Abstract
Background
Sepsis, the dysregulated host response to infection, triggers abnormal pro-coagulant and pro-inflammatory host responses. Limitations in early disease intervention highlight the need for effective diagnostic and prognostic biomarkers. Protein C’s role as an anticoagulant and anti-inflammatory molecule makes it an appealing target for sepsis biomarker studies. This meta-analysis aims to assess the diagnostic and prognostic value of protein C (PC) as a biomarker for adult sepsis.
Methods
We searched MEDLINE, PubMed, EMBASE, CINAHL and Cochrane Library from database inception to September 12, 2021. We included prospective observational studies of (1) adult patients (> 17) with sepsis or suspicion of sepsis that; (2) measured PC levels with 24 h of study admission with; and (3) the goal of examining PC as a diagnostic or prognostic biomarker. Two authors screened articles and conducted risk of bias (RoB) assessment, using the Quality in Prognosis Studies (QUIPS) and the Quality Assessment in Diagnostic Studies-2 (QUADAS-2) tools. If sufficient data were available, meta-analysis was conducted to estimate the standardized mean difference (SMD) between patient populations.
Results
Twelve studies were included, and 8 were synthesized for meta-analysis. Pooled analysis demonstrated moderate certainty of evidence that PC levels were less reduced in sepsis survivors compared to non-survivors (6 studies, 741 patients, SMD = 0.52, 95% CI 0.24–0.81, p = 0.0003, I2 = 55%), and low certainty of evidence that PC levels were less reduced in septic patients without disseminated intravascular coagulation (DIC) compared to those with DIC (3 studies, 644 patients, SMD = 0.97, 95% CI 0.62–1.32, p < 0.00001, I2 = 67%). PC could not be evaluated as a diagnostic tool due to heterogeneous control populations between studies.
Conclusion and relevance
Our review demonstrates that PC levels were significantly higher in sepsis survivors compared to non-survivors and patients with sepsis but not disseminated intravascular coagulation (DIC). Our evaluation is limited by high RoB in included studies and poor reporting of the sensitivity and specificity of PC as a sepsis biomarker. Future studies are needed to determine the sensitivity and specificity of PC to identify its clinical significance as a biomarker for early sepsis recognition.
Trial Registration PROSPERO registration number: CRD42021229786. The study protocol was published in BMJ Open.
Similar content being viewed by others
Introduction
Rationale
Sepsis, a life-threatening organ dysfunction caused by a dysregulated host response to infection, is a leading cause of mortality worldwide [1, 2]. With 48.9 million sepsis cases and 11 million sepsis-related deaths in 2017, the disease has a mortality rate of approximately 22% and represents 19.7% of deaths worldwide [2]. To address the high mortality rate, the Surviving Sepsis campaign emphasizes the importance of early therapeutic interventions and improved screening for high-risk patients [3].
Diagnosing early-stage sepsis and identifying those at high-risk for mortality remain a challenge due to the disease’s heterogeneous presentation. Sepsis presentation and outcome are affected by multiple factors, including patient characteristics, the causative microorganism and the site of infection [4]. While the current Sepsis-3 definition uses the Sequential Organ Failure Assessment (SOFA) scale to diagnose patients, the use of a highly specific and sensitive diagnostic biomarker could aid timely and appropriate treatment. In addition, the use of SOFA and quick SOFA (qSOFA), as risk stratification models, is limited by low specificity, and the sensitivity and specificity of qSOFA have been shown to vary widely across studies [5,6,7]. These limitations drive the need for a new rapid and sensitive diagnostic and prognostic test.
Biomarkers are an appealing research target because their rapid quantification has the potential to diagnose disease, predict prognosis and guide early therapeutic interventions. Although the Surviving Sepsis Committee (SSC) highlights the potential value of a biomarker for aiding in the prognosis and diagnosis of sepsis, they do not provide any recommendations for a biomarker when evaluating patients [8]. Identifying an ideal biomarker for sepsis is challenging due to the complex intersection between pro-coagulant, pro-inflammatory and anti-inflammatory mechanisms in sepsis pathology [9, 10]. Given the complex pathophysiology of sepsis, it can be theorized that the optimal biomarker would play a role in multiple septic pathways.
