXA-1, blaTEM-1 was highly prevalent in isolates from factories C2 (100%) and C3 (100%). Among 17 isolates resistant to both β-lactams and aminoglycosides, the most common multilocus sequence type was ST399 (13isolates, 76.5%). Furthermore, 2 (11.8%) and 12 (70.6%) isolates belonged to the phylogenetic groups B2 and D, respectively, which are invasive strains that cause intestinal infections, respectively. The predominant serogroup was O15 (70.6%), which is a globally distributed extraintestinal pathogen. Interestingly, one isolate from factory A1 belonged to O157 and carried six virulence genes, simultaneously.
Conclusions
Although E. coli isolates were isolated from bulk tank milk, and not the clinical mastitis samples, the presence of the phylogenetic groups B2 and D, and the serogroups O15 and O157, which harbor antimicrobial resistance genes and virulence factors, can pose a threat to public health.
Similar content being viewed by others
Background
Beta-lactam (β-lactam) antibiotics contain the β-lactam moiety in their molecular structure and they include clinically important antibiotics such as the penicillins, cephalosporins and carbapenems. Beta-lactam antibiotics kill bacteria by inhibiting penicillin-binding proteins (PBPs) essential for the cross-linking process during cell wall biosynthesis [1, 2]. Since the discovery of the first β-lactam antibiotic, penicillin, in 1928, the sale of β-lactam antibiotics has been recorded to be more than half of all the other commercially available antibiotics in human and veterinary medicine [3].
In Korea, β-lactam antibiotics are also widely used for treating bacterial infections in food-producing livestock [4, 5]. However, resistance to β-lactam antibiotics in a variety of pathogens from livestock has been continuously reported in recent years, and these bacteria are also considered as the primary reservoir of zoonotic pathogens [6, 7]. The production of β-lactamases, which inactivates the drug by hydrolyzing the β-lactam ring, is a major cause of multi-resistance to β-lactam antibiotics, and this resistance can be easily transferred by conjugation to other bacteria [8]. In particular, aminoglycosides, which are also an important class of antibiotics used frequently, are primarily used in combination with β-lactams to treat severe infections caused by gram-negative bacteria [9]. The resistance to aminoglycosides is generally due to the production of aminoglycoside modifying-enzymes (AMEs) or the ribosomal modification by the acquired 16S rRNA methyltransferase [9, 10]. Recently, the co-occurrence of β-lactamase and AME genes in gram-negative bacteria has been continuously reported worldwide [11, 12].
Escherichia coli is a common organism in the gastrointestinal tract of humans and animals [13], but it is one of the common causes of environmental mastitis in the dairy industry [14]. In particular, bovine mastitis caused by E. coli induces chronic, subclinical or clinical infection based on cow-dependent factors such as age and lactation stage [15]. Therefore, antimicrobial approach must be the first option for limiting the growth of E. coli in the mammary gland. Although β-lactams are also widely used in the intramammary treatment of bovine mastitis in Korea, the β-lactam resistance of E. coli isolated from milk and milk products, including bovine mastitis has not been completely investigated in Korea. Therefore, the present study was conducted to analyze the genetic characteristics of β-lactam-resistant E. coli isolated from bulk tank milk of dairy companies in Korea.
Materials and methods
Bacterial strains
A total of 1,160 batches of bulk tank milk were collected from 290 dairy farms in five factories (A1, B1, C1, C2 and C3) operated by three dairy companies (A, B, and C) in Korea. A total of 183 E. coli were isolated according to the standard microbiological protocols published by the Ministry of Food and Drug Safety (MFDS, 2018) [16], and identified by polymerase chain reaction (PCR) as described previously [17]. Among them, 41 E. coli isolates that showed resistance to penicillins, cephalosporins, or carbapenems by the disk diffusion method were analyzed in this study.
Antimicrobial susceptibility testing
According to the Clinical and Laboratory Standards Institute guidelines (CLSI, 2019) [18]. 41 β-lactam-resistant E. coli were examined for antimicrobial susceptibility using antimicrobial disc (BD biosciences, San Jose, CA, USA) as follows: ampicillin (AM, 10 μg), amoxicillin-clavulanate (AMC, 20 μg), chloramphenicol (C, 30 μg), ceftazidime (CAZ, 30 μg), cefadroxil (30 μg), cephalothin (CF, 30 μg), ciprofloxacin (5 μg), colistin (CL, 10 μg), cefotaxime (CTX, 30 μg), cefuroxime (CXM, 30 μg), cefazoline (30 μg), cefepime (FEP, 30 μg), cefoxitin (30 μg), cefpirome (30 μg), gentamicin (GM, 10 μg), imipenem (10 μg), nalidixic acid (30 μg), trimethoprim/sulfamethoxazole (SXT, 1.25 μg), and tetracycline (TE, 30 μg). E. coli ATCC 25,922 was included for quality control. Multidrug resistance (MDR) was defined as resistance to at least one agent of three or more antimicrobial classes [19].
