Abstract
Objectives
To investigate current situation of minimal information implementation highlighted by minimal information for studies of extracellular vesicles 2018 (MISEV2018) guidelines, and explore technological advances towards mass production and functional modification in aesthetic, plastic and reconstructive surgery.
Methods
Original articles on extracellular vesicles (EVs) of adipose stem cells (ASCs) were identified. Statistics upon minimal information for EVs research, such as species, cell types, culture conditions, conditioned media harvesting parameters, EVs isolation/storage/identification/quantification, functional uptake and working concentration, were analyzed.
Results
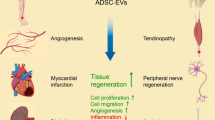
The items of cell culture conditions such as passage number, seeding density, conditioned media harvesting time, functional uptake and working concentration were poorly documented, with a reporting percentage of 47.13%, 54.02%, 29.89%, 62.07% and 36.21%, respectively. However, there were some studies not reporting information of ASCs origin, culture medium, serum, EVs isolation methods, quantification and identification of EVs, accounting for 3.45%, 10.34%, 6.90%, 3.45%, 18.39% and 4.02%, respectively. Serum deprivation and trophic factors stimuli were attempted for EVs mass production. Several technological advances towards functional modification included hypoxia pre-condition, engineering EVs and controlled release. Presently, ASCs EVs have been applied in multiple fields, including diabetic/non-diabetic wound healing, angiogenesis, inflammation modulation, fat grafting, hair regeneration, antiaging, and healing and regeneration of cartilage/bone/peripheral nerve/tendon.
Conclusion
Our results highlight normative reporting of ASCs EVs in functional studies to increase reliability and reproducibility of scientific publications. The advances towards mass production and functional modification of ASCs EVs are also recommended to enhance therapeutic effects.
Similar content being viewed by others
Introduction
Adipose stem cells (ASCs) isolated from adipose tissues have emerged as a promising therapy for the healing of multiple tissues, such as wound healing [1], fat grafting [2], skin rejuvenation [3], cartilage [4] and bone regeneration [5]. The paracrine effect of ASCs is partly attributed to the extracellular vesicles (EVs) secretion. As a cell-free therapy, stem cell-derived EVs-associated intercellular communication has been widely studied for promoting regeneration and reconstruction of multiple tissues such as tendon [6] and bone regeneration [7]. EVs is the generic term for several subtypes of particles naturally released from the native cells, such as “exosome”, “microparticle/microvesicle”, “ectosome”, “oncosome”, “apoptotic body” and many other names [8]. With a size of about 50–200 nm, exosome is a subset of endosome-origin small EVs, known as a heterogeneous mixture of microRNA-assembled, protein-decorated and lipid-bound nanoparticles [9,10,11]. The last decades has witnessed a dramatically increasing number of scientific publications on ASCs EVs, opening new frontiers for a next-generation drug delivery platform in ASCs-based regenerative [10].
In 2018, the “minimal information for studies of extracellular vesicles 2018 (MISEV2018) guidelines” has sensitized researchers to follow normative outlines when reporting extracellular vesicles-associated studies [8]. However, some of the current scientific publications associated with ASCs EVs poorly followed these guidelines to clearly report minimal information, involving passage number [12], the name of culture medium [13], the source of species and adipose tissue [14], ASCs seeding density [15], conditioned media collection time [16] and working concentration [17], which would affect reliability and reproducibility of published results especially in the face of skepticism by researchers outside EVs. When translating EVs-therapy to clinical and industrial practices, the primary hurdle is the low yield. Several strategies, such as serum deprivation [18] and precondition of platelet-derived growth factor (PDGF) [19] have been used to stimulate ASCs EVs release. Another hurdle is the unsatisfactory therapeutic effects. Sometimes functional modification for EVs is necessary to enhance therapeutic roles, including but not limited to precondition of PDGF [19], hypoxia stimulus [20] and genetically engineered EVs through cell transfection [21] or electroporation [22]. It seems to be the productivity paradox between the remarkable advances in EVs research and the relatively slow growth of productivity.
