Abstract
Background
The present study aimed to investigate the prevalence and molecular characterization of extended-spectrum β-lactamase (ESBL)—producing Escherichia coli (E. coli) isolated from dairy cattle with endometritis in China. The prevalence of ESBL-producing E. coli in sample was detected using ChromID ESBL agar, and genotyping of the ESBL producers was performed by PCR and DNA sequencing.
Results
The results revealed that the proportion of positive pathogens tested was 69.76% (180/258) in samples obtained from cows diagnosed with clinical endometritis, with E. coli accounting for 170 out of the 180 positive samples. The infection rate of isolated E. coli was 39.14% (101/258), and co-infections with other pathogens were prevalent. Furthermore, among the 158 E. coli isolates, 50 strains were identified as ESBL producers, with TEM and CTX-M prevalence rates at 78.00% and 32.00%, respectively. Drug sensitivity experiments indicated that 50 isolates of ESBL- producing E. coli were multidrug resistance (MDR), with 48.0% of them exhibiting positive results for both the class 1 integron gene and five gene cassettes associated with resistance to trimethoprim (dfr1 and dfrA17) and aminoglycosides (aadA1, aadA5, and dfrA1), respectively.
Conclusion
This investigation demonstrated a substantial prevalence and heightened level of antimicrobial resistance among ESBL-producing E. coli isolates derived from dairy cattle infected with endometritis in China.
Similar content being viewed by others
Background
Endometritis is one of the most important reproductive diseases that damages the reproductive performance of cows around the world [1]. Bacterial infection is primary pathogenic factor that caused endometritis in cows, previous studies reported that Escherichia coli (E. coli), Streptococcus, and Staphylococcus are the most common pathogens in cow breeding farms [2,3,4]. The effective treatment measures for endometritis in dairy cows remain the intrauterine infusion of antibiotics. However, there is a gradual increase in the prevalence of multidrug resistance (MDR) strains of E. coli due to prolonged and irregular antibiotic usage, accompanied by continuously strengthening level of drug resistance [5, 6]. Therefore, the investigation on the prevalence of E. coli strains in dairy farms and their impact on β-lactamase drug resistance is of great significance for maintaining healthy dairy farming practice and reducing economic losses.
Bacteria that carry genes encoding extended-spectrum β-lactamases (ESBLs) have the ability to enzymatically break down a wide range of β-lactamase antibiotics, including penicillins and cephalosporins. E. coli strains that produce ESBLs exhibited MDR, which suggested that they are not only resistant to β-lactam antibiotics but also non-β-lactam antibiotics. The extensive MDR poses significant challenges in the clinical management of both human and animal E. coli infection [7,8,9]. Previous studies have indicated that the ESBL genes are responsible for resistance encompass CTX-M, TEM, and SHV, which are easily transmissible and promote the dissemination of resistance genes. In China, the CTX-M-type strain is the most predominant ESBLs, with a predominance of CTX-M-14 [10, 11]. The recent study has revealed that integron, which is hereditary units possessing gene capture and expression function, plays a pivotal role in the mechanism of bacteria resistance [12]. The prevalence of integron, especially class 1 integron, among multidrug resistant E. coli strains facilitates the horizontal transfer of resistance genes as mobile genetic elements, which contributes to the widespread emergence of MDR [13]. Extensive researches have been conducted on E. coli integrons and gene cassettes. However, the majority of these studies have primarily focused on non-pathogenic strains that isolated from healthy animals or the environment, with limited attention given to investigating pathogenic strains [14,15,16,17]. Interestingly, the majority of ESBL-producing E. coli strains were predominantly identified within cattle herds comprising less than or equal to 2, 000 cows, potentially indicated a lack of knowledge and skills among producers associated with these farms [18]. Therefore, there are a dearth of comprehensive reports on the molecular characterization of ESBL producing E. coli isolates that is associated with endometritis in cattle from large-scale dairy farms located in western of China.
The objective of this study was to detect the prevalence and characterize ESBL- producing E. coli strains isolated from bovine endometritis cases in Gansu province recently, then the drug resistance pattern, the associations between resistance phenotypes and genotypes were explored. Our results provided valuable insights for preventing and controlling E. coli infection in both livestock, as well as providing novel therapeutic strategies in future research.