Detection of β-lactamase, AME, and virulence genes
The presence of β-lactamase genes (blaCTX-M, blaTEM, blaSHV, and blaOXA), AME genes [aac(6′)-Ib, aac(3)-II, ant(2″)-I, aph(3″)-Ib, and ant(4′)-IIa], and virulence genes (eaeA, escV, stx1, fimH, iucC, iutA, and fyuA) was detected by PCR using primers listed in Table 1. The PCR product of β-lactamase genes was also sequenced using an automatic sequencer (Cosmogenetech, Deajeon, Korea) and compared with those in the GenBank nucleotide database using the Basic Local Alignment Search Tool (BLAST) program available at the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov/BLAST).
Detection of integrons and associated gene cassettes
The presence of intl1 and intl2 integrase genes was also detected by PCR using primers listed in Table 1. Moreover, E. coli isolates harboring the integrase gene were the amplification of variable regions using primers, and the PCR product was sequenced with an automatic sequencer (Cosmogenetech, Deajeon, Korea) after purification using the GFX PCR DNA and Gel Band Purification Kit (Amersham Bioscience, Freiburg, Germany). The DNA sequence data were compared with those in the GenBank nucleotide database using the BLAST program available at the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov).
Phylogenetic groups and serogrouping
E. coli isolates exhibiting resistance to both β-lactams and aminoglycosides were classified into phylogenetic groups and serogrouping using PCR-based typing, as described by Clermont et al. (2000) [31] and Iguchi et al. (2015) [32], respectively.
Molecular typing
The genetic relationship of E. coli isolates showing resistance to both β-lactams and aminoglycosides was analyzed by pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST). PFGE was conducted by digesting genomic DNA using the XbaI enzyme (Takara Bio Inc., Shiga, Japan) according to a standard protocol of the Centers for Disease Control and Prevention (CDC, USA) [33], using a CHEF-MAPPER apparatus (Bio-Rad Laboratories, Hercules, CA), as described previously [34], and analyzed using the BioNumerics Software (Applied Maths, Kortrijk, Belgium). Moreover, PCR amplification of seven housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) was performed to identify MLST as described by Tartof et al. (2005) [35]. The PCR products of these seven housekeeping genes were purified using the GFX PCR DNA and Gel Band Purification Kit (Amersham Bioscience, Freiburg, Germany) and sequenced with an automatic sequencer (Cosmogenetech, Deajeon, Korea). Sequence types (STs) were obtained by combination at the E. coli database (https://pubmlst.org/organisms/escherichia-spp).
Results
Phenotypic and genotypic characteristics
The Characteristics of the 41 β-lactam-resistant E. coli isolates are shown in Table 2. The β-lactam-resistant E. coli isolates demonstrated different antimicrobial profiles by factory origin. Isolates from factories A1 and C1 showed the high resistance to CF (76.9 and 100%, respectively), the first-generation cephalosporin, but each of the six isolates from factories C2 and C3 of the same company as C1 showed no resistance to CF. Furthermore, one isolate from factory A1 simultaneously showed the resistance to CXM, CTX, and FEP, which are the second-, third-, and fourth-generation cephalosporins, respectively, and one isolate from factory C1 showed the resistance to CAZ, which is the third-generation cephalosporin. However, resistance to TE was shown only by isolates from factories B1 (60.0%), C2 (66.7%) and C3 (100%). Resistance to CL was exhibited by isolates from factories A1 (23.1%) and C1 (16.7%), and resistance to C was shown by isolates from factories B1 (50.0%) and C1 (16.7%).
The prevalence of MDR was the highest in isolates from factory C3 (100%), followed by C2 (83.3%), B1 (50.0%) and A1 (7.7%). Isolates from factory C1 showed no MDR. The patterns of MDR also showed differences between factories. In particular, an MDR pattern with simultaneous resistance to AM, GM, and TE was highly prevalent in factories C2 (66.7%) and C3 (100%). Otherwise, isolates from factories A1 and B1 showed a pattern of MDR to a combination of cephalosporins, AM, AMC, G, GM and SXT.