In the wake of these hurdles, we carry out a systematic survey of scientific publications on ASCs EVs. We will critically discuss the status quo of minimal information implementation. Besides, we will outline the current technological advances towards mass production and functional modification for the potential off-on-shelf alternative to cell therapy. We also list the functional roles of ASCs EVs in the fields of aesthetic, plastic and reconstructive surgery.
Methods
Search strategy
We performed a systematic search in the PubMed, EMBASE and Cochrane Library databases involving ASCs EVs, without restrictions of language, publication year and publication status. A search strategy was generated using the following terms: “adipose stem cells,” “adipose stem cells,” “exosome,” and “extracellular vesicles”. We also reviewed reference lists of eligible studies and relevant reviews for additional articles. Those reviews, letters, comments, abstracts and publications irrelevant to ASCs EVs were excluded.
Study selection
Two authors (J.G.C. and T.Y.H.) independently reviewed titles and abstracts of identified records, and full texts of potentially useful studies were reviewed. We resolved any disagreements through discussion with another author (H.Y.J.), and based on consensus, included or excluded those studies that we have discussed. The study was organized based on investigation of the minimal information and functional roles in aesthetic, plastic and reconstructive surgery.
Results
Search results
A total of 173 pre-clinical and clinical studies [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183] between 2011 to 2021 were included for the statistical analysis of minimal information implementation, mass production, functional modification and functional roles in aesthetic, plastic and reconstructive surgery. The screening process is shown in Fig. 1.
ASCs culture parameters
There were 3.45% of studies not reporting ASCs origin. The top three reporting derived types of ASCs were “homo, rat and mouse” adipose tissues (Fig. 2A). There were 10.34% of studies not clearly reporting the types of ASCs culture medium. The top three types were DMEM, DMEM/F-12 and MEM (Fig. 2B). Of the studies reporting DMEM, only 20.00% disclosed the use of high-glucose or low-glucose. 6.90% of studies did not document the use of serum for harvesting conditioned medium. 50.00% of studies used serum-free medium or serum replacement medium while 24.71% used EVs-depleted serum. However, the remaining studies used native serum without process of EVs-depletion (Fig. 2C). Almost half of studies did not document the passage number for EVs isolation. The top five reporting passage number were passage 3, passage 3 to 5, passage 3 to 6, passage 2 and passage 4 (Fig. 2D). Notably, there were more than half of studies not reporting ASCs seeding density. 28.16% of studies preferred to report degree of ASCs confluency as seeding density. (Fig. 2E).
Technological advances towards mass production
All included studies performed 2D-cell culture platforms for EVs production, without reporting use of hyperflasks, roller bottles, or 3D culture methods (e.g. perfusion, fixed bed or spinner flasks). Several physical or chemical stimulation was tried in 51.15% of studies to optimize EVs production. Serum deprivation was mostly used, accounting for 50.00%. Only one study reported precondition of ASCs with platelet-derived growth factor (PDGF). A study has evidenced that ASCs EVs could be stored in the form of lyophilized powder that could be helpful for stable storage and subsequent large scale production. There were no studies reporting methods of low pH, heat shock, glucose deprivation, ethanol, or ultrasounds for mass production. (Fig. 3A).
The percentage (%) of minimal information for A mass production, B conditioned medium harvesting time, C EVs isolation methods, D EVs identification, E EVs morphology, F EVs size distribution, and G EVs protein markers. NR: percentage of “not reporting” minimal information. PDGF platelet-derived growth factor, UC ultracentrifugation, UF ultrafiltration, TEM transmission electron microscope, SEM scanning electron microscope, NTA nanoparticle tracking analysis, DLS dynamic light scattering, WB western blotting, FCM flow cytometry
Conditioned media harvesting parameters
29.89% of studies did not report the conditioned media harvesting time, but some of them document harvest in a cell confluence of 70% to 90%. The top three harvesting time were 48-h, 24-h and 72-h (Fig. 3B). Almost all studies chose to store the conditioned media at – 80 °C, or firstly isolated EVs and then stored it at – 80 °C.
