Abstract
Background
Sepsis and septic shock are frequently accompanied by coagulopathy. Since the sepsis-induced coagulopathy (SIC) score was first described, subsequent studies from Asia revealed a SIC prevalence of 40–60%. In Europe, however, SIC prevalence in patients fulfilling sepsis criteria according to the third international consensus definition (SEPSIS-3) has not yet been evaluated.
Methods
The Critical Care Trials Group of the German Sepsis Competence Network (SepNet) conducted a secondary analysis of two randomized controlled trials. Only patients fulfilling sepsis criteria according SEPSIS-3 were included in this secondary analysis. In a two step approach, SIC prevalence was determined in 267 patients with sepsis but not septic shock (at the time of inclusion) from the “Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis” (HYPRESS) trial. Then, we estimated SIC prevalence in 1,018 patients from the “Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients With Severe Sepsis or Septic Shock” (SISPCT) trial using a simplified SIC score based on the platelet-SIC-subscore (PSSC). Study aims were to assess (i) the prevalence of SIC in patients with SEPSIS-3, (ii) the association of SIC with 90-day mortality and morbidity, (iii) the time when patients become SIC positive during the course of sepsis, and (iv) the value of the PSSC for predicting SIC.
Results
In the HYPRESS trial, SIC prevalence was 22.1% (95% confidence interval [CI] 17.5–27.5%). The estimated SIC prevalence in the SISPCT trial was 24.2% (95% CI 21.6–26.9%). In the HYPRESS trial, SIC was associated with significantly higher 90-day mortality (13.9% vs. 26.8%, p = 0.027) and morbidity. Logistic regression analysis adjusted for age, sex, treatment arm, and (SIC-adapted) SOFA score confirmed the negative association of SIC with survival (p = 0.011). In the SISPCT trial, increased PSSCs were associated with higher 90-day mortality (PSSC 0: 34.4%, PSSC 1: 40.5%, PSSC 2: 53.3%; p < 0.001). In both trials, SIC was already present at sepsis diagnosis or occurred during the following 4 days.
Conclusions
SIC is a clinically relevant complication of sepsis. Although it might be less frequent than previously reported, its occurrence is associated with higher morbidity and mortality and should be interpreted as an early warning sign.
Similar content being viewed by others
Background
In patients with sepsis or septic shock, the coagulation system is regularly impaired [1]. Due to the physiological interaction of inflammation and coagulation, disorders of the coagulation system are understood to be an integral part of the “life threatening host response to infection” that defines sepsis [2, 3]. In 2017, the Scientific Standardization Committee on Disseminated Intravascular Coagulopathy (DIC) of the International Society on Thrombosis and Haemostasis (ISTH) established the term sepsis-induced coagulopathy (SIC), introducing a new screening and diagnostic tool called the SIC score [4, 5]. The SIC score provides criteria to diagnose coagulopathy caused by sepsis easier and in an earlier stage [4, 5]. Therefore, in contrast to other DIC-screening tools like the ISTH overt-DIC score [6] and the Japanese Association for Acute Medicine (JAAM) score [7], the SIC score only relies on three components: (1) an adapted Sequential (sepsis-related) Organ Failure Assessment (SOFA) score, (2) the platelet count, and (3) the international normalized ratio (INR; or the prothrombin time [PT]) [4, 8]. Both fibrinogen plasma levels (a component of the ISTH score) and D-dimer plasma levels (a component of both the ISTH and JAAM scores) are no longer integrated (see Additional file 1: Table S1). As a result, the SIC score detects a coagulopathy that does not (yet) necessarily reflect overt DIC. Systemic inflammatory response syndrome (SIRS) criteria, which are part of the JAAM score, were replaced by the SOFA score in order to adapt the SIC score to the Third International Consensus Definitions for Sepsis and Septic Shock (SEPSIS-3) [3]. A SIC score ≥ 4 is considered positive. Moreover, as an additional condition the sum of the Platelet SIC subscore (PSSC) and the INR SIC subscore (ISSC) has to be ≥ 3 [4]. The performance of the SIC score has been validated retrospectively in several Japanese cohorts [5, 9, 10]. However, it remains unclear whether the determined incidences and outcomes are transferable to other, non-Japanese cohorts. The original work through which the SIC score was established used highly preselected patients fulfilling the criteria of severe sepsis and DIC according to the criteria of the Japanese Ministry of Health, Labor and Welfare [4, 11]. Moreover, in contrast to the Surviving Sepsis Campaign (SSC), the Japanese sepsis guidelines recommend the use of antithrombin and thrombomodulin in patients with suspected DIC [12, 13]. As a result, about 50% of the patients included in these validation studies had been treated with at least one of these drugs [5, 10].