The distribution of β-lactamase and AME genes was also different between factories. Although all the 41 β-lactam-resistant E. coli isolates were positive for blaOXA-1, blaTEM-1 was highly prevalent in isolates from factories C2 (100%) and C3 (100%), followed by B1 (60.0%) and A1 (7.7%). In addition, all isolates from factories C2 and C3 carried both aac(3)-II and aph(3″)-Ib genes, but only one (7.7%) and four (40.0%) isolates from factories A1 and B1, respectively, carried AME genes, and isolates from factory C1 did not carry any of the AME genes. In particular, one and three isolates from factories A1 and B1, respectively, carried aac(6′)-Ib, aac(3)-II, and aph(3″)-Ib genes, simultaneously.
Twenty (48.8%) among 41 β-lactam-resistant E. coli isolates harbored class 1 integrons, and the prevalence of class 1 integrons was the highest in isolates from factory B1 (70.0 %), followed by C1 (50.0%), A1 (46.2%), C2 (33.3%) and C3 (33.3%). Moreover, four different gene cassette types were detected in six isolates, which were dfrA12 + aadA2 (2 isolates), aadA4 (2 isolates), dfrA17 + aadA5 (1 isolate), and aacA4 (1 isolate), from factories A1, B1, and C1.
The distribution of virulence genes also showed the differences between factories. Among seven virulence genes, the highest prevalence was fimH (32 isolates, 78.0%), followed by eaeA (11 isolates, 26.8%) and iucC (8 isolates, 19.5%). Interestingly, isolates from factories C2 and C3 only harbored both eaeA and fimH (100%) and fimH alone (83.3%), respectively, and iucC only revealed in isolates from factories A1 (23.1%), B1 (30.0%) and C1 (33.3%). Moreover, one isolate from factory A1 simultaneously harbored six virulence genes, eaeA, escV, fimH, iucC, iutA and stx1.
Differentiation of genotypes and serogrouping scheme
Distribution of genotypes and serogroups of 17 isolates which were resistant to both β-lactams and aminoglycosides, were composed of 1 (7.7%), 4 (40.0%), 6 (100%) and 6 (100%) isolates from factories A1, B1, C2, and C3, respectively, are shown in Fig. 1. PFGE patterns were divided into nine clusters by 85% similarity. Although four isolates from factory C3 showed significant genetic relatedness, even within the same factory, most isolates showed grouped into a variety of clusters. A total of four different MLST types were also identified. The most common type was ST399 (76.5%), which included all isolates from factories C2 and C3, and ST306, ST409 and ST9624 were found in one, one, and two isolates, respectively. In the distribution of phylogenetic groups, the most predominant group was D (70.6%), which was included all isolates from factory C3, and two (11.8%) isolates belonged to B2. Among five different serogroups, the highest prevalence was O15 (70.6%), which was detected in isolates from factories B1 (1 isolate), C2 (5 isolates) and C3 (6 isolates). Interestingly, one isolate from factory A1 belonged to serogroup O157.
Discussion
Bovine mastitis is the most common disease in the udder of dairy cows that causes high economic losses due to reduction of milk output and additional cost incurred in the treatment mastitis. In particular, E. coli one of the more important etiological organisms responsible for bovine mastitis [36]. Although several studies have reported the characteristics of antimicrobial resistance in E. coli from bovine mastitis in Korea [37,38,39], to our knowledge, this is the first study to investigate a comparative analysis of β-lactam-resistant E. coli isolated from bulk tank milk in different dairy companies. In Korea, five major dairy companies produce 84% of the total milk and dairy products (ATFIS 2020) [40], and β-lactam-resistant E. coli isolated from five factories operated by three dairy companies were compared in this study. Interestingly, the phenotypic characteristics of β-lactam-resistant E. coli revealed a difference for each factory even within the same company. In particular, all β-lactam-resistant E. coli isolates from factory C1 showed high resistance to CF, the first-generation cephalosporins, whereas no isolates from factories C2 and C3 of the same company showed resistance to CF. Isolates from company A also showed high resistance to CF, moreover, one isolate simultaneously exhibited resistance to second-, third-, and fourth-generation cephalosporins. Especially, the third- and fourth-generation cephalosporins have been categorized by the World Health Organization (WHO 2019) [41] as high-priority and critically important antibiotics for human medicine. Therefore, transmission of antimicrobial-resistant E. coli from the dairy industry should be considered a grave public health concern.
Unlike isolates from factory C1, isolates from factories C2 and C3 showed high resistance to TE. Tetracycline is also a widely used antibiotic in the treatment of bovine mastitis in Korea [38]. In addition, although isolates from factory C1 showed no MDR, isolates from factories C3 and C2 showed the highest MDR prevalence. Dairy factories are primarily located in different regions, therefore, even the same company appears to have differences in dairy product management, including antibiotic use by the factory.