Results
The identification of the causative agent responsible for endometritis
The uterine secretions that diagnosed with clinical endometritis were collected from 258 cows, and pathogen detection was performed using PCR. The results revealed that diverse pathogens were detected with the percentage of 69.76% (180/258) in collected samples, with E. coli being identified in 170 out of the 180 positive samples. The infection rate of isolated E. coli was 39.14% (101/258), while the co-infection rate of E. coli with Streptococcus dysgalactiae (S. dysgalactiae), S. agalactiae (S. agalactiae), Mycoplasma bovis (M. bovis), Klebsiella Trevisan (K. Trevisan), S. agalactiae + Streptococcus dysgalactiae (S. dysgalactiae), M.bovis + S. dysgalactiae, K. Trevisan + S. dysgalactiae, S. agalactiae + M.bovis, K. Trevisan + M.bovis, K. Trevisan + S. dysgalactiae + M.bovis, S. agalactiae + M.bovis + S. aureus, S. agalactiae + M.bovis + S. dysgalactiae, S. agalactiae + M.bovis + S. dysgalactiae + K. Trevisan, S. agalactiae + M.bovis + S. dysgalactiae + K. Trevisan was 2.71% (7/258), 4.26% (11/258), 7.36% (19/258), 3.88% (10/258), 0.39% (1/258), 1.94% (5/258), 0.39% (1/258), 1.55% (4/258), 0.78% (2/258), 0.39% (1/258), 1.16% (3/258), 0.78% (2/258), 0.39% (1/258), and 0.78% (2/258) in turn. These results indicated that E. coli is one of the most widespread pathogens in dairy farms and co-infection with other pathogens were also common (26.76%), as shown in Fig. 1.
Isolation and identification of E. coli and ESBLs
In this study, a total of 158 clinical strains were isolated from 258 samples in clinical endometritis uterine secretions that obtained from dairy cows. A total of 158 clinical strains were identified as E. coli based on colony morphology analysis on blood agar, gram staining, biochemical identification, and 16S rDNA sequencing. Additionally, the ChromID ESBL agar results showed that 23.04% samples were tested positive for ESBL production based on the ChromID ESBL agar culture among these isolates, as shown in Table 1.
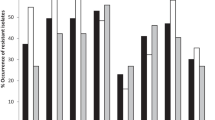
The susceptibility profiles of β-lactamase and the associated antimicrobial resistance of ESBL-producing isolates are summarized in Table 2. All isolates exhibited MDR and were resistant to ampicillin. The majority of isolated strains showed resistance to gentamicin (78%), amoxicillin (66%), ceftiofur (62%), and tetracycline (58%). Notably, a total of 26 isolates (52%) were resistant to all tested penicillins, cephalosporins, and monobactams. Except for β-lactamase resistance testing, these isolates were subjected to antimicrobial susceptibility testing in various categories. It is worth noting that a high level of resistance was observed against tetracycline (58%) as well as erythromycin (56%), trimethoprim (52%), oxytetracycline (50%), enrofloxacin (42%) and streptomycin (38%). Importantly, E. coli is intrinsically resistant to erythromycin.
Characterization of ESBLs genes and class 1 integrons
The TEM gene was detected in 78% of the isolates, whereas CTX-M was identified in 32% of the ESBL-producing E. coli isolates (16 out of 50). Specifically, CTX-M-14 tested positive in 26% (13 out of 50), and CTX-M-27 exhibited positivity in 6% (3 out of 50), as depicted in Fig. 2. Furthermore, the co-occurrence of TEM and CTX-M genes was observed with 10% (5/50) in the E. coli isolates, while SHV gene was not detected in all isolates. Moreover, the presence of intI-1 genes was detected in 48% (24/50) of all isolates, while none of the isolates exhibited the occurrence of either intI-2 or intI-3. However, amplification of gene cassettes from three intI1- positive isolates was not successful, indicating the absence of amplifiable genetic material in these isolates. The class 1 integrons contained five distinct gene cassettes: dfrA1, dfrA17-aadA5, aadA1, aadA5 and dfr1-aadA1. Among the gene cassettes identified within these integrons, dfrA17-aadA5 exhibited the highest prevalence (26%, 13 isolates), followed by dfrA1 (10%, 5 isolates), aadA1 (6%, 3 isolates), aadA5 (4%, 2 isolates) and dfr1-aadA1 (4%, 2 isolates), as presented in Table 3.