Here, we present a secondary analysis of the “Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis: The HYPRESS Randomized Clinical Trial”, and the “Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients With Severe Sepsis or Septic Shock” (SISPCT) trial [14, 15]. The aims of this analysis were to assess (i) the prevalence of SIC in patients with sepsis (SEPSIS-3), (ii) the association of SIC with mortality and morbidity, (iii) the onset of SIC positivity during the course of sepsis. In a two step approach we first analysed the HYPRESS trial including patients with sepsis but not septic shock (about 22% of these patients developed septic shock during the 14 day observation period) [14]. Then, we analysed the SISPCT trial using a simplified SIC-score based on the SIC-adapted SOFA and PSSC.
Methods
This study was a secondary analysis of the “The HYPRESS Randomized Clinical Trial” [14, 15] and the SISPCT trial [14, 15]. Only patients fulfilling SEPSIS-3 critera (i.e., infection + SOFA score ≥ 2 points) at the time of inclusion in the original trial were included.
The HYPRESS trial was an investigator-initiated, multicenter, placebo-controlled, double-blind randomized controlled trial (RCT). It was supported by the German Federal Ministry of Education and Research and conducted between January 2009 and February 2014 at 34 intermediate care (IMCs) or intensive care units (ICUs) of university and community hospitals in Germany. A detailed description of the methodology can be found in the primary publication of the trial [14]. Briefly, adult patients with severe sepsis who were not in septic shock (at the time of inclusion) were included and randomized 1:1 either to receive a continuous infusion of 200 mg hydrocortisone (HC) for 5 days followed by dose tapering until day 11 or to receive placebo. The primary outcome, “development of septic shock within 14 days”, was met by 75 of 340 (22.06%) patients [14]. All patients were treated according to the (at that time) valid guidelines of the German Sepsis Society [16]. SIC scores were calculated according to the previously published definition of Iba et al. [4] (see Additional file 1: Table S1). For a SIC score to be considered positive, two conditions had to be met: i) the SIC score had to be ≥ 4 and ii) the sum of the PSSC and the INR SIC subscore (ISSC) had to be ≥ 3 [4]. The SIC score uses an adapted SOFA score only taking into account the sum of four subscores, namely respiratory, cardiocirculatory, hepatic, and renal [4, 8].
The SISPCT trial was an investigator-initiated, multicenter RCT performed at 33 ICUs in Germany [15]. The trial included patients with sepsis and septic shock (according to the SEPSIS-2 definition) and was conducted from November 6, 2009, to June 6, 2013, including a 90-day follow-up period [15, 17]. It was designed to investigate the effect of sodium selenite and procalcitonin guidance of anti-infective therapy on the 28-day mortality of patients with sepsis and septic shock. The international normalized ratio (INR) was not recorded during the SISPCT trial. Therefore, SIC prevalence in SISPCT was estimated using a “simplified SIC score”. The simplified SIC score was considerd positive, if the sum of the SIC-adapted SOFA sub score and PSSC was 4.
Before including the first patient, the trial protocols of both trials had been registered (clinicaltrials.gov Identifier: NCT00670254 [18], NCT00832039 [19]) and approved by the leading ethics board of Jena University Hospital and the institutional review boards of all participating institutions [15]. Both trials were carried out according to the Declaration of Helsinki (October 2013) and in both trials, written informed consent, including secondary analyses, was obtained from all study participants [20].
Statistical analyses
Primary endpoints were SIC prevalence in HYPRESS and the estimated SIC prevalence in SISPCT. Secondary endpoints were the association of SIC with mortality in HYPRESS and SISPCT, as well as the association of SIC with mean SOFA score until day 14, the need of renal replacement therapy (RRT) until day 28 and ICU length of stay (ICU-LOS) in HYPRESS.
Study participant characteristics were compared using the χ2 test, Fisher’s exact test, the Mann–Whitney U test, the Kruskal–Wallis H test, the Kaplan–Meier estimator, or the log-rank test, as appropriate. Multivariate logistic regression was performed to compare survival of SIC positive and SIC negative patients adjusting for age, sex, treatment arm, and the SIC-adapted SOFA score at sepsis onset (= the time of sepsis diagnosis). The SOFA score was adapted according to Iba et al. [4]. All reported p values are two sided. Statistical analyses were performed using SPSS version 28.0 (IBM Corp., Armonk, NY, USA). Data analysis was performed between September 2021 and May 2022. Because the SEPSIS-2 definition was still valid at the time the HYPRESS and the SISPCT trial were conducted, the SOFA score was not part of the mandatory baseline parameters. Likewise, it was not mandatory to provide platelet counts or the INR. If SOFA scores were missing at sepsis onset (the day of diagnosis), they were imputed as the SOFA scores for day 1. If a SOFA score was missing for sepsis onset and day 1, the patient was excluded. If an INR value or platelet count was missing at sepsis onset, the patient was excluded.