Resistance to C showed the high prevalence in isolates from factory B1. Although C is no longer used in food-producing livestock and humans in Korea because of side effects in humans such as bone marrow suppression and fetal aplastic anemia [42, 43], other amphenicols, such as florfenicol, are commonly recommended for the treatment of bacterial pneumonia and associated respiratory infections in cattle.
In this study, only two genes, blaTEM-1 and blaOXA-1, among four β-lactamase genes tested were detected, but the distribution of genes also revealed a difference between factories. All the 41 β-lactam-resistant E. coli harbored blaOXA-1. The blaOXA-1 has generally been identified in ampicillin-resistant enterobacterial strains such as E. coli, moreover, it has been able to impart resistance to cephalosporins [44, 45]. In this study, isolates mostly from factories A1 and C1 only harbored blaOXA-1 gene, and showed resistance to CF. However, all isolates from factories C2 and C3 simultaneously harbored both blaTEM-1 and blaOXA-1, and showed no resistance to CF. Therefore, blaOXA-1 in isolates from factories A1 and C1 seems to be deeply related to the resistance to cephalosporins, whereas blaOXA-1 in factories C2 and C3 appeared to predominate the resistance to AM. The gene, blaTEM-1, was also reported to be the most prevalent in ampicillin-resistant E. coli isolates from food-producing animals [30, 46]. However, any type of blaCTX-M, which is the most common extended-spectrum β-lactamase (ESBL) gene, was not detected.
In this study, 41.6% of β-lactam-resistant E. coli isolates harbored AME genes. In particular, isolates from factories A1 and B1 simultaneously carried the genes, aac(6′)-Ib, aac(3)-II and aph(3″)-Ib, except one isolate, otherwise, all isolates from factories C2 and C3 showed a combination of aac(3)-II and aph(3″)-Ib. In general, AAC(3) and APH(3′) are associated with broad-spectrum β-lactamases, followed by AAC(6′), which is associated with ESBL [11]. Interestingly, 17 among 19 isolates, including both blaOXA-1 and blaTEM-1, harbored AME genes. Carattoli (2009) [47] have reported that the harboring of blaTEM-1, blaOXA-1, and aac(6′)-Ib-cr on plasmids has been well established, and Bodendoerfer et al. (2020) [11] also reported that MDR plasmids, encoding combinations of OXA-1/TEM-1/ AAC(3)/APH(3′)/AAC(6′)-Ib-cr may be responsible for the co-resistance to β-lactams and aminoglycosides.
Integrons are important genetic elements for harboring and spreading of antimicrobial resistance determinants, because they are capable of carrying gene cassettes containing antimicrobial resistance genes [48, 49]. In this study, 20 (48.8%) of the 41 isolates carried class 1 integrons, which are widely distributed among plasmids in different bacterial species [50], and five isolates carried aadA cassettes, which are determinants conferring resistance to aminoglycosides [51]. Ali et al. (2016) [52] and Li and Zhao (2018) [53] have already reported that aadA families frequently detected in class 1 integrons gene cassette from bovine mastitis milk.
In this study, fimH, which is the adhesion portion of type 1 fimbriae in E. coli [54], was found to be the most prevalent virulence gene (78.0%). Ombarak et al. (2019) [55] also reported that fimH was the most prevalent in pathogens isolated from subclinical bovine mastitis milk sample in Egypt (93%). Although fimH does not always cause severe illness, it may be a potential opportunistic factor. Moreover, the intimin gene, eaeA, showed a prevalence of 26.8% among isolates, which was higher than that of isolates from subclinical or clinical mastitis milk in China (0%), Iran (0%) and Egypt (7.1%) [55,56,57]. Interestingly, all isolates from factory C2 harbored eaeA, whereas only five among isolates from other factories harbored eaeA.
In this study, a total of 17 isolates showed resistance to both β-lactams and aminoglycosides. In particular, all isolates from factories C2 and C3 showed resistance to both β-lactams and aminoglycosides, simultaneously, but none of the isolates from factory C1 exhibited resistance to aminoglycosides. Moreover, some of E. coli strains have been generally divided into four phylogenetic groups, and invasive strains that caused intestinal infections mainly belonged to groups B2 and D, whereas symbiotic and diarrhea-causing strains belonged to groups A and B1 [31]. However, previous studies have reported that E. coli associated with bovine mastitis mainly belong to the phylogenetic groups A and B1 [56, 58, 59]. Interestingly, in the present study, 2 (11.8%) and 12 (70.6%) of the 17 isolates exhibiting resistance to both β-lactams and aminoglycosides belonged to groups B2 and D, respectively.