Discussion
Endometritis is a prevalent perinatal ailment in dairy cattle, which serves as the primary cause of bovine infertility and significantly hinders the progress of the dairy cattle breeding industry. Pathogenic bacterial infection emerges as a prominent factor contributing to cow endometritis, with mixed infections involving multiple pathogenic bacteria being the primary etiological determinant of this disease [19,20,21]. The present study systematically investigated pathogenic microorganisms associated with cow endometritis in Gansu Province, China. The results indicated that bovine Endometritis caused by E. coli exhibited multiple drug resistance, and E. coli and ESBLs in cows are the dominant strains in western China. These findings provided valuable insights for the prevention and control of E. coli infection in livestock and poultry, as well as for the exploration of innovative treatment strategies.
Currently, conventional pathogen detection methods such as culture medium plate coating and ordinary PCR are plagued by issues of time-consuming procedures, instrument limitations, and the need for specialized personnel. Therefore, high-throughput rapid pathogen detection is crucial for early prevention, control, and treatment in cow endometritis. In this study, a total of 258 endometritis cow samples were detected using a nucleic acid test kit for multiple pathogens, and the predominant pathogens were identified. The findings revealed that E. coli emerged as the predominant pathogen, constituting the largest proportion among all pathogenic microorganisms. Both single and mixed infections were observed, with a lower incidence of mixed infections compared to those caused by E. coli alone. Furthermore, Mycoplasma bovis exhibits a significantly elevated detection rate, thereby presenting substantial challenges in the management of mixed infections in cattle and requiring considerable attention from livestock farms.
The β-lactam antibiotics have been used to treat various infectious diseases in humans and animals since the 1940s. However, the emergence of bacterial resistance has led to a decreased trend in the clinical efficacy of these antibiotics, particularly against Enterobacteriaceae. As a result, there has been an increase in the number of ESBL-producing E. coli strain [22, 23]. In this study, a total of 217 E. coli isolates were collected from the case of bovine endometritis in China, and the prevalence of ESBL producers observed in this study was found to be 23.04%, which is consistent with previous studies conducted on bovine E. coli in China, while surpassing the reported values from other countries [24,25,26,27]. Previous researches confirmed that CTX-M- type ESBLs have replaced TEM and SHV-type ESBLs as the predominant type among Enterobacteriaceae members in Asia, Europe, and Canada [28,29,30]. Our findings revealed that the TEM genotype was the most prevalent, followed by CTX-M in the western of China. Our study has identified the presence of CTX-M-14 and CTX-M-27 producers among bovine- associated E. coli isolates, which is a novel finding, these producers have previously been reported to be frequently found in clinical isolates of E. coli from healthcare facilities [31]. Furthermore, the use of Meropenem and imipenem in animals has been prohibited, and all ESBL-producing E. coli isolates have shown susceptibility to these two antibiotics. Our findings are line with previous study that indicated the majority of ESBL producers identified from livestock exhibit MDR [32]. Specifically, we also observed that all ESBL-producing E. coli isolates were MDR and most were resistant to aminoglycosides, trimethoprim, tetracyclines, chloramphenicol or quinolones.
The prevalence rate of positive integrons in this study was determined to be 48%, which aligns with the reported value (45.5%) observed in Inner Mongolia, China. The variable regions within class 1 integron gene cassette arrays exhibited five distinct gene combinations, which are likely responsible for conferring additional resistance traits onto our isolates. Notably, three intI1-positive amplicons were unable to produce any gene cassettes potentially due to the lack of a 3’-CS region within these particular integrons [33]. The cassette dfrA17-aadA5 was identified as the predominant gene array, which is consistent with previous study conducted in China [34]. The presence of class 1 integrons in pathogenic bacteria has been found to be closely associated with bacterial resistance according to recent research [35, 36]. The variable region of Class 1 integrons exhibited a remarkable level of gene diversity and demonstrated a strong association with resistant phenotypes. Further comprehensive investigations about class 1 integrons can provide valuable insights into the occurrence and dissemination of pathogenic bacteria, thereby establishing a foundation for effective prevention and control measures against epidemics, bacterial infections in breeding enterprises, as well as facilitating the selection of antimicrobial agents.