One example of a such a biomarker is protein C (PC), the zymogen of activated protein C (aPC). aPC is a vitamin K-dependent glycoprotein that circulates through the blood plasma [11]. Primarily known as an anticoagulant, aPC negatively regulates the coagulation cascade by preventing fibrin formation, and platelet and coagulation cofactor activation through inhibition of Factor V and VIII [12]. It also contributes to anti-inflammation through upregulation of anti-inflammatory mediators, control of danger-associated molecular patterns and downregulation of leukocyte adhesion and migration [13, 14]. In sepsis, dysregulation of PC contributes to excessive thrombosis and inflammation [11]. Previous research has demonstrated that septic patients display a reduction in endogenous PC levels [15]. This is attributed to increased consumption, decreased protein synthesis in the liver and proteolytic degradation by neutrophil elastase [16].
Given PC’s involvement in sepsis pathology, recent investigations have focused on PC’s utility as a prognostic and diagnostic biomarker in patients with sepsis. To date, the use of PC as a biomarker for sepsis has only been reported in individual clinical studies. Clinicians caring for septic patients require a comprehensive evaluation of PC’s utility as a biomarker for sepsis to inform evidence-based practices.
Objective
This systematic review and meta-analysis will synthesize existing knowledge and evaluate PC’s utility as a prognostic and diagnostic biomarker in adult sepsis patients.
Methods
Protocol/registration
This systematic review and meta-analysis was performed in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [17]. The protocol of this systematic review has been published in BMJ Open [18]. The systematic review was registered in PROSPERO (CRD42021229786).
Eligibility criteria
We included prospective observational studies that examined one of the following: (1) PC as a diagnostic marker of sepsis or sepsis-induced DIC or (2) PC as a prognostic marker for sepsis-related mortality. Eligible participants were male and female adults (> 17 years of age) with sepsis (including severe sepsis, septic shock or sepsis with DIC) or suspicion of sepsis. Studies enrolling patients with any of the three consensus definitions were included [1, 19, 20]. Only studies measuring PC in the blood of adult sepsis patients within 24 h of study enrollment were included (Table 1).
We excluded retrospective observational studies, abstracts, editorials, poster presentations and non-English studies. Studies on pediatric populations or animal studies were also excluded. We also excluded studies for which there were insufficient data to examine one of the following two: (1) mean PC biomarker levels or (2) the sensitivity, specificity and area under the receiver operating curve (AU-ROC) of PC.
Information source and search strategy
We initially searched PubMed, EMBASE, CINAHL, Medline and CENTRAL databases from their date of inception to January 20, 2021, and then reran our search September 12, 2021, to include any more recent studies. A full search strategy is detailed in Additional file 1 (See search strategy). The citations of included studies were screened to identify additional studies for inclusion.
Study selection
We screened studies in a two-step process on Covidence: first by title and abstract, and then by full text, according to the defined inclusion/exclusion criteria. All articles were screened independently in duplicate (VC & KP), and disagreements regarding the eligibility of studies were resolved by discussion or consultation with a third reviewer (FS). We also screened the references of included studies for additional studies.
Data extraction and management
Two reviewers (VC and KP) extracted study data independently and in duplicate using a standardized data abstraction table, created in Excel. Study authors were contacted for further information if necessary. Any disagreements between reviewers during the extraction were resolved by discussion or consultation with a third reviewer (FS). The following information was extracted from published articles and the corresponding supplemental material: (1) bibliographic details: first author, publication year, study setting, type of study (prognostic or diagnostic) and country; (2) demographic and clinical information: study size, mean age, sepsis definition used, patient population description (severity of sepsis), mortality proportion and follow-up duration for mortality; (3) protein C measurement: time point of measurement and protein C assay used; and (4) study outcomes: Mean biomarker levels, PC threshold values, area under receiver operating curve (AU-ROC), sensitivity, specificity, positive and negative predictive values.