In the distribution of serogroups of 17 E. coli isolates resistant to both β-lactams and aminoglycosides, 12 (70.6%) isolates were classified into serogroup O15. Although serogroup O15 was described as a causative factor for septicemia in newborn calves [60] and as a clonal group of uropathogenic E. coli that caused cystitis and bacteremia in humans [61], the identification of serogroup O15 in milk and dairy products was first reported in this study. Moreover, one isolate from factory A1 was classified into serogroup O157, which is known to cause human illness by producing several Shiga toxins [62]. Interestingly, one isolate classified into O157 uniquely harbored six virulence genes (eaeA, escV, fimH, iucC, iutA and stx1) in this study.
In this study, four STs were identified in 17 E.coli isolates resistant to both β-lactams and aminoglycosides, and all isolates from company C were classified into ST399. However, PFGE analysis revealed higher differentiation, therefore, isolates from the same factory exhibited a variety of pulsotypes according to different genetic characteristics.
Conclusions
Although E. coli were isolated from bulk tank milk, and not clinical mastitis, different phenotypic and genotypic characteristics could be identified for each factory. Especially, the presence of phylogenetic groups B2 and D, and serogroups O15 and O157, which habor antimicrobial resistance genes and virulence factors, can pose a threat to public health.
Availability of data and materials
Not applicable.
References
Waxman DJ, Strominger JL. Penicillin-Binding Proteins and the Mechanism of Action of Beta-Lactam Antibiotics1. Annu Rev Biochem. 1983;52:825–69. https://doi.org/10.1146/annurev.bi.52.070183.004141.
De Angelis G, Del Giacomo P, Posteraro B, Sanguinetti M, Tumbarello M. Molecular Mechanisms, Epidemiology, and Clinical Importance of β-Lactam Resistance in Enterobacteriaceae. Int J Mol Sci. 2020;21:5090 Available from: (https://www.mdpi.com/1422-0067/21/14/5090).
Li X-Z, Mehrotra M, Ghimire S, Adewoye L. β-Lactam resistance and β-lactamases in bacteria of animal origin. Vet Microbiol. 2007;121:197–214 Available from: (https://linkinghub.elsevier.com/retrieve/pii/S037811350700034X).
Noh EB, Kim Y, Bin, Jeon HY, Seo KW, Son SH, Lee YJ. Antimicrobial resistance and genetic diversity of salmonella serotypes recovered from edible pork offal from Korea. Microb Drug Resist. 2019;25:1514–20. https://doi.org/10.1089/mdr.2019.0010.
Na SH, Moon DC, Kang HY, Song HJ, Kim SJ, Choi JH, et al. Molecular characteristics of extended-spectrum β-lactamase/AmpC-producing Salmonella enterica serovar Virchow isolated from food-producing animals during 2010–2017 in South Korea. Int J Food Microbiol. 2020;322: 108572. https://doi.org/10.1016/j.ijfoodmicro.2020.108572.
Shin SW, Jung M, Won HG, Belaynehe KM, Yoon IJ, Yoo HS. Characteristics of transmissible CTX-M- and CMY-type β-lactamase-producing escherichia coli isolates collected from pig and chicken farms in South Korea. J Microbiol Biotechnol. 2017;27:1716–23. https://doi.org/10.4014/jmb.1610.10006.
Song J, Oh S-S, Kim J, Park S, Shin J. Clinically relevant extended-spectrum β-lactamase–producing escherichia coli isolates from food animals in South Korea. Front Microbiol. 2020;11:1–9 Available from: (https://pubmed.ncbi.nlm.nih.gov/32390965/).
Bush K. Proliferation and significance of clinically relevant β-lactamases. Ann N Y Acad Sci. 2013;1277:84–90.
Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist Updat. 2010;13:151–71. https://doi.org/10.1016/j.drup.2010.08.003.
Doi Y, Wachino J, Arakawa Y. Aminoglycoside resistance. Infect Dis Clin North Am. 2016;30:523–37. https://doi.org/10.1016/j.idc.2016.02.011.
Bodendoerfer E, Marchesi M, Imkamp F, Courvalin P, Böttger EC, Mancini S. Co-occurrence of aminoglycoside and β-lactam resistance mechanisms in aminoglycoside- non-susceptible Escherichia coli isolated in the Zurich area. Switzerland Int J Antimicrob Agents. 2020;56:106019 Available from: (https://pubmed.ncbi.nlm.nih.gov/32422315/).
Latifi B, Tajbakhsh S, Ahadi L, Yousefi F. Coexistence of aminoglycoside resistance genes in CTX-M-producing isolates of Klebsiella pneumoniae in Bushehr province. Iran Iran J Microbiol. 2021;13:161–70 Available from: (https://ijm.tums.ac.ir/index.php/ijm/article/view/2993).
Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–40 Available from: (http://www.nature.com/articles/nrmicro818).
Saini V, McClure JT, Léger D, Keefe GP, Scholl DT, Morck DW, et al. Antimicrobial resistance profiles of common mastitis pathogens on Canadian dairy farms. J Dairy Sci. 2012;95:4319–32 Available from: (https://linkinghub.elsevier.com/retrieve/pii/S0022030212004213).
Sumon S, Parvin M, Ehsan M, Islam M. Dynamics of somatic cell count and intramammary infection in lactating dairy cows. J Adv Vet Anim Res. 2020;7:314 Available from: (https://www.ejmanager.com/fulltextpdf.php?mno=87529).
Ministry of Food and Drug Safety (MFDS). Processing standards and ingredient specifications for livestock products. Cheongju, Korea; 2018. Available from: https://www.mfds.go.kr/eng/index.do.
Sobur MA, Sabuj AAM, Sarker R, Rahman AMMT, Kabir SML, Rahman MT. Antibiotic-resistant Escherichia coli and Salmonella spp. associated with dairy cattle and farm environment having public health significance. Vet World. 2019;12:984–93 Available from: (http://www.veterinaryworld.org/Vol.12/July-2019/9.html).
Clinical and Laboratory Standards Institute (CLSI). CLSI M100-ED29 : 2019 Performance standards for antimicrobial susceptibility testing, 29th Edition.
Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
Pitout JDD, Hossain A, Hanson ND. Phenotypic and molecular detection of CTX-M-β-lactamases produced by Escherichia coli and Klebsiella spp. J Clin Microbiol. 2004;42:5715–21. https://doi.org/10.1128/JCM.42.12.5715-5721.2004.
Dallenne C, da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65:490–5.
Briñas L, Zarazaga M, Sáenz Y, Ruiz-Larrea F, Torres C. β-Lactamases in Ampicillin-resistant Escherichia coli isolates from foods, humans, and healthy animals. Antimicrob Agents Chemother. 2002;46:3156–63 Available from: (https://aac.asm.org/content/46/10/3156).
Jiang Y, Zhou Z, Qian Y, Wei Z, Yu Y, Hu S, et al. Plasmid-mediated quinolone resistance determinants qnr and aac(6′)-Ib-cr in extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in China. J Antimicrob Chemother. 2008;61:1003–6.
Sandvang D, Aarestrup FM. Characterization of aminoglycoside resistance genes and class 1 integrons in porcine and bovine gentamicin-resistant Escherichia coli. Microb Drug Resist. 2000;6:19–27.
Ojdana D, Sieńko A, Sacha P, Majewski P, Wieczorek P, Wieczorek A, et al. Genetic basis of enzymatic resistance of E. coli to aminoglycosides. Adv Med Sci. 2018;63:9–13.
Kagambèga A, Martikainen O, Lienemann T, Siitonen A, Traoré AS, Barro N, et al. Diarrheagenic Escherichia coli detected by 16-plex PCR in raw meat and beef intestines sold at local markets in Ouagadougou, Burkina Faso. Int J Food Microbiol. 2012;153:154–8. https://doi.org/10.1016/j.ijfoodmicro.2011.10.032.
Tarchouna M, Ferjani A, Ben-Selma W, Boukadida J. Distribution of uropathogenic virulence genes in Escherichia coli isolated from patients with urinary tract infection. Int J Infect Dis. 2013;17:e450-3. https://doi.org/10.1016/j.ijid.2013.01.025.
Chapman TA, Wu XY, Barchia I, Bettelheim KA, Driesen S, Trott D, et al. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl Environ Microbiol. 2006;72:4782–95.
Kerrn MB, Klemmensen T, Frimodt-Møller N, Espersen F. Susceptibility of Danish Escherichia coli strains isolated from urinary tract infections and bacteraemia, and distribution of sul genes conferring sulphonamide resistance. J Antimicrob Chemother. 2002;50:513–6. https://doi.org/10.1093/jac/dkf164.
Sáenz Y, Briñas L, Domínguez E, Ruiz J, Zarazaga M, Vila J, et al. Mechanisms of resistance in multiple-antibiotic-resistant escherichia coli strains of human, animal, and food origins. Antimicrob Agents Chemother. 2004;48:3996–4001. https://doi.org/10.1128/AAC.48.10.3996-4001.2004.
Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli Phylogenetic Group. Appl Environ Microbiol. 2000;66:4555–8 Available from: (https://aem.asm.org/content/66/10/4555).
Iguchi A, Iyoda S, Seto K, Morita-Ishihara T, Scheutz F, Ohnishi M. Escherichia coli O-Genotyping PCR: a comprehensive and practical platform for molecular O Serogrouping. J Clin Microbiol. 2015;53:2427–32 Available from: (https://jcm.asm.org/content/53/8/2427).