Conclusion
In summary, the investigation has revealed that ESBL-producing E. coli isolates obtained from dairy cattle with endometritis in China exhibited a significant prevalence and elevated antimicrobial resistance level, thereby establishing a theoretical foundation for the prevention and treatment of bovine endometritis.
Materials and methods
Sample collection area and experimental animals
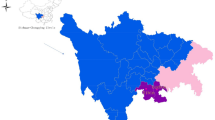
The dairy cows selected in this study were obtained from a total of 17 dairy herds located in Gansu Province, as shown in Fig. 3. These cows ranged in age from 3 to 6 years old, with weights ranging between 650 and 700 kg. None of the participants had a history of retained placenta or septicemia, although some may have encountered initial postpartum challenges such as delayed uterine involution and endometritis. The cows in these 17 dairy farms were vaccinated against BVDV and foot-and-mouth disease vaccines according to standard immunization protocol prior to being utilized for uterine fluid collection. The cow utilized in our study originated from different ranch, where it was internally raised and selectively bred by the ranch itself.
Samples collection and cases definition
In our study, a total of 258 samples of uterine secretions were collected from dairy cows diagnosed with clinical endometritis from Sep 2020 to Feb 2023. The procedure for sample collection is outlined as follows: The perineal areas of the restrained cows were disinfected with a 75% ethyl alcohol solution. Subsequently, a plastic infusion pipette with a protective sheath was inserted into the cranial vagina and carefully guided through the cervix and into the uterus after rupturing the sheath. A sterile saline solution was gently injected and agitated within the uterus before being aspirated for sampling purposes. The volume of collected samples ranged from 5 to 15 mL and was transported on ice for analysis in a laboratory within 24 h (h).
The inflammation of the endometrium, referred to as endometritis, is characterized by the presence of purulent cervical discharge that can be detected in the vagina 21 days or more postpartum. In most cases, no obvious clinical signs were observed and milk production remains normal. Lochia (the presence of malodorous cervical discharge) and rectal palpation (an enlarged, atonic uterus) were evaluated according to the diagnostic method of clinical endometritis, which was made by a veterinarian with extensive experience in treating cow diseases to ensure an accurate clinical diagnosis.
DNA amplification and sequencing
The Multiplex TaqMan real-time fluorescent quantitative PCR kit (Shenzhen, China) was employed for pathogen detection according to the manufacturer’s instruction, including E. coli, Staphylococcus aureus (S. aureus), S. agalactiae, M. bovis, K. Trevisan, S. dysgalactiae and Pseudomonas aeruginosa (P. aeruginosa) in clinical endometritis samples. Bovine respiratory tract pathogen nucleic acid detection kit (fluorescence PCR method) was purchased from Shenzhen Anieasy Biotechnology Co., Ltd. In brief, the Bacterial DNA was extracted using the Bacterial DNA Kit (Omega Bio-Tek, USA) following the manufacturer’s instructions. The different solutions were melt on ice after being removed from the reagent kit, add the mixture of samples and PCR reaction fluids. PCR reaction program as follows: Decontamination for 3 min (min) at 50 °C, pre-denaturation for 3 min at 95 °C, amplification for 10 s at 95 °C and signal collection for 30 s at 60 °C (40 cycles). The judgment of the result is shown in Supplemental Table 1.
The TEM, SHV, and CTX genes, which are prominent members of the β-lactamase gene family, were amplified using multiplex PCR with previously described primers and amplification conditions [25]. The integrase gene fragments were specifically amplified using PCR to identify the presence of integrons in all isolates. Subsequently, the detection of class 1, class 2, and class 3 integrons, as well as their associated gene cassettes, was performed following previously reported PCR conditions. PCR products were confirmed by bi-directional sequencing after being purifying with a QIAquick PCR Purification Kit (Qiagen, Hilden, Germany). The DNA sequences obtained were compared with those in GenBank using the BLAST program (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The primers used in this work are listed in Supplemental Table 2.