EVs isolation
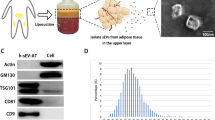
There were 3.45% of studies not reporting EVs isolation methods. The top five isolation techniques were differential ultracentrifugation (UC), ExoQuick-TC reagent from System Biosciences (SBI), ultrafiltration (UF), total EVs isolation kit from Invitrogen, and UC plus isolation kit (Fig. 3C).
EVs identification
There were 4.02% of studies not reporting the information of EVs identification. The reporting percentages in terms of morphology, size distribution and protein markers were 81.61%, 56.90%, and 82.18%, respectively (Fig. 3D). Transmission electron microscope (TEM) was mostly used for detecting morphology (Fig. 3E). The top three size assessment tools were nanoparticle tracking analysis (NTA), dynamic light scattering (DLS) and qNano devices (Fig. 3F). Protein markers were mostly identified by western blotting. Particularly, the top five reporting markers were CD9, CD63, CD81, TSG101 and HSP70/90. Flow cytometry or flow cytometry combined with western blotting were also used for protein markers identification (Fig. 3G).
Quantification, functional uptake through fluorescence labelling and working concentration of EVs
There were 18.39% of studies not reporting quantification of EVs. Most of studies quantified EVs using BCA protein assay while only 6.90% of studies only using NTA. (Fig. 4A) There were 62.07% of studies not reporting functional uptake assays. The remaining studies mainly used PKH26, PKH67 and Dil as fluorescence labelling dyes (Fig. 4B). 36.21% of studies did not report working concentration in functional studies. The working concentration generally used in in vitro and in vivo studies are shown at Fig. 4C, D.
The percentage of “not reporting” minimal information: before and after the publication of MISEV2018
We also conducted a comparison on the percentage of “not reporting” minimal information before and after the publication of MISEV2018. The results could be seen at Table 1. We found that the “not reporting” percentage of several parameters such as ASCs origin, isolation methods, EVs morphology/size/protein markers, EVs quantification and working concentration decreased to some extent after the publication of MISEV2018, indicating that MISEV2018 was favorable to promote the reporting of minimal information when performing ASCs EVs studies.
Technological advances towards functional modification
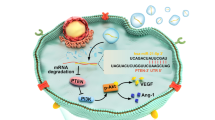
THE modified strategies for enhancing loading and targeted delivery of EVs have been reported. Engineering EVs were mostly carried out either via transfecting functional molecules into ASCs [14, 17, 21, 26, 34, 35, 44, 45, 49, 72, 77, 79, 82, 113, 125, 181] or directly transfecting functional molecules into EVs [22, 37, 142]. Six studies [20, 67, 85, 86, 102, 103] reported the strategy of hypoxia culture precondition of ASCs. These hypoxia-preconditioned ASCs EVs shown superiority in RNA sequencing and functional assays such as fat grafting survival, neovascularization, inflammation inhibition, extracellular matrix regeneration, and pro-metabolism/pro-survival abilities.
Biomaterials laden with EVs were a promising strategy for controlled EVs release, which was especially helpful for chronic wound healing and bone regeneration [50, 88, 139, 140, 144, 145]. Seven kinds of regenerative biomaterials laden with EVs have been reported, including polypeptide-based FHE hydrogel, antioxidant polyurethane, hyaluronic acid, thermosensitive multifunctional polysaccharide-based dressing, alginate-based hydrogel, biohybrid bovine bone matrix and human acellular amniotic membrane.
Another strategy was the targeted differentiation induction. EVs released from osteogenic or chondrogenic induction of ASCs could specifically promote osteogenesis or chondrogenesis differentiation of MSCs [104, 151, 160, 182]. ASCs EVs could be modified in bone healing and regeneration via giving a stimulus of TNF-α or low-level laser irradiation (LLLI) to parent ASCs [114, 152]. The anti-inflammatory and immunosuppressive functions of ASCs EVs could be modified via giving an inflammatory stimulus of IFNγ and TNFα [92]. Stimulus of Platelet-derived growth factor (PDGF) could triggered the EVs secretion from parent ASCs and enhanced the angiogenic potential [19]. ASCs EVs from lean volunteers even were different from those from obese individuals in terms of protein markers, size, contents of cargo and functional effects [11, 159]. Conclusive information on functional modification was shown at Table 2.