Results
Study participant characteristics and SIC prevalence in in the HYPRESS trial
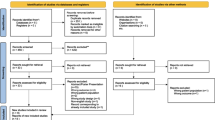
From 380 patients that had been randomized in the HYPRESS trial, 27 had to be excluded from the intention-to-treat (ITT) analysis. The remaining 353 patients (ITT) were eligible for our secondary analysis. Eighty-six had to be excluded due to missing sepsis onset data. All remaining 267 patients had “severe sepsis” according to SEPSIS-2 [17] and fulfilled the criteria for “sepsis” according to SEPSIS-3 [3] (Fig. 1). At sepsis onset, 45 (16.9%) patients were SIC positive. During the 14 day observation period, 14 additional patients were SIC positive at least once. Therefore, the SIC prevalence during the observation period was 22.1% (95% confidence interval [CI] 17.5–27.5%).
Flow diagram detailing the selection of patient groups analyzed from the HYPRESS trial [14]. #SOFA scores missing at sepsis onset and day 1 (if SOFA subscores were missing at sepsis onset they were imputed as day 1). If the INR or platelet counts were missing at sepsis onset, patients were excluded. INR international normalized ratio, SIC sepsis-induced coagulopathy; SOFA Sequential (sepsis-related) Organ Failure Assessment
Patients with and without SIC were comparable regarding the distribution of age and sex (Table 1). However, a positive SIC score at sepsis onset was associated with a higher mean SOFA score at sepsis onset (8 [interquartile range, IQR, 7–10] vs. 5 [IQR 4–7]; Table 1). SIC positive patients more often fulfilled SEPSIS-2 criteria for “coagulation abnormalities” (INR 1.5 or activated prothrombin time [aPTT] > 60 s) at sepsis onset [17].
Onset of SIC positivity
Of 222 patients who were SIC negative at sepsis onset, 14 patients (6.3%) developed SIC during the following days. Twelve of these 14 patients (85.7%) became SIC positive during the first 4 days following sepsis onset (Fig. 1).
SIC and clinical outcomes of patients of the HYPRESS trial
Mortality rates (ICU, 28 day, 90 day, and 180 day) differed significantly between patients with and without SIC (Table 2 and Fig. 2). The positive predictive value of SIC (within the 14-d-observation period) to predict 180-day mortality was 37.5% (95%-CI 26.0–50.6%], whereas the negative predictive value was 81.7% [95%-CI 75.7–86.5%]. Logistic regression revealed a negative association of SIC with survival (p = 0.011; Additional file 1: Table S2). Of note, there was no difference regarding the necessity of mechanical ventilation between SIC positive and SIC negative patients with sepsis. However, SIC positive patients had a higher mean SOFA score until day 14 (p < 0.001) and needed renal replacement therapy (RRT) significantly more often until day 28 (p < 0.001; Table 2). Moreover, SIC was not associated with an increased ICU length of stay (ICU-LOS). There was no difference regarding gastro-intestinal bleeding events (defined as an acute bleeding which required transfusion of more than one unit of red blood cells within 24 h [14]) between SIC positive and SIC negative patients (Table 2).
Kaplan–Meier plots of patients included in the HYPRESS trial [15]: 90-day survival (p = 0.014) Blue line: SIC negative patients. Green line: patients who were SIC positive at least once during the observation period. SIC sepsis-induced coagulopathy
Of the 45 patients who were SIC positive on admission to the ICU, 34 recovered from SIC during their stay on the ICU. In 11 patients SIC was persistent (defined as last documented values corresponding to a positive SIC score). Persistence of SIC was associated with a higher mortality (ICU, in-hospital, 14 day, 28 day, 90 day) as well as with a higher mean SOFA score until day 14 and a higher percentage of patients needing RRT until day 28 (Additional file 1: Table S3).
PSSC prediction of SIC
An increased ISSC (> 0 points) at sepsis onset had a sensitivity of 93.2% and a specificity of 54.3% to predict SIC during the 14-day observation period (Additional file 1: Table S4). The positive predictive value (PPV) was 36.7%, the negative predictive value (NPV) 96.6%. In contrast, an increased PSSC reached a sensitivity of 84.8%, a specificity of 83.7%, a PPV of 59.5%, and a NPV of 95.1% Additional file 1: Tables S5 andS6.