Assessment of risk of bias
Two reviewers independently performed quality assessments (VC and KP). Agreement between the two reviewers for the assessment of methodological quality was evaluated using Cohen’s kappa statistic. The Quality in Prognostic Studies (QUIPS) tool and the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool were used for studies evaluating the prognostics and diagnostic outcome, respectively [21, 22]. Any discrepancies were resolved through discussion with a third author (FS). For studies with insufficient information to make a category decision, the RoB was classified as unclear.
Data analysis
All statistical analyses were performed using RevMan V5.4.1 [23]. We present study and summary estimates as standard mean difference (SMD) for continuous outcomes with 95% confidence intervals. If studies reported mean and standard error, the SD was computed using the formula provided by the Cochrane collaboration [24]. If mean biomarker data were not reported, we used the method by Wan et al. to estimate the mean and SD using the median and interquartile range (IQR) or median and range [25]. Heterogeneity was assessed using visual inspection of forest plots, the I2 statistic and the Chi-squared test for homogeneity (significance at p < 0.05). Meta-analyses were performed using fixed effects models if there was no significant heterogeneity (I2 < 50%); otherwise, a random effects model was used.
The following outcomes were pooled for meta-analysis: (1) SMD in PC levels in sepsis survivors and non-survivors; and (2) SMD in PC levels in sepsis patients with and without DIC. PC differences in septic vs. non-septic patients could not be synthesized for meta-analysis due to heterogeneous control groups. A p value of < 0.05 was considered significant.
We planned to conduct H-SROC analysis to evaluate sensitivity and specificity for each outcome [19]; however, a limited number of studies reported the data needed for this analysis. Instead, AUC, sensitivity, specificity, positive predictive value (PPV) and/or negative predictive value (NPV) values reported in the included studies were summarized.
Subgroup and sensitivity analysis
We performed subgroup analysis on the prognostic outcome to examine 28-day sepsis-related mortality. We planned to conduct subgroup analysis to evaluate only the studies using the recent Sepsis-3 definition; however, insufficient studies were available for this analysis. Many studies began before 2016 and the implementation of the Sepsis-3 definition, and therefore relied on Sepsis-1 and Sepsis-2 definitions for study enrollment.
Sensitivity analysis was performed for our prognostic outcome to assess the effect of removing studies with high RoB. Studies evaluated by QUIPS were determined to be high RoB if they had a “high” distinction for any category or two “moderate” distinctions. Sensitivity analysis was not performed for our diagnostic outcome evaluating the difference in protein C levels between septic patients with and without DIC, as all studies included in meta-analysis had been designated as high RoB.
Assessment of certainty of the evidence
We assessed the certainty of the evidence for pooled outcomes according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology [26]. Assessments were made independently and in duplicate with a third reviewer to resolve conflicts.
Results
Study selection
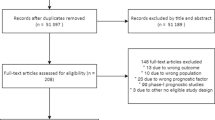
The systematic literature search returned 706 articles, with 455 original articles after the removal of duplicates. Figure 1 shows the study selection process. After initial title and abstract and full-text screening, 9 studies were eligible for analysis. In addition, 5 potential articles were identified through citation screening, 3 of which were eligible for inclusion in the study. In total, 12 articles were included in our systematic review [27,28,29,30,31,32,33,34,35,36,37,38], and 8 articles were combined for meta-analysis [27,28,29,30,31,32,33,34,35, 38]. The remaining 4 articles were not included in the meta-analysis due to limited available data; however, the study characteristics and available results have been reported in table format [28, 32, 36, 37].
Study/patient characteristics
Twelve prospective observational studies with a total of 2471 patients were included. Study characteristics are presented in Table 2. All twelve studies were prospective observational and published between 1993 and 2021. Five studies were conducted in North America [28, 31, 33, 35, 37], four in Eastern Asia [27, 29, 32] and three in Europe [30, 34, 38]. The number of participants across studies ranged from 48 to 971 [31, 34].