The isolation and identification of ESBL-producing E. coli
E. coli isolated from endometritis cases were identified as strains of ESBL-producing E. coli through morphological characterization and biochemical in our laboratory. In brief, the collected samples were inoculated into fresh blood agar plates and incubated for 24 h at 37 °C under aerobic and anaerobic conditions. The colonies were observed after being cultured for 24 h and conducted Gram staining. Then, the typical colonies were purified for secondary cultivation. Purified colonies were inoculated into 2 mL of nutrient broth and cultured at at 37 °C overnight. Then bacterial DNA was extracted with OMEGA genomic DNA extraction kit according to the instructions. 16S rDNA fragments were amplified using 16S rDNA bacterial identification PCR kit, and the nucleic acid electrophoresis was performed. The target fragment was sequenced by Beijing Liuhe Huada Gene Technology Co., Ltd.
Testing for antimicrobial susceptibility
ESBL-producing E. coli isolates were tested for susceptibility against 14 antimicrobial agents using the disc diffusion method, following CLSI recommendations [37]. The antibiotics (Oxoid, United Kingdom) that were tested included ampicillin (10 μg), amoxicillin (30 μg), ceftiofur (30 μg), cefuroxime (30 μg), trimethoprim (25 μg), tetracycline (10 μg), gentamicin (10 μg), oxytetracycline (30 μg), florfenicol (30 μg), erythromycin (10 μg), meropenem (10 μg), imipenem (10 μg), streptomycin (10 μg) and enrofloxacin (5 μg). The quality control strain used in this study was E. coli ATCC 25922 and exhibiting resistance to three or more antimicrobial categories were categorized as MDR [38].
Statistical analysis
All experiments were performed with at least three replications. Statistical analysis was performed using Prism 7.0 Software (GraphPad, La, Jolla, CA), and differences are evaluated by Student’s t-test. For all comparisons, P < 0.05 was considered statistically significant, P < 0.01 was considered to be extremely significant.
Availability of data and materials
The data supporting this study’s findings are available on request from the corresponding author.
References
Benzaquen ME, Risco CA, Archbald LF, Melendez P, Thatcher MJ, Thatcher WW. Rectal temperature, calving-related factors, and the incidence of puerperal metritis in postpartum dairy cows. J Dairy Sci. 2007;90(6):2804–14.
Bicalho MLS, Machado VS, Oikonomou G, Gilbert RO, Bicalho RC. Association between virulence factors of Escherichia coli, Fusobacterium necrophorum and Arcanobacterium pyogenes and uterine diseases of dairy cows. Vet Microbiol. 2012;25:125–31.
Brick TA, Schuenemann GM, Bas S, Daniels JB, Pinto CR, Rings DM, Rajala-Schultz PJ. Effect of intrauterine dextrose or antibiotic therapy on reproductive performance of lactating dairy cows diagnosed with clinical endometritis. J Dairy Sci. 2012;95(4):1894–905.
Westermann S, Drillich M, Kaufmann TB, Madoz LV, Heuwieser W. A clinical approach to determine false positive findings of clinical endometritis by vaginoscopy by the use of uterine bacteriology and cytology in dairy cows. Theriogenology. 2010;74:1248–55.
Senosy W, Hussein HA. Association among energy status, subclinical endometritis postpartum and subsequent reproductive performance in Egyptian buffaloes. Anim Reprod Sci. 2013;140(1–2):40–6.
Levy B, Marshall B. Antibacerial resistance worldwide:Causes, challengeand responses. Nat Med. 2004;10:S122–9.
Hijazi SM, Fawzi MA, Ali FM, Galil KH. Prevalence and characterization of extended- spectrum beta-lactamases producing Enterobacteriaceae in healthy children and associated risk factors. Ann Clin Microbiol Antimicrob. 2016;15:3.
Mikhayel M, Leclercq SO, Sarkis DK, Doublet B. Occurrence of the Colistin Resistance Gene mcr-1 and Additional Antibiotic Resistance Genes in ESBL/AmpC-Producing Escherichia coli from Poultry in Lebanon: A Nationwide Survey. Microbiol Spectr. 2021;9(2):e0002521.
Tekiner H, Ozpinar H. Occurrence and characteristics of extended spectrum beta-lactamases- producing Enterobacteriaceae from foods of animal origin. Braz J Microbiol. 2016;47:444–51.
Liu WE, Chen LM, Li HL, Duan HL, Zhang YL, Liang XH, Li X, Zou MX, Xu L, Hawkey PM. Novel CTX-M {beta}-lactamase genotype distribution and spread into multiple species of Enterobacteriaceae in Changsha, Southern China. J Antimicrob Chemother. 2009;63:895–900.