Estimating SIC prevalence and mortality using the PSSC in patients of the SISPCT trial
We analyzed 1,018 of 1,089 patients included in the SISPCT trial fulfilling SEPSIS-3 criteria (Fig. 3). At sepsis onset, 318 of 1018 (31.2%) patients had a PSSC of 1 or 2 (Fig. 3). Moreover, 112 of 700 (16.0%) patients having a sepsis-onset PSSC of 0 developed a PSSC of 2 (while having a SOFA score ≥ 2) during the 14-day observation period. In addition, 85 of 157 (54.1%) patients with PSSC of 1 at sepsis-onset developed a PSSC of 2 (while having a SOFA score ≥ 2) during the observation period. Using this modified SIC score (SOFA score ≥ 2 and PSSC = 2), the estimated SIC prevalence in the SISPCT trial was 24.2% (95% CI 21.6–26.9%). Additional file 1: Table S7 details the characteristics of patients having a PSSC of 0, 1, or 2. The PSSC was independent of the SISPCT intervention arms. There were no differences regarding sex or age. Like SIC scores in the HYPRESS trial, a higher PSSC in the SISPCT trial was associated with a higher SOFA score (PSSC 0: SOFA 9 [IQR 7–11]; PSSC 1: SOFA 11 [IQR 9–13]; PSSC 2: 13 [IQR 11–16]). In addition, a higher PSSC was associated with significantly higher 90-day mortality (PSSC 0: 34.5% [95%-CI 30.9–38.0%] vs. PSSC 1: 40.5% [95%-CI 33.1–48.4%] and PSSC 2: 53.3% [95%-CI 45.4–61.1%]; p < 0.001) (Fig. 4).
Flow diagram detailing the selection of patient groups analyzed from the SISPCT trial [15]. ITT intention to treat, SIC sepsis-induced coagulopathy; SOFA Sequential (sepsis-related) Organ Failure Assessment
Kaplan–Meier plots of patients included in the SISPCT trial, stratified for the platelet-sepsis-induced coagulopathy subscore (PSSC) at sepsis onset. N = 675 patients having a PSSC of 0 (blue line), n = 153 patients having a score of 1 (green line), and n = 150 patients having a score of 2 (red line); p < 0.001
Discussion
In this secondary analysis of the HYPRESS and SISPCT trials conducted in German ICUs, we observed a significantly lower SIC prevalence (16.9% at sepsis onset and 22.1% during the observation period) in patients with sepsis but not septic shock (at the time of inclusion) and 24.2% in patients with septic shock than had been reported in previous studies from Asia (40–60%) [4, 10, 14, 21]. Furthermore, SIC was either already present at sepsis onset or developed within the first 4 days. This observation is in line with the pathophysiology of SIC being most likely triggered by a dysregulated interaction between the innate immune response and the coagulation system [22]. To address these special features of SIC and to differentiate SIC from other DIC subtypes, Iba and colleagues introduced the SIC score [4, 21]. By excluding the parameters D-dimer and fibrinogen, the SIC score is more likely to detect sepsis-induced coagulopathy at an earlier stage than previous screening tools [23]. However, this also means that not all SIC positive patients suffer from overt DIC. The SIC score was developed “based on the results of logistic regression analyses” using data from 1,498 patients [4]. However, the included patients were highly preselected, since all patients suffered from “DIC according to the Japanese Ministry Health and Labor Welfare Diagnostic Criteria for DIC” and were treated with thrombomodulin alpha [4, 11]. In consequence, SIC prevalence was > 60%. [4]. Therefore, it is not surprising that the observed prevalences in our study are lower although the median SIC-adapted SOFA scores (at the time of inclusion) of these patients were comparable to those of the patients analyzed from the HYPRESS trial (5 [IQR 3–7] vs. 5 [IQR 4–7]) [4]. However, there are several more studies reporting high SIC prevalences. Another study from Japan reported a SIC prevalence of 61.4% [5], and Ding and colleagues [5, 24] even found a SIC prevalence of 67.9% in a Chinese cohort of SEPSIS-3 patients. The most recent validation of the SIC sore is from 2021 [10]. In their secondary analysis, Tanaka and colleagues included only patients fulfilling sepsis criteria according to SEPSIS-3 [10]. The reported SIC incidences were 42.2% in patients not needing vasopressor therapy during their ICU stay and 66.4% in patients requiring vasopressors [10]. In the only European study that calculated SIC prevalence in patients with sepsis and vasopressor requirements, Julie Helms and colleagues reported a positive SIC Score in even 84.2% of the cases [25]. However, the SIC prevalence could have been overestimated in the latter study, since the additional condition introduced by Iba and colleagues (PSSC + ISSC ≥ 3) was not taken into account by Helms et al. when calculating the SIC score [4, 25]. In summary, the prevalence of SIC seems to vary significantly not only depending on the sepsis definition used, but also depending on the composition of the underlying cohort. As the HYPRESS trial included patients with severe sepsis but not shock with only about one fifth of the patients developing septic shock during the observation period (median SOFA Score = 5) and the SISPCT trial included patients with sepsis and septic shock with a median SOFA score of 10 (including 86.7% of patients with shock [15]), our secondary analysis includes two cohorts with different disease severities. However, in both trials SIC prevalence is considerably lower than previously reported. This is surprising as most of the prevalence studies mentioned above referred to patients with severe sepsis and septic shock according to the SEPSIS-2 definition and the patient groups covered by the definitions of “severe sepsis” (according to SEPSIS-2) and “sepsis” (according to SEPSIS-3) overlap widely [26]. Against this background, it is remarkable that the prevalence observed in both of our cohorts are lower by a factor of 2–3.