Systematic survey in aesthetic, plastic and reconstructive surgery
Diabetic/non-diabetic wound healing (n = 26)
ASCs EVs delivered functional molecules for non-diabetic/diabetic wound healing via enhancing skin collagen production/angiogenesis/cell proliferation/migration/expression of wound healing-related growth factors, inhibiting apoptosis, promoting skin barrier function repair, reducing inflammation and scar formation, as well as regulating extracellular matrix remodeling [12, 14, 15, 32, 40, 51, 53, 73, 87, 88, 108, 119, 138,139,140,141,142,143,144,145,146,147,148,149, 166, 175]. The underlying mechanisms of action were shown as follows. For the capacity of promoting diabetic wound healing, ASCs EVs have been reported to regulate several axes such as mmu_circ_0000250/miR-128-3p/SIRT1 axis [14] in endothelial progenitor cell, miR-21-5p/Wnt/β-catenin signaling in keratinocytes [142], or transferring transcription factor nuclear factor-E2-related factor 2 (Nrf2) to endothelial progenitor cells [143]. The healing capability of ASCs EVs in non-diabetic wound involved the modulation of multiple signaling, such as the lncRNA H19/miR-19b/SOX9 axis in human skin fibroblast (HSF) cell [12], and miR-19b/CCL1/TGF-β pathway axis [40], AKT/HIF‑1α axis [53], Wnt/β‐catenin signaling [87], lncRNA MALAT1/miR-124/Wnt/β-catenin axis [141], miR-21/PI3K/AKT axis in HaCaT cells [148], as well as ERK/MAPK pathway in skin dermal fibroblasts [108]. The functional modification of ASCs EVs went through several processes from the simple to the complex. Initially, ASCs EVs without modification could be used topically or systemically. Then, a variety of regenerative biomaterials built up the concept of controlled EVs release, effectively matching with the complicated and long healing process of chronic wound [88, 139, 140, 144, 145]. Engineered EVs were another direction of achieving gene therapy by loading functional non-code RNA into the patent ASCs or EVs [14, 142].
Other skin diseases and medical cosmetology (n = 12)
The pre-clinical studies indicated that ASCs EVs could promote epidermal barrier repair on the treatment of atopic dermatitis via increasing stratum corneum hydration, reducing the levels of multiple inflammatory cytokines, and enhancing de novo synthesis of ceramides [61, 97]. ASCs EVs could promote genes expression involved in skin barrier, lipid metabolism, cell cycle, and inflammatory response in the diseased area [61]. Only one study revealed that the intravenous injections of ASCs EVs could effectively slow-down the course of the systemic sclerosis via regulating miR-29a-3p/Dnmt3a/Pdgfrbb/Bcl2/Bcl-xl axis [35]. Two studies found that ASCs EVs could inhibit the proliferation/migration, and promote the apoptosis of keloid/hypertrophic scar fibroblasts via the regulation of miR-192-5p/IL-17RA/Smad axis [23] or inhibiting TGF-β1/Smad pathway [174]. Another two studies reported the essential roles of ASCs EVs in promoting the vascularization of skin flaps [163, 176], and one study found that ASCs EVs were comparable to parent ASCs in the inhibition of alloimmune response for vascularized composite allotransplantation [13]. Recently, ASCs EVs have been investigated in the antiaging of photoaged skin by increasing the mRNA expression of type I collagen, corresponding to the antiaging properties of parent ASCs [36].
Only three clinical studies have been reported in Korea for testing the therapeutic functions of hASCs EVs. Park et al. [16] tentatively applied ASCs EVs to the treatment of atopic dermatitis, and found that EVs could serve as an effective agent in the management of dupilumab facial redness. Two randomized controlled trials have indicated the safety and efficacy of hASCs EVs on the treatment of facial acne scars and skin brightening [136, 137].