Li L, Wang B, Feng S, Li J, Wu C, Wang Y, Ruan X, Zeng M. Prevalence and characteristics of extended-spectrum β-lactamase and plasmid-mediated fluoroquinolone resistance genes in Escherichia coli isolated from chickens in Anhui province, China. PLoS ONE. 2014;9(8):e104356.
Hall MA, Paauw A, Box ATA, Blok HEM, Verhoef J, Fluit AC. Presence of integron associated resistance in the community is widespread and contributes to multidrug resistance in the hospital. J Clin Microbiol. 2002;40:3038–40.
O’Brien TF. Emergence, spread, and environmental effect of antimicrobial resistance: how use of an antimicrobial anywhere can increase resistance to any antimicrobial anywhere else. Clin Infect Dis. 2002;1:34.
Odetoyin BW, Labar AS, Lamikanra A, Aboderin AO, Okeke IN. Classes 1 and 2 integrons in faecal Escherichia coli strains isolated from mother-child pairs in Nigeria. PLoS ONE. 2017;12(8):e0183383.
Cavicchio L, Dotto G, Giacomelli M, Giovanardi D, Grilli G, Franciosini MP, Trocino A, Piccirillo A. Class 1 and class 2 integrons in avian pathogenic Escherichia coli from poultry in Italy. Poult Sci. 2015;94:1202–8.
Siqueira AK, Michael GB, Domingos DF, Ferraz MMG, Ribeiro MG, Schwarz S, Leite DS. Diversity of class 1 and 2 integrons detected in Escherichia coli isolates from diseased and apparently healthy dogs. Vet Microbiol. 2016;194:79–83.
Venturini C, Hassan KA, Chowdhury PRI, Paulsen IT, Walker MJ, Djordjevic SP. Sequences of two related multiple antibiotic resistance virulence plasmids sharing a unique IS26-related molecular signature isolated from different Escherichia coli pathotypes from different hosts. PLoS ONE. 2013;8:e78862.
Kivaria FM, Noordhuizen JP, Msami HM. Risk factors associated with the incidence rate of clinical mastitis in smallholder dairy cows in the Dares Salaam region of Tanzania. Vet J. 2007;173(3):623–9.
Pande M, Das GK, Khan FA, Sarkar M, Pathak MC, Prasad JK, Kumar H. Endometritis impairs luteal development, function, and nitric oxide and ascorbic acid concentrations in buffalo (Bubalus bubalis). Trop Anim Health Prod. 2013;45(3):805–10.
Gonen S, Bishop SC, Houston RD. Exploring the utility of cross-laboratory RAD-sequencing datasets for phylogenetic analysis. BMC Res Notes. 2015;8:299.
Song H, Bae Y, Kwon H, Kwon Y, Joh S. Loop-mediated isothermal amplification assays for Enterococcussp., Escherichiacoli and Staphylococcusaureus in chicken. FEMS Microbiol Lett. 2019;366(5):fnz042.
Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010;8(4):251–9.
Gutkind GO, Conza DJ, Power P, Radice M. β-lactamase-mediated resistance: a biochemical, epidemiological and genetic overview. Curr Pharm Des. 2013;19(2):164–208.
Ali T, Ur Rahman S, Zhang L, Shahid M, Zhang S, Liu G, Gao J, Han B. ESBL-Producing Escherichia coli from Cows Suffering Mastitis in China Contain Clinical Class 1 Integrons with CTX-M Linked to ISCR1. Front Microbiol. 1931;2016:7.
Yang F, Zhang SD, Shang XF. Prevalence and characteristics of extended spectrum β-lactamase-producing Escherichia coli from bovine mastitis cases in China. J Integr Agric. 2018;17:1246–51.
Geser NS, Stephan R, Hächler H. Occurrence and characteristics of extended-spectrum b-lactamase (ESBL) producing Enterobacteriaceae in food producing animals, minced meat and raw milk. BMC Vet Res. 2015;8:21.
Freitag C, Michael GB, Kadlec K, Hassel M, Schwarz S. Detection of plasmid-borne extended-spectrum beta-lactamase (ESBL) genes in Escherichia coli isolates from bovine mastitis. Vet Microbiol. 2016;200:151–6.