In this context, it is striking that almost all of the studies on the SIC score mentioned (even the one published in 2021) included patients that were treated for sepsis between 2011 and 2014 [4, 5, 10, 24]. At the same time, it is important to know that the recommendation for routine VTE prophylaxis was not included in the Japanese Sepsis Guideline before 2016 [13, 27]. The chapter did not exist in the 2014 version [27]. It was not until 2012 that the SCC recommended routine VTE prophylaxis for patients with sepsis for the first time [28]. There is no published data on how and whether pharmaceutical VTE prophylaxis was administered during this period. It can be assumed that it was heterogeneous and this fact could have had an impact on the SIC prevalence.
At the same time, mortality of SIC positive patients in our study was comparable to previous reports. SIC was associated with a 90-day mortality between 26.8% (HYPRESS) and 53.3% (SISPCT). This is comparable to the 28-day-mortality in SIC positive patients reported by Iba and collegues ranging between 30% in patients with a SIC score of 4–45% in patients with a SIC score of 6 [4]. Tanaka and colleagues also observed increased in-hospital-mortalities in SIC positive patients (requiring vasopressors: 35.8% vs. 27.9%; not requiring vasopressors: 15.6% vs. 12.2%) [10].
The fact that mortality of SIC positive patients in our observation was comparable to those of SIC positive patients in previous reports is remarkable knowing that the SSC guidelines and the Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock differ substantially with regard to their recommendations for anticoagulation [13, 29]. While both the German guidelines and the SSC guidelines recommend pharmaceutical prophylaxis of venous thromboembolism (VTE) with unfractionated heparin or low molecular weight heparins, they do not provide any recommendation regarding the treatment of SIC or overt DIC [29, 30]. In contrast, the Japanese guidelines recommend screening for DIC and the replacement of antithrombin as well as the administration of thrombomodulin alpha in case of SIC [12]. Moreover, older versions of the Japanese guidelines for the management of sepsis suggested the use of protease inhibitors, or heparinoids at doses exceeding VTE prophylaxis [27]. Only since 2016 there has been a recommendation against the use of heparin as standard treatment for patients with sepsis and DIC and against the use of protease inhibitors [13]. As a result, about 50% of patients included in the major Japanese studies received at least one of the following medications: antithrombin, thrombomodulin alpha, protease inhibitors, or heparinoids at doses exceeding VTE prophylaxis [10]. In contrast, most patients in the HYPRESS and SISPCT trials received only pharmacological VTE prophylaxis, most likely because the German sepsis guidelines recommend against the use of antithrombin due to low evidence and augmented risk for severe bleeding events [30].
Our work has several strengths as well as limitations. Using two well-characterized cohorts of patients, which were included in two German multicenter RCTs within the SepNet Critical Care Trials network, strengthens the internal validity of our study. In both trials, patients were treated at more than 30 study sites, supporting the generalizability of our findings to other health care settings respecting the SSC guidelines. This is the first work to evaluate the performance of the SIC score in a population of sepsis patients receiving anticoagulation and prophylaxis of venous thromboembolism (VTE) according to the International Guidelines for Management of Sepsis and Septic Shock by the SSC [13, 16, 17]. Moreover, this is the first work that has been able to discuss the onset of SIC, because detailed daily data during the 14-day observation period were available.