Angiogenesis/inflammation/fat grafts/hair regeneration (n = 18)
ASCs EVs could promote angiogenesis mainly via transferring functional microRNAs to targeted cells [19, 56, 85, 103, 122, 168, 169, 173, 177, 180, 181]. The underlying mechanisms for angiogenesis potential of ASCs EVs were shown as follows. Platelet-derived growth factor pre-conditioned ASCs EVs could load c-kit, SCF and matrix metalloproteinases that played a role in angiogenesis [19]. EVs derived from hypoxia-treated hADSCs showed angiogenesis capacity in fat grafting probably via regulating VEGF/VEGF-R signaling [85] and PKA signaling [103]. micro-RNAs derived from ASCs EVs also played an important role in angiogenesis. ASCs EVs could promote angiogenesis of endothelial cells by regulating miR-125a/DLL4 axis [122], miR-181b-5p/TRPM7 axis [173], miR-199-3p/sema3A axis [177] or miR-21/PTEN/AKT/ERK/HIF-1α/SDF-1 axis [181]. Xu et al. [169] found miR-423-5p from ASCs EVs mediated the proangiogenic activity of hADSCs by targeting Sufu. EVs isolated from Sirtuin 1 (SIRT1)-overexpressing ASCs unregulated Nrf2/CXCL12/CXCR7 signaling and promoted migration and tube formation of endothelial progenitor cells [180].
ASCs EVs also showed potential in attenuating inflammation and immune reactions probably via transferring functional molecules such as miR-34a, miR-124 and miR-135b [41, 84, 92]. Evidence has shown that ASCs could promote the survival rate of fat grafting via EVs secretion [27, 76, 85, 102]. Hao et al. [27] found that ASCs EVs could downregulate the level of transcription factor CCAAT/enhancer-binding protein via transferring let-7c. Corresponding to the poor angiogenesis/hypoxia in the early phase of fat grafting, the hypoxia-preconditioned ASCs EVs were superior to ASCs EVs in neovascularization and inflammation attenuation [102]. Recently, Wu et al. [183] indicted that that ASCs EVs could increase terminal hairs regeneration via promoting the expression of PDGF and VEGF in skin tissues.
Cartilage and bone (n = 19)
A total of five studies investigated the functional roles of EVs from undifferentiated ASCs and chondrogenic ASCs in cartilage regeneration through modulating inflammation, promoting chondrocyte differentiation of ASCs, stimulating the migration/proliferation, and chondrogenic/osteogenic differentiation of BMSCs [29, 100, 105, 160, 161]. Zhao et al. [161] found that ADCs EVs could transfer miR‑145 and miR‑221 which could enhance cell proliferation and chondrogenic potential. In addition, proteomics analysis reveals that ASCs EVs could induce cartilage/bone regeneration probably by regulating signaling pathways including focal adhesion, ECM-receptor interaction, actin cytoskeleton, cAMP, and PI3K-Akt signaling pathways [29]. EVs LncRNA sequencing was also conducted to investigated the expression profile of lncRNAs, and several neighboring genes of differentially expressed lncRNAs that were involved in cartilage regenerations, such as TBX6, CHD4, and TRPV2 were identified [160].
A total of 14 studies have been published for investigating the functional effects on bone healing and tissue-engineered bone [50, 58, 63, 69, 75, 77, 90, 104, 114, 151, 152, 155, 156, 182]. ASCs EVs played an essential role of modulating functions of osteocytes and osteoclasts. Several studies have evidenced that ASCs EVs could be applied in the treatment of some bone damage-related pathologies such as diabetic osteoporosis, hypoxia/ischemia induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis [58, 90, 182]. The underlying mechanisms for these treatments could be attributed to inhibiting NLRP3 inflammasome activation in osteoclast [58], upregulating the radio of Bcl-2/Bax and diminishing the production of reactive oxygen species/cytochrome/caspase-9/caspase-3 [90]. Notably, Yang et al. [182] conducted EVs-miRNA sequencing in osteogenic differentiation of ADSCs and found some differentially expressed miRNAs connected osteogenic differentiation to processes such as axon guidance, MAPK signaling and Wnt signaling. In addition, ASCs could be pre-conditioned with tumor necrosis factor-alpha or low-level laser irradiation to mimic the inflammatory phase upon bone injury [114, 152].