Centers for Disease Control and Prevention (CDC). Centers for Disease Control and Prevention [Internet]. USA; 2020. Available from: https://www.cdc.gov/.
Liu J-H, Wei S-Y, Ma J-Y, Zeng Z-L, Lü D-H, Yang G-X, et al. Detection and characterisation of CTX-M and CMY-2 β-lactamases among Escherichia coli isolates from farm animals in Guangdong Province of China. Int J Antimicrob Agents. 2007;29:576–81 Available from: (https://linkinghub.elsevier.com/retrieve/pii/S0924857907000386).
Tartof SY, Solberg OD, Manges AR, Riley LW. Analysis of a uropathogenic Escherichia coli clonal group by multilocus sequence typing. J Clin Microbiol. 2005;43:5860–4. https://doi.org/10.1128/JCM.43.12.5860-5864.2005.
Gao J, Barkema HW, Zhang L, Liu G, Deng Z, Cai L, et al. Incidence of clinical mastitis and distribution of pathogens on large Chinese dairy farms. J Dairy Sci. 2017;100:4797–806. https://doi.org/10.3168/jds.2016-12334.
Nam HM, Lim SK, Kang HM, Kim JM, Moon JS, Jang KC, et al. Prevalence and antimicrobial susceptibility of gram-negative bacteria isolated from bovine mastitis between 2003 and 2008 in Korea. J Dairy Sci. 2009;92:2020–6. https://doi.org/10.3168/jds.2008-1739.
Tark D-S, Moon DC, Kang HY, Kim S-R, Nam H-M, Lee H-S, et al. Antimicrobial susceptibility and characterization of extended-spectrum β-lactamases in Escherichia coli isolated from bovine mastitic milk in South Korea from 2012 to 2015. J Dairy Sci. 2017;100:3463–9. https://doi.org/10.3168/jds.2016-12276.
Yun M-J, Yoon S, Lee YJ. Monitoring and characteristics of major mastitis pathogens from bulk tank milk in Korea. Animals. 2020;10:1562 Available from: (https://www.mdpi.com/2076-2615/10/9/1562).
Korea Agro-Fisheries & Food Trade Corporation Food Information Statistics System (ATFIS) [Internet]. Available from: https://www.atfis.or.kr/home/M000000000/index.do
World Health Organization (WHO). Highest priority critically important antimicrobials [Internet]. 2019. Available from: https://www.who.int/foodsafety/cia/en/
Schwarz S, Kehrenberg C, Doublet B, Cloeckaert A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol Rev. 2004;28:519–42.
Sood S. Chloramphenicol – A potent armament against multi-drug resistant (MDR) gram negative bacilli? J Clin Diagnostic Res. 2016;10:DC01-3.
Poirel L, Naas T, Nordmann P. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob Agents Chemother. 2010;54:24–38 Available from: (https://aac.asm.org/content/54/1/24).
Evans BA, Amyes SGB. OXA –Lactamases. Clin Microbiol Rev. 2014;27:241–63 Available from: (https://cmr.asm.org/content/27/2/241).
Jouini A, Ben Slama K, Sáenz Y, Klibi N, Costa D, Vinué L, et al. Detection of multiple-antimicrobial resistance and characterization of the implicated genes in Escherichia coli isolates from foods of animal origin in Tunis. J Food Prot. 2009;72:1082–8 Available from: (https://meridian.allenpress.com/jfp/article/72/5/1082/172277/Detection-of-MultipleAntimicrobial-Resistance-and).
Carattoli A. Resistance plasmid families in enterobacteriaceae. Antimicrob Agents Chemother. 2009;53:2227–38 Available from: (https://aac.asm.org/content/53/6/2227).
Kar D, Bandyopadhyay S, Bhattacharyya D, Samanta I, Mahanti A, Nanda PK, et al. Molecular and phylogenetic characterization of multidrug resistant extended spectrum beta-lactamase producing Escherichia coli isolated from poultry and cattle in Odisha. India Infect Genet Evol. 2015;29:82–90. https://doi.org/10.1016/j.meegid.2014.11.003.
Seo KW, Lee YJ. Prevalence and characterization of β-lactamases genes and class 1 integrons in multidrug-resistant Escherichia coli isolates from chicken meat in Korea. Microb Drug Resist. 2018;24:1599–606. https://doi.org/10.1089/mdr.2018.0019.