Kar D, Bandyopadhyay S, Bhattacharyya D, Samanta I, Mahanti A, Nanda PK. Molecular and phylogenetic characterization of multidrug resistant extended spectrum beta-lactamase producing Escherichia coli isolated from poultry and cattle in Odisha, India. Infect Genet Evo. 2015;29:82–90.
Chandramohan L, Revell PA. Prevalence and molecular characterization of extended-spectrum- β-lactamase-producing Enterobacteriaceae in a pediatric patient population. Antimicrob Agents Chemother. 2012;56:4765–70.
Dahmen S, Métayer V, Gay E, Madec JY, Haenni M. Characterization of extended-spectrum beta-lactamase (ESBL) -carrying plasmids and clones of Enterobacteriaceae causing cattle mastitis in France. Vet Microbiol. 2013;162:793–9.
Kim J, Lim YM, Rheem I, Lee YH, Lee JC, Seol SY, Cho DT. CTX-M and SHV-12 beta-lactamases are the most common extended-spectrum enzymes in clinical isolates of Escherichia coli and Klebsiella pneumoniae collected from 3 university hospitals within Korea. FEMS Microbiol Lett. 2005;245:93–8.
Yu T, He T, Yao H, Zhang JB, Li XN, Zhang RM, Wang GQ. Prevalence of 16S rRNA Methylase Gene rmtB Among Escherichia coli isolated from Bovine Mastitis in Ningxia, China. Foodborne Pathog Dis. 2015;12(9):770–7.
Lu L, Dai L, Wang Y, Wu C, Chen X, Li L, Qi Y, Xia L, Shen J. Characterization of antimicrobial resistance and integrons among Escherichia coli isolated from animal farms in Eastern China. Acta Trop. 2010;113:20–5.
Xu G, An W, Wang H, Zhang X. Prevalence and characteristics of extended-spectrum beta-lactamase genes in Escherichia coli isolated from piglets with post-weaning diarrhea in Heilongjiang province, China. Front Microbiol. 2015;6:1103.
Yu G, Li Y, Liu X. Role of integrons in antimicrobial resistance: a review. Afr J Microbiol Res. 2013;7:1301–10.
Li B, Zhao ZC, Wang MH, Huang XH, Pan YH, Cao YP. Antimicrobial resistance and integrons of commensal Escherichia coli strains from healthy humans in China. J Chemother. 2014;26:190–2.
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI document M100–S23. Wayne, PA: Clinical and Laboratory Standard Institute (CLSI); 2015.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olssonliljequist B. Multidrug-resistant, extensively drugresistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81.
Acknowledgements
None.
Funding
This work was financially supported by the earmarked fund for CARS (No. 36), the National Natural Science Foundation of China (No. 32202848 & No. 32172903), Gansu Province Science and Technology Foundation for Youths (No. 21JR7RA030), The Basic service Fund of CAAS (No. 1610322023023), The Science and Technology Innovation Project of CAAS Collaborative Innovation (No. CAAS-XTCX2016011-01–09), Innovation Project of Traditional Chinese Veterinary Medicine and Clinical Science (No. CAAS -ASTIP-2015-LIHPS) and National Key Research and Development Plan (No. 2022YFD1801102).
Author information
Authors and Affiliations
Contributions
Kang Zhang, Haipeng Feng: Conceptualization, Investigation, Writing – review & editing. Jingyan Zhang: Methodology. Xuezhi Wang, Zhiting Guo, Guibo Wang, Zunxiang Yan: Formal analysis, Data curation. Lei Wang and Jianxi Li: Conceptualization, Investigation, Writing – review & editing, Project administration. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experimental protocols and procedures in current study were authorized and approved by the Animal Care and Use Committee, Lanzhou Institute of Husbandry and Pharmaceutical Sciences of the Chinese Academy of Agricultural Sciences (the permission number: SYXK (Gan) 2019–0002).
Animal owner’s informed consent was obtained before collecting sample, and all methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
No applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, K., Feng, H., Zhang, J. et al. Prevalence and molecular characterization of extended-spectrum β–lactamase—producing Escherichia coli isolates from dairy cattle with endometritis in Gansu Province, China. BMC Vet Res 20, 19 (2024). https://doi.org/10.1186/s12917-023-03868-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-023-03868-x