One limitation is that cases of “late-onset” SIC with an onset after 14 days were not captured. However, our data highlight SIC rather as a complication of early sepsis, making the first 4 days after sepsis diagnosis the most important. Considering the described crosstalk between the innate immune response and the coagulation system, it seems reasonable that patients becoming SIC positive after 14 days might have had either a second infection or another medical condition (e.g. severe bleeding complication), which are accompanied by a drop in platelet count or a rise in INR. Moreover, such a hypothetical “late-onset” case of SIC after day 14 would have been without a major clinical impact as the median ICU-LOS in SIC negative patients was 8 days with a range of [5–15]. Another limitation is that we were unable to calculate the SIC prevalence in the SISPCT trial, because INR data had not been collected. The SIC score requires two conditions to be met to be considered positive: First, the total SIC score has to be ≥ 4 points, second, as an additional condition, the sum of PSSC and ISSC has to be ≥ 3 points. To fulfill the second condition, SIC positive patients must have a PSSC > 0. By counting only patients who had a PSSC of 2 at onset (while having a SOFA score ≥ 2), we only counted patients with a high probability of having or developing SIC. On the one hand, we might have underestimated the SIC prevalence by missing some patients with a PSSC of 1 and an ISSC of 2 during the course of the disease. On the other hand, SIC prevalence was likely overestimated because not all patients with a PSSC of 2 necessarily have an ISSC > 0. However, as both effects balance each other to a certain extent, our estimation seems to be quite exact.
Conclusion
With a prevalence of 22.1% (HYPRESS)—24.2% (SISPCT) and an association with mortality, SIC is relevant in patients with sepsis and septic shock, although the prevalence is lower than previously reported. If SIC occurred, it was either present at the time of sepsis diagnosis or occurred during the first 4 days following sepsis diagnosis. In comparison with the ISSC, the PSSC appears to be more specific in order to predict SIC during the course of the disease. SIC was associated with mortality in two study populations representing different sepsis severity. Moreover, SIC was associated with a higher morbidity and as its occurrence within the first days of hospitalization should be perceived as a warning sign.
Availability of data and materials
Access to study data may be granted following a formal request to the Study Management Committee (Studienleitkommission) of the SepNet Critical Care Trials group for approval. It can be contacted at [14].
Abbreviations
- CI:
-
Confidence interval
- DIC:
-
Disseminated intravascular coagulopathy
- HYPRESS:Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis:
-
The HYPRESS randomized clinical trial
- INR:
-
International normalized ratio
- IQR:
-
Interquartile range
- ISTH:
-
International Society on Thrombosis and Haemostasias
- ISSC:
-
INR sepsis induced coagulopathy subscore
- ITT:
-
Intention to treat
- JAAM:
-
Japanese Association for Acute Medicine
- PSSC:
-
Platelet sepsis-induced coagulopathy subscore
- PPV:
-
Positive predictive value
- RCT:
-
Randomized controlled trial
- SD:
-
Standard derivation
- SepNet:
-
German sepsis competence network
- SIC:
-
Sepsis-induced coagulopathy
- SISPCT:
-
Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock
- SOFA:
-
Sequential (sepsis-related) organ failure assessment
- SSC:
-
Surviving sepsis campaign
- VTE:
-
Venous thromboembolism
References
Saito S, Uchino S, Hayakawa M, Yamakawa K, Kudo D, Iizuka Y. Epidemiology of disseminated intravascular coagulation in sepsis and validation of scoring systems. J Crit Care. 2019;50:23–30.
Lupu F, Keshari RS, Lambris JD, Mark CK. Crosstalk between the coagulation and complement systems in sepsis. Thromb Res. 2014;133:S28-31.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10.
Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9):e017046.
Yamakawa K, Yoshimura J, Ito T, Hayakawa M, Hamasaki T, Fujimi S. External validation of the two newly proposed criteria for assessing coagulopathy in sepsis. Thromb Haemost Februar. 2019;119(2):203–12.
Taylor FB, Toh CH, Hoots WK, Wada H, Levi M, Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–30.
Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625–31.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. 1996;22(7):707–10.
Iba T, Arakawa M, Levy JH, Yamakawa K, Koami H, Hifumi T. Sepsis-induced coagulopathy and Japanese association for acute medicine DIC in coagulopathic patients with decreased antithrombin and treated by antithrombin. Clin Appl Thromb Off J Int Acad Clin Appl Thromb. 2018;24(7):1020–6.
Tanaka C, Tagami T, Kudo S, Takehara A, Fukuda R, Nakayama F. Validation of sepsis-induced coagulopathy score in critically ill patients with septic shock: post hoc analysis of a nationwide multicenter observational study in Japan. Int J Hematol. 2021;114(2):164–71.
Kanno N, Kaneko M, Yatomi Y. Japanese ministry of health, labour and welfare DIC diagnostic criteria. Rinsho Byori. 2011;147:31–5.