Zhang AN, Li L-G, Ma L, Gillings MR, Tiedje JM, Zhang T. Conserved phylogenetic distribution and limited antibiotic resistance of class 1 integrons revealed by assessing the bacterial genome and plasmid collection. Microbiome. 2018;6:130. https://doi.org/10.1186/s40168-020-00950-6.
White PA. Current status of the aadA and dfr gene cassette families. J Antimicrob Chemother. 2001;47:495–6. https://doi.org/10.1093/jac/47.4.495.
Ali T, ur Rahman S, Zhang L, Shahid M, Zhang S, Liu G, et al. ESBL-producing escherichia coli from cows suffering mastitis in China contain clinical class 1 integrons with CTX-M linked to ISCR1. Front Microbiol. 2016;7:1–11. https://doi.org/10.3389/fmicb.2016.01931/full.
Li L, Zhao X. Characterization of the resistance class 1 integrons in Staphylococcus aureus isolates from milk of lactating dairy cattle in Northwestern China. BMC Vet Res. 2018;14:59. https://doi.org/10.1186/s12917-018-1376-5.
Zhang W, Xu L, Park H-B, Hwang J, Kwak M, Lee PCW, et al. Escherichia coli adhesion portion FimH functions as an adjuvant for cancer immunotherapy. Nat Commun. 2020;11:1187 Available from: (http://www.nature.com/articles/s41467-020-15030-4).
Ombarak RA, Zayda MG, Awasthi SP, Hinenoya A, Yamasaki S. Serotypes, pathogenic potential, and antimicrobial resistance of Escherichia coli Isolated from subclinical bovine mastitis milk samples in Egypt. Jpn J Infect Dis. 2019;72:337–9 Available from: (https://www.jstage.jst.go.jp/article/yoken/72/5/72_JJID.2018.538/_article).
Liu Y, Liu G, Liu W, Liu Y, Ali T, Chen W, et al. Phylogenetic group, virulence factors and antimicrobial resistance of Escherichia coli associated with bovine mastitis. Res Microbiol. 2014;165:273–7 Available from: (https://pubmed.ncbi.nlm.nih.gov/24705087/).
Moradli GA, Salehii TZ, Jamshidian M, Mosakhani F. Molecular identification virulence genes of Escherichia coli isolated from bovine clinical mastitis. Int J Life Sci. 2014;8:1–3 Available from: (https://www.nepjol.info/index.php/IJLS/article/view/10219).
Zhang D, Zhang Z, Huang C, Gao X, Wang Z, Liu Y, et al. The phylogenetic group, antimicrobial susceptibility, and virulence genes of Escherichia coli from clinical bovine mastitis. J Dairy Sci. 2018;101:572–80. https://doi.org/10.3168/jds.2017-13159.
Cruz-Soto AS, Toro-Castillo V, Munguía-Magdaleno CO, Torres-Flores JE, Flores-Pantoja LE, Loeza-Lara PD, et al. Relación genética, formación de biopelículas, movilidad y virulencia de Escherichia coli aislada de mastitis bovina. Rev Mex Ciencias Pecu. 2020;11:167–82 Available from: (https://cienciaspecuarias.inifap.gob.mx/index.php/Pecuarias/article/view/4998).
Ørskov F. Antigenic relationships between H Antigens 1–22 Of E. coli And Wramby’s H Antigens 23W–36W1. Acta Pathol Microbiol Scand. 1953;32:241–4. https://doi.org/10.1111/j.1699-0463.1953.tb00248.x.
Beutin L, Tao J, Feng L, Krause G, Zimmermann S, Gleier K, et al. Sequence analysis of the escherichia coli O15 antigen gene cluster and development of a PCR assay for rapid detection of intestinal and extraintestinal pathogenic E coli O15 strains. J Clin Microbiol. 2005;43:703–10 Available from: (https://jcm.asm.org/content/43/2/703).
Erickson MC, Doyle MP. Food as a vehicle for transmission of Shiga Toxin–producing Escherichia coli. J Food Prot. 2007;70:2426–49 Available from: (https://meridian.allenpress.com/jfp/article/70/10/2426/170872/Food-as-a-Vehicle-for-Transmission-of-Shiga).
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, H.-R. and Y.J.L.; methodology, H.-R. and Y.J.L; software, H.-R.; formal analysis, H.-R. and K.K.; investigation, H.-R.; data curation, H.-R and K.K.; writing—original draft preparation, H.-R.; writing—review and editing, Y.J.L.; supervision, Y.J.L.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jung, HR., Kim, K. & Lee, Y.J. Comparative analysis of genetic characterization of β-lactam-resistant Escherichia coli from bulk tank milk in Korea. Ir Vet J 74, 26 (2021). https://doi.org/10.1186/s13620-021-00203-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13620-021-00203-4