Protein C levels were measured using two different biochemical techniques. Five studies used antibody-based techniques, such as ELISAs [28, 31, 33, 35, 37], while the remainder of studies measured PC using functional clotting assays [27, 29, 30, 32, 35, 36]. All studies reported their PC measurements as a percentage of healthy PC levels, with the exception of Shapiro et al., which reported PC measurements in ug/mL [37]. Of the studies included, 3 examined our prognostic outcomes [30, 31, 33], 3 examined our diagnostic outcomes [27,28,29], and the remaining 6 studies examined both outcomes simultaneously [32, 34,35,36,37,38].
The patient characteristics within each study are shown in Table 3. The mean age of the patients varied from 54.9 to 71.7 [27, 37]. SOFA score was reported in 7 studies and ranged from 5.73 to 8.44 [30, 33]. Most studies did not investigate the source of the infection, but those that did identified the lungs, abdomen and urinary tract to be the most common sources of infection [31,32,33, 36,37,38]. Finally, the mortality within each study ranged widely, from 7% to 52.1% [34, 37].
Risk of bias
QUIPS was used to evaluate prognostic studies, while QUADAS-2 was used to evaluate diagnostic studies. The inter-rater agreement for QUIPS and QUADAS-2 was κ = 0.74 and κ = 0.68, respectively. The overall results of quality assessment are displayed in Figs. 2 and 3.
QUIPS
We evaluated 8 prognostic studies using QUIPS. Study participation and prognostic factor (PF) measurement bias was identified as a concern in > 50% of the papers (Fig. 2). Many studies failed to specify whether they followed consecutive or random enrollment, reported no exclusion criteria or were missing information on the time period and location of recruitment. For PF measurement, risk of bias occurred due to missing measurements of PC, data-dependent calculations of sensitivity/specificity and partial reporting of PF information. For study attrition and confounding measurement, > 3 studies had bias concerns. This was due to failure to clarify why some biomarker samples were not taken after enrollment and failure to account for confounding concerns in their study exclusion criteria. Risk of bias for each outcome with justification is found in Additional file 1 (Table S1).
QUADAS-2
Eight studies with diagnostic outcomes were evaluated using QUADAS-2. For the patient selection domain, 50% of studies were scored as unclear as they did not provide enough information on their patient enrollment strategy, [27,28,29], while 3 studies scored high because they used a case–control mechanism when comparing septic patients to healthy control samples [34,35,36]. Healthy controls are not representative of the patient populations being assessed for sepsis; therefore, the difference in protein C levels between septic and non-septic patients may be overestimated. For the reference standard domain, all studies were marked as unclear as they did not report if the interpretation of the reference standard was blinded to the measurement of the index test. For flow and timing, 5 studies were given a high or unclear risk of bias due to their time intervals between reference and index standards [27, 34, 36,37,38] (Fig. 3).
All studies were marked as “low” RoB for reference test and index test applicability, as all studies used one of the gold standard sepsis consensus definitions and measured PC within 24 h of hospital admission. Further, 75% of studies scored low in concerns regarding applicability. The remaining two examined specific populations within sepsis (i.e., severe sepsis, septic shock), so there were concerns about the applicability of the studies to our research question. Risk of bias and applicability ratings for each outcome with justification are provided in Additional file 1 (Tables S2 & S3).
Summary of findings and GRADE certainty
The certainty of evidence is summarized in Additional file 1 (Tables S4 and S5).
PC biomarker levels in survivors versus non-survivors
Nine studies examined PC’s ability to predict sepsis-related mortality, with six reporting PC biomarker levels. Of these six studies, three used functional PC assays [30, 35, 37], while three used immunoassays [31, 33, 34]. There was a statistically significant difference between PC levels in survivors and non-survivors (751 patients, SMD 0.52 (95% CI 0.24–0.81), p = 0.0003). There was also significant heterogeneity between the studies (I2 = 55%, p = 0.05); therefore, a random effects model was used for analysis (Fig. 4a).