Egi M, Ogura H, Yatabe T, Atagi K, Inoue S, Iba T. The Japanese clinical practice guidelines for management of sepsis and septic shock 2020 (J-SSCG 2020). J Intensive Care. 2021;9(1):53.
Nishida O, Ogura H, Egi M, Fujishima S, Hayashi Y, Iba T. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2016 (J-SSCG 2016). Acute Med Surg. 2018;5(1):3–89.
Keh D, Trips E, Marx G, Wirtz SP, Abduljawwad E, Bercker S. Effect of hydrocortisone on development of shock among patients with severe sepsis: the HYPRESS randomized clinical trial. JAMA. 2016;316(17):1775–85.
Bloos F, Trips E, Nierhaus A, Briegel J, Heyland DK, Jaschinski U. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med. 2016;176(9):1266–76.
Reinhart K, Brunkhorst F, Bone H, Gerlach H, Gründling M, Kreymann G. Diagnosis and therapy of sepsis: guidelines of the German Sepsis Society Inc. and the German interdisciplinary society for intensive and emergency medicine. Anaesthesist. 2006;55:43–56.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29(4):530–8.
Keh D. Placebo-controlled, randomised, double-blind study to investigate the efficacy and safety of low dose hydrocortisone to prevent the development of septic shock in patients with severe sepsis. clinicaltrials.gov. 2013 [zitiert 2. Januar 2022]. Report No.: NCT00670254. Verfügbar unter: https://clinicaltrials.gov/ct2/show/NCT00670254. Accessed 10 Oct 2022
Kompetenznetz Sepsis. prospective, randomized multicenter trial of adjunctive intravenous therapy with sodium-selenite(Selenase®, double-blinded) and a procalcitonin guided causal therapy (open) of severe sepsis or septic shock. Clinicaltrials.gov. 2016. [zitiert 14. April 2022]. Report No.: NCT00832039. Verfügbar unter: https://clinicaltrials.gov/ct2/show/NCT00832039. Accessed 10 Oct 2022
The World Medical Association (WMA). Declaration of Helsinki—ethical principles for medical research involving human subjects—64th WMA General Assembly, Fortaleza, Brazil. 2013 [zitiert 15. August 2019]. Verfügbar unter: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 10 Oct 2022
Iba T, Levy JH, Warkentin TE, Thachil J, van der Poll T, Levi M. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17(11):1989–94.
Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res. 2016;118(9):1392–408.
Iba T, Arakawa M, Di Nisio M, Gando S, Anan H, Sato K. Newly proposed sepsis-induced coagulopathy precedes international society on thrombosis and haemostasis overt-disseminated intravascular coagulation and predicts high mortality. J Intensive Care Med. 2018. https://doi.org/10.1177/0885066618773679.
Ding R, Wang Z, Lin Y, Liu B, Zhang Z, Ma X. Comparison of a new criteria for sepsis-induced coagulopathy and International Society on thrombosis and Haemostasis disseminated intravascular coagulation score in critically ill patients with sepsis 3.0: a retrospective study. Blood Coagul Fibrinolysis Int J Haemost Thromb. 2018;29(6):551–8.
Helms J, Severac F, Merdji H, Clere-Jehl R, François B, Mercier E. Performances of disseminated intravascular coagulation scoring systems in septic shock patients. Ann Intensive Care. 2020;10(1):92.
Shankar-Hari M, Harrison DA, Rubenfeld GD, Rowan K. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br J Anaesth. 2017;119(4):626–36.
Oda S, Aibiki M, Ikeda T, Imaizumi H, Endo S, Ochiai R. The Japanese guidelines for the management of sepsis. J Intensive Care. 2014;2:55.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021. https://doi.org/10.1007/s00134-021-06506-y.
Brunkhorst FM, Weigand MA, Pletz M, Gastmeier P, Lemmen SW, Meier-Hellmann A. S3 Guideline Sepsis-prevention, diagnosis, therapy, and aftercare: long version. Med Klin Intensivmed Notfallmedizin. 2020;115:37–109.
Acknowledgements
Not applicable.
Funding
The HYPRESS trial was supported by Charité–Universitätsmedizin Berlin and by grant 01KG0701 from the German Federal Ministry of Education and Research [14]. The funding agencies had no role in the design, data collection, data management, data analysis, data interpretation, preparation, review, or approval of the manuscript of the HYPRESS trial or the secondary analysis presented in this paper [15]. Parts of the study infrastructure of the SISPCT trial were funded by Grant 01 KI 0106 from the German Federal Ministry of Education and Research. Biosyn (Germany) and ThermoFisher Germany) provided study medication and financial support via unrestricted Grants [18]. The funding organizations had no role in the design, data collection, data management, data analysis, data interpretation, preparation, review, or approval of the manuscript of the SISPCT trial or the secondary analysis presented in this paper.