Another essential role of ASCs EVs was to induce osteogenic differentiation, promote MSCs adhesion/migration/proliferation of MSCs via entrapping EVs on the surface of biohybrid bovine bone matrix [50], mineral-doped poly(L-lactide) acid scaffolds [63], or titanium [69]. The EVs proteome demonstrated that EVs carried proteins involving various integrins and integrin ligands, growth factors and growth factor receptors, as well as Wnts and MAPKs, which were related to adhesion, structure, morphology and GF activity [69]. In addition, Yang et al. [155] also found miR-130a-3p derived from ASCs EVs would regulate osteogenic differentiation of MSCs through mediating SIRT7/Wnt/β-catenin axis.
Engineered EVs s could also be designed specifically for osteogenic induction via altering expression of EVs-miRNAs. The simple methods were to induce the osteogenic differentiation of parent ASCs [104, 151, 182]. Other methods were directly loading specific miRNA such as miR‐375 into parent ASCs or EVs [77]. The EVs miR‐375 would inhibit insulin‐like growth factor binding protein 3 (IGFBP3) by binding to its 3′UTR and then improved the osteogenic differentiation of hBMSCs [77].
Peripheral nerve injury (n = 8)
A total of eight studies have evidenced that ASCs EVs could exert therapeutic effects for peripheral nerve injury via increasing neurite outgrowth, improving neurotransmission function, modulating proliferation/migration/myelination of Schwann cells, and increasing secretion of neurotrophic factors [81, 96, 98, 124, 164, 165, 171, 172]. Notably, Ching et al. [165] found that Schwann cell-like phenotype-differentiated ASCs EVs contained mRNAs and miRNAs known to play a role in nerve regeneration. These EVs RNA could be transferred to neurons and promoted neurite outgrowth via down-regulating intrinsic inhibitors of regeneration.
Discussion
The past decades have witnessed an upsurge in EVs research, primarily focusing on either disease markers or paracrine mediators for regenerative therapy. Every year, tremendous research funding is pouring into the preclinical and clinical studies of EVs. EVs even have been reported as potential regenerative cell-free medicine for COVID-19 treatments [184, 185]. Indeed, obstacles have concurrently emerged when launching the clinical and industrial translation of EVs [186]. We give three hurdles urgently needing to be solved: poor follow to MISEV2018 guidelines, low yield, and unsatisfactory functional effects.
The findings are summarized at Fig. 5. Overall, the principal finding of our study was that the current studies were poorly recording minimal information for EVs study. First, some studies did not or unclearly disclose the species and cell types for EVs secretion. The functional effects of EVs depended largely on their parent cells. Notably, EVs form different types of MSCs such ASCs, BMSCs, umbilical cord blood MSCs (UCB-MSCs), and endometrium-derived MSCs (EnMSCs) could play different roles in tissue healing and regeneration [30, 115]. Secondly, we found that several minimal information for cell culture conditions, such as passage number, cell seeding density, and conditioned media collection time, were also poorly reported. These parameters could affect the yield or biological functions of ASCs EVs. Previous study indicated that both increasing frequency of collection and decreasing cell seeding density could increase EVs production, while the passage number beyond passage 4 was less effective in pro-vascularization bioactivity [187].
Additionally, culture medium components, such as basal medium, serum, growth factors, glucose and antibiotics, were the essential influence factors deserved special attention. However, there were still some studies not reporting the kinds of medium they used. The traditional culture for ASCs is the DMEM. However, most of them did not report the information of low-glucose or high-glucose. DMEM for ASCs culture easily caused low proliferation rate, early cell senescence and multi-lineage differentiation loss that were not helpful for mass production of ASCs EVs. DMEM/F-12, MEM α, specific MSC serum free medium and MSCM could be used for solving these obstacles. Using serum free medium or EVs-depleted serum could reduce the influence of serum-derived EVs to the functional assays. However, we found that there some of studies using serum without EVs depletion. Overall, we highlight the necessity for careful consideration of cell culture parameters.