Forest plots of SMD in PC biomarker measurements in survivors vs non-survivors. Standardized mean difference (SMD) estimate favoring survivors indicates that normal PC levels favor survival in sepsis patients. A SMD of PC levels in septic survivors vs. non-survivors, B SMD of PC in survivors vs. non-survivors for 28-day mortality. C Sensitivity analysis conducted by removing high RoB studies
Five studies reported 28-day mortality. There was a statistically significant difference in PC levels between survivors and non-survivors at the 28-day mortality (657 patients, SMD 0.44 (95% CI 0.26–0.61), p < 0.00001). Further, the heterogeneity was reduced in this subgroup analysis (I2 = 0%. p = 0.70) (Fig. 4b).
Studies that had a high RoB or two moderate RoB designations using the QUIPS tool were removed for sensitivity analysis. This resulted in reduced heterogeneity (I2 = 0%. p = 0.47) and a statistically significant difference in PC levels between survivors and non-survivors (3 studies, 609 patients, SMD 0.44 (95% CI 0.25–0.63), p < 0.00001) (Fig. 4c).
AUC analysis of protein C as a predictor for mortality
All reported results including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+) and negative likelihood ratio (LR−) are presented in Table 4.
Differences in PC biomarker levels in septic versus non-septic patients
Five studies examined the difference between septic patients and their control populations (Additional file 1: Table S6). Three studies used functional PC assays [29, 35, 38] and two used immunoassays [34, 37]. These data could not be synthesized for meta-analysis due to the variability in control groups between the studies. Individually, each study demonstrated a higher PC biomarker level in control patients compared to septic patients, although these values varied widely between studies.
AUC analysis of protein C as a diagnostic predictor for sepsis
Only Ishikura et al. reported the sensitivity and specificity of PC as a diagnostic tool for sepsis (Sn = 78%, Sp = 81%) [29]. Any other results regarding the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+) and negative likelihood ratio (LR−) are presented in Additional file 1: Table S7.
Differences in PC biomarker levels in septic patients with and without DIC
Four studies examined PC’s diagnostic accuracy for the identification of sepsis-induced DIC. Two studies used a functional assay [32, 35], one used an immunoassay [28], and the last did not state their methodological technique [26]. There was a statistically significant difference between PC levels in septic patients with and without DIC (644 patients, SMD 0.97 (95% CI 0.62–1.32), p < 0.00001). There was heterogeneity within the meta-analysis (I2 = 67%, p = 0.03); therefore, a random effects model was used (Fig. 5). Further sensitivity analyses were not conducted, as each study had a high RoB designation in at least one QUADAS-2 category.
AUC analysis of protein C as a diagnostic predictor for sepsis-induced DIC
Three of the studies conducted ROC analysis, with the AUC values ranging from 0.67 to 0.86 [27, 28]. The results regarding sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+) and negative likelihood ratio (LR−) are presented in Table 5.
Discussion
Sepsis remains a global health concern and the lack of accurate diagnostic and prognostic criteria for sepsis remains a huge limitation in early sepsis treatment and risk management [39]. Although the Surviving Sepsis Campaign acknowledges the value of biomarkers for septic patient treatment, the 2021 updated guidelines provide no recommendation for the use of any biomarker in the prognosis or diagnosis of sepsis [3]. This systematic review and meta-analysis is the first comprehensive meta-analysis to date that assesses the diagnostic and prognostic accuracy of PC as a biomarker for sepsis. Using rigorous search criteria, this study identified only prospective observational studies to minimize RoB from study design. Further, studies were extensively evaluated using QUIPS and QUADAS-2, which were specifically developed to assess the RoB in prognostic and diagnostic studies, respectively. While we were unable to synthesize data examining PC was a diagnostic marker for sepsis due to study heterogeneity, we identified a large effect size with low certainty demonstrating PC levels are less reduced in septic patients without DIC compared to those with DIC. Further, the meta-analysis demonstrated a moderate effect size with moderate certainty for differences in PC levels within 24 h of sepsis diagnosis in survivors compared to non-survivors. Sensitivity and subgroup analyses failed to demonstrate that the quality of studies or primary outcome affected the prognostic value of PC. Overall, these results indicate that the sensitivity and specificity of protein C should be further investigated as a measure to guide clinical evaluations in identifying sepsis-related DIC and sepsis survival outcomes.