Author information
Authors and Affiliations
Consortia
Contributions
TS designed the study, analyzed the data, and wrote the manuscript. PMö, PM, JB, MB, FB, MW, GE, and TB were involved in the conception, hypothesis delineation, as well as the design of the study. They also critically reviewed the manuscript. DK was responsible for the design and conduct of the HYPRESS trial. HB participated in the design of the study, performed the statistical analyses, and was involved in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Before including the first patient, the trial protocols of both trials had been registered (clinicaltrials.gov Identifier: NCT00670254 [19], NCT00832039 [15]) and approved by the leading ethics board of Jena University Hospital and the institutional review boards of all participating institutions [20]. Both trials were carried out according to the Declaration of Helsinki (October 2013) and in both trials, written informed consent, including secondary analyses, was obtained from all study participants [14].
Consent for publication
All authors critically reviewed and approved the final submitted version of the manuscript. All authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Competing interests
T. Schmoch reports that he has received lecture fees from Mitsubishi Tanabe Pharma GmbH, CSL Behring GmbH (Germany). T. Brenner reports that he has received honoraria for lectures or advisory boards: Baxter Deutschland GmbH, Schöchl medical education GmbH (Germany), Boehringer Ingelheim Pharma GmbH (Germany), CSL Behring GmbH (Germany), Astellas Pharma GmbH (Germany), B. Braun Melsungen AG (Germany), Lücke Kongresse GmbH (Germany), Sedana medical Germany GmbH (Germany), Shionogi GmbH (Germany) and MSD Sharp & Dohme GmbH (Germany) and advisory board and consulting activity for Baxter Deutschland GmbH (Germany). Furthermore, he received research funding from Deutsche Forschungsgemeinschaft (DFG), Dietmar Hopp Stiftung, Innovationsfonds of the Gemeinsamer Bundesausschuss (G-BA), and Stiftung Universitätsmedizin Essen. Patrick Möhnle reports that he has received reimbursement of conference fees and travel and accommodation expenses, and lecture fees from Bayer (Germany), Biotest (Germany), CSL Behring (Germany), NovoNordisk (Germany), Pfizer (Germany), Roche (Germany), Shire/Takeda, and SOBI (Germany) and advisory board member at CSL Behring (Germany). M.A. Weigand reports that he has received lecture fees from GE Healthcare (Germany), Gilead (Germany), Köhler Chemie (Germany), MSD Sharp & Dohme (Germany), Pfizer Pharma (Germany), and Boehringer Ingelheim (Germany) and was member of advisory boards at B. Braun (Germany), Gilead (Germany), MSD Sharp & Dohme (Germany), and Shionogi (Germany). P. Meybohm and/or the Department of Anesthesiology, Intensive Care, Emergency and Pain Medicine, University Hospital Wuerzburg (Wuerzburg, Germany) received research grants from the German Research Foundation (ME 3559/1-1, ME 3559/3-1), BMBF (01KG1815), BMG (ZMVI1-2520DAT10E); grants from B. Braun Melsungen, CSL Behring, Fresenius Kabi, and Vifor Pharma for the implementation of Frankfurt’s Patient Blood Management Program; honoraria for scientific lectures from Biotest AG, Vifor Pharma, CSL Behring, and Pharmacosmos. Michael Bauer, Frank Bloos, Holger Bogatsch, Josef Briegel, Gunnar Elke, Didier Keh and Markus Löffler declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Comparison of scores measuring sepsis associated coagulopathy (adapted from Saito et al. [1], Iba et al. [2], and Schmoch et al. [3]). Table S2. Multivariate regression analysis 180 d mortality of HYPRESS patients. Table S3. Association of SIC persistence with mortality and morbidity. Table S4. Performance of the ISSC as test to predict SIC. Table S5. Performance of the PSSC as test to predict SIC. Table S6 Maximum platelet SIC subscore after onset grouped by platelet SIC subscore at onset. Table S7. Part 1: Sepsis onset characteristics of SISPCT patients [6] grouped by PSSC.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmoch, T., Möhnle, P., Weigand, M.A. et al. The prevalence of sepsis-induced coagulopathy in patients with sepsis – a secondary analysis of two German multicenter randomized controlled trials. Ann. Intensive Care 13, 3 (2023). https://doi.org/10.1186/s13613-022-01093-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-022-01093-7