Dysregulation of protein C contributes to excessive thrombosis and inflammation seen in sepsis patients. The cytokine storm produced in early innate immune response decreases the production of negative acute-phase proteins, such as PC [40, 41]. PC levels decrease further due to proteolytic degradation by neutrophil elastase [16]. As sepsis progresses, protein C levels remain low due to impaired transcription, and aPC levels begin to decrease. The release of histones from neutrophil extracellular traps (NETs) impairs thrombin–thrombomodulin binding interaction, a requirement for PC activation [42]. Further, pro-inflammatory cytokines cause increases in soluble endothelial protein C receptor (sEPCR), which binds to PC and acts as a competitive inhibitor to its activation [43]. This reduction in PC and aPC over the course of sepsis causes excessive coagulation and inflammation. Given PC’s clear involvement in sepsis pathology, and limited current knowledge as a diagnostic biomarker, it warrants future in-depth investigation.
There are several limitations to our meta-analysis. We were unable to conduct H-SROC analysis as originally planned due to limited reporting on the AUC, sensitivity and specificity of PC as a prognostic or diagnostic biomarker. Further, we were unable to evaluate heterogeneity by conducting subgroup analysis based on sepsis definition, geographical location or decade of study publication due to a limited number of studies. The inability to analyze studies that use only the most recent Sepsis-3 definition highlights the need for future studies evaluating PC’s effectiveness as a biomarker. In addition, our study quality assessment demonstrated high RoB in many of the studies included. This was largely due to underreporting of information on patient selection, lack of index text blinding, reporting of AUC values that were based on data-dependent cutoffs and missing patient biomarker samples. Finally, this study identified that papers generally use one of two ways to measure protein C: immunoassays to evaluate PC antigen levels and coagulation assays to measure PC functional activity levels. We chose to combine all studies for meta-analysis regardless of the assay used to measure PC, as they all presented their PC measurements as a % of healthy controls. However, this should be regarded with caution, and future studies should make a clearer distinction the method of protein C measurement used.
Despite these limitations, the results demonstrated that protein C measurements offer guidance as an indicator of the presence of sepsis-induced DIC and risk of mortality. Further, this meta-analysis helps to inform future researchers of the potential of PC as a part of a multi-biomarker panel for sepsis prognosis. Going forward, studies are needed to evaluate whether PC can be used as a biomarker in early diagnostic settings, such as the emergency department [44]. Further, future studies must include more robust data on the sensitivity, specificity, PPV and NPV of PC as a biomarker to ascertain the clinical utility of PC for sepsis prognosis and/or diagnosis.
Conclusion
Our systematic review and meta-analysis of current literature suggests that PC levels are less reduced in sepsis survivors compared to non-survivors and in septic patients without DIC compared to those with DIC. There are insufficient data in the current literature to determine whether PC levels are different in septic vs. non-septic patients. More robust studies on the sensitivity and specificity of PC as a biomarker are needed to determine clinical significance of this biomarker as a prognostic or diagnostic tool for sepsis.
Availability of data and materials
All data generated and/or analyzed during the current study are included within the published article and its additional files.
Abbreviations
- AU-ROC:
-
Area under the receiver operating characteristic
- CI:
-
Confidence interval
- CINAHL:
-
Cumulative Index to Nursing and Allied Health Literature
- DIC:
-
Disseminated intravascular coagulation
- ED:
-
Emergency department
- ELISA:
-
Enzyme-linked immunoassay
- GRADE:
-
Grading of Recommendations, Assessment, Development and Evaluation
- HSROC:
-
Hierarchical summary receiver operating characteristic
- ICU:
-
Intensive care unit
- IDC:
-
Infectious disease center
- LR+:
-
Positive likelihood ratio
- LR−:
-
Negative likelihood ratio
- MC:
-
Multicenter
- NPV:
-
Negative predictive value
- PC:
-
Protein C
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PPV:
-
Positive predictive value
- RoB:
-
Risk of bias
- SC:
-
Single center
- SD:
-
Standard deviation
- SMD:
-
Standardized mean difference
- Sn:
-
Sensitivity
- SOFA:
-
Sequential organ failure assessment scale
- Sp:
-
Specificity
- Q1:
-
Quartile 1
- Q3:
-
Quartile 3
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet. 2020;395(10219):200–11.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. Intensive Care Med. 2021;2021:1–67.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–77.
Leligdowicz A, Matthay MA. Heterogeneity in sepsis: new biological evidence with clinical applications. Crit Care. 2019;23(1):80.
Gaini S, Relster MM, Pedersen C, Johansen IS. Prediction of 28-days mortality with sequential organ failure assessment (SOFA), quick SOFA (qSOFA) and systemic inflammatory response syndrome (SIRS)—a retrospective study of medical patients with acute infectious disease. Int J Infect Dis. 2019;78:1–7.
April MD, Aguirre J, Tannenbaum LI, Moore T, Pingree A, Thaxton RE, et al. Sepsis clinical criteria in emergency department patients admitted to an intensive care unit: an external validation study of quick sequential organ failure assessment. J Emerg Med. 2017;52(5):622–31.
Williams JM, Greenslade JH, McKenzie JV, Chu K, Brown AFT, Lipman J. Systemic inflammatory response syndrome, quick sequential organ function assessment, and organ dysfunction: insights from a prospective database of ED patients with infection. Chest. 2017;151(3):586–96.
Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38(4):709–25.
Toltl LJ, Beaudin S, Liaw PC, Canadian Critical Care Translational Biology Group. Activated protein C up-regulates IL-10 and inhibits tissue factor in blood monocytes. J Immunol. 2008;181(3):2165–73.
Danese S, Vetrano S, Zhang L, Poplis VA, Castellino FJ. The protein C pathway in tissue inflammation and injury: pathogenic role and therapeutic implications. Blood. 2010;115(6):1121–30.
Dahlbäck B, Villoutreix BO. The anticoagulant protein C pathway. FEBS Lett. 2005;579(15):3310–6.
Sturn DH, Kaneider NC, Feistritzer C, Djanani A, Fukudome K, Wiedermann CJ. Expression and function of the endothelial protein C receptor in human neutrophils. Blood. 2003;102(4):1499–505.
Frommhold D, Tschada J, Braach N, Buschmann K, Doerner A, Pflaum J, et al. Protein C concentrate controls leukocyte recruitment during inflammation and improves survival during endotoxemia after efficient in vivo activation. Am J Pathol. 2011;179(5):2637–50.
Shorr AF, Bernard GR, Dhainaut J-F, Russell JR, Macias WL, Nelson DR, et al. Protein C concentrations in severe sepsis: an early directional change in plasma levels predicts outcome. Crit Care. 2006;10(3):R92.
Liaw PCY. Endogenous protein C activation in patients with severe sepsis. Crit Care Med. 2004;32(5 Suppl):S214-218.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Catenacci V, Sheikh F, Patel K, Fox-Robichaud A. Diagnostic and prognostic accuracy of Protein C in adult patients with sepsis: protocol for a systematic review and meta-analysis. BMJ Open. 2021;11(9):e050754.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med. 2003;29(4):530–8.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55.
Grooten WJA, Tseli E, Äng BO, Boersma K, Stålnacke B-M, Gerdle B, et al. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS—aspects of interrater agreement. Diagn Progn Res. 2019;3(1):1–11.
Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
RevMan [Internet]. [cited 2021 Aug 30]. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley; 2019. p. 726.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
Schünemann, Holger, Bròzek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group [Internet]. 2013. www.guidelinedevelopment.org/handbook.
Masuda T, Shoko T. Clinical investigation of the utility of a pair of coagulation-fibrinolysis markers for definite diagnosis of sepsis-induced disseminated intravascular coagulation: a single-center, diagnostic, prospective, observational study. Thromb Res. 2020;192:116–21.
Jackson Chornenki NL, Dwivedi DJ, Kwong AC, Zamir N, Fox-Robichaud AE, Liaw PC, et al. Identification of hemostatic markers that define the pre-DIC state: a multi-center observational study. J Thromb Haemost. 2020;18(10):2524–31.
Ishikura H, Nishida T, Murai A, Nakamura Y, Irie Y, Tanaka J, et al. New diagnostic strategy for sepsis-induced disseminated intravascular coagulation: a prospective single-center observational study. Crit Care (London, England). 2014;18(1):R19.
Mihajlovic D, Brkic S, Lendak D, Novakov Mikic A, Draskovic B, Mitic G. Endogenous thrombin potential as marker of procoagulant response that can be useful in early stage of sepsis. Blood Coagul Fibrinol Int J Haemost Thrombsis. 2017;28(6):460–7.
Dwivedi DJ, Toltl LJ, Swystun LL, Pogue J, Liaw K-L, Weitz JI, et al. Prognostic utility and characterization of cell-free DNA in patients with severe sepsis. Crit Care. 2012;16(4):R151.
Umemura Y, Yamakawa K, Kiguchi T, Yoshikawa Y, Ogura H, Shimazu T, et al. Design and evaluation of new unified criteria for disseminated intravascular coagulation based on the Japanese Association for Acute Medicine Criteria. Clin Appl Thromb Hemost. 2016;22(2):153–60.
Liaw PC, Fox-Robichaud AE, Liaw K-L, McDonald E, Dwivedi DJ, Zamir NM, et al. Mortality risk profiles for sepsis: a novel longitudinal and multivariable approach. Crit Care Explor. 2019;1(8):e0032.
Lorente JA, García-Frade LJ, Landín L, de Pablo R, Torrado C, Renes E, et al. Time course of hemostatic abnormalities in sepsis and its relation to outcome. Chest. 1993;103(5):1536–42.
Walborn A, Rondina M, Fareed J, Hoppensteadt D. Development of an algorithm to predict mortality in patients with sepsis and coagulopathy. Clin Appl Thromb Hemost. 2020;26:1076029620902849.
Koyama K, Madoiwa S, Nunomiya S, Koinuma T, Wada M, Sakata A, et al. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Crit Care (London, England). 2014;18(1):R13.
Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, Osborn TM, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med. 2009;37(1):96–104.
Karamarkovic A, Radenkovic D, Milic N, Bumbasirevic V, Stefanovic B. Protein C as an early marker of severe septic complications in diffuse secondary peritonitis. World J Surg. 2005;29(6):759–65.
Farrah K, McIntyre L, Doig CJ, Talarico R, Taljaard M, Krahn M, et al. Sepsis-associated mortality, resource use, and healthcare costs: a propensity-matched cohort study*. Crit Care Med. 2021;49(2):215–27.
Dhainaut J-F, Marin N, Mignon A, Vinsonneau C. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med. 2001;29(7):S42.
Vary TC, Kimball SR. Regulation of hepatic protein synthesis in chronic inflammation and sepsis. Am J Physiol. 1992;262(2 Pt 1):C445-452.
Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9(9):1795–803.
Menschikowski M, Hagelgans A, Eisenhofer G, Tiebel O, Siegert G. Reducing agents induce thrombomodulin shedding in human endothelial cells. Thromb Res. 2010;126(2):e88-93.
Arora J, Klowak JA, Parpia S, Zapata-Canivilo M, Faidi W, Skappak C, et al. Diagnostic potential of coagulation-related biomarkers for sepsis in the emergency department: protocol for a pilot observational cohort study. Crit Care Explor. 2021;3(4):e0414.
Acknowledgements
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
VC and FS contributed to the conception and design of this review, VC and KP performed the literature screening, VC and KP extracted the data and performed the quality assessment, VC and FS interpreted and synthesized the data, VC drafted the manuscript, VC, FS, KP, AFR critically revised the manuscript, and AFR provided general supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
AFR has a patent pending for PC as a biomarker in combination with other biomarkers for sepsis prognosis. All other authors have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Search strategy and Table S1–S7. The prognostic utility of protein C as a biomarker for adult sepsis: a systematic review and meta-analysis - Additional data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Catenacci, V., Sheikh, F., Patel, K. et al. The prognostic utility of protein C as a biomarker for adult sepsis: a systematic review and meta-analysis. Crit Care 26, 21 (2022). https://doi.org/10.1186/s13054-022-03889-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-03889-2