Almost all studies stored the conditioned media at − 80 °C, or firstly isolated EVs and then stored it at − 80 °C. Whether the long-term cryopreserved EVs is different from those freshly isolated in terms of morphology and function deserves special attention. ASCs EVs freeze-dried powder may be safe and a long-term storage alternative. After rehydration, ASCs EVs were still stable in the membrane morphology and components.
The obstacles to large-scale production and clinical translation of ASCs EVs are the inefficient isolation techniques along with the high costs and low purification. Ultracentrifugation still remains the gold standard to concentrate EVs, despite the defects of low yield and time consumption. The EVs isolation kit only could be used to isolate EVs from little conditioned medium. Ultrafiltration combined with ultracentrifugation could be translated to large-scale EVs manufacturing. Recently, the tangential flow filtration (TFF) and size exclusion chromatography (SEC) have been proposed as an effective concentration methods for large volumes of conditioned media [186]. Another strategy for large-scale manufacturing is to increase the number of secretion by stimulating ASCs. In our study, we found several methods for optimal production of ASCs EVs, such as hypoxia pre-condition, PDGF pre-condition and serum starvation.
Next, EVs identification via several complementary techniques, such as TEM, NTA and protein markers, is essential to quality control of EVs. However, there was still 4.02% of studies not reporting any identification methods. Only 56.90% of studies reported the size distribution while 81.61% of studies provided images of single EVs at high resolution. In addition, there were 82.18% of studies evaluated the protein markers mainly involving those transmembrane/lipid-bound protein and cytosolic protein. Actually, a study by Mathieu et al. [188] has evidenced that exosomes might specifically bear CD63 combined with some late endosome proteins but little CD9. Notably, our study found that there was 18.39% of publication not reporting the quantification of EVs. The BCA for total proteins yield was most used to reported EVs quantification.
Some preclinical and clinical studies were included in our systematic survey, involving one case series [16] and two randomized controlled trials [136, 137]. Overall, the current articles have given some therapeutic evidence for the functional roles of ASCs EVs in aesthetic, plastic and reconstructive surgery. In our study, we found three kinds of strategies could be used for optimizing the functional roles of ASCs EVs: engineering EVs, targeted precondition of parent ASCs and controlled EVs release.
We found several obstacles to the promotion of EVs research. Firstly, the functional roles were attributed to uptake of ASCs EVs by receipt cells rather than soluble non- EVs associated mediators from conditioned media. This was especially right when isolating EVs from polymer-based concentration kits. However, in our study, we found 62.07% of included studies did not reported any assays related to functional uptake. Besides, we found that there were 36.21% of included studies not reporting the working concentration. The clear reporting of working concentration undoubtedly increased the reliability and reproducibility of published results.
Conclusion
Our study highlights a normative reporting for EVs research, referring to MISEV2018 guidelines to increase robustness of results. Technological advances towards mass production and functional modification should be further improved for the translation of clinical practices and industrial manufacturing.
Availability of data and materials
Not applicable.
References
Burmeister DM, Stone R II, Wrice N, et al. Delivery of allogeneic adipose stem cells in polyethylene glycol-fibrin hydrogels as an adjunct to meshed autografts after sharp debridement of deep partial thickness burns. Stem Cells Transl Med. 2018;7(4):360–72.
Philips BJ, Grahovac TL, Valentin JE, et al. Prevalence of endogenous CD34+ adipose stem cells predicts human fat graft retention in a xenograft model. Plast Reconstr Surg. 2013;132(4):845–58.
Jo H, Brito S, Kwak BM, Park S, Lee MG, Bin BH. Applications of mesenchymal stem cells in skin regeneration and rejuvenation. Int J Mol Sci. 2021;22(5):2410.
Xie A, Peng Y, Yao Z, Lu L, Ni T. Effect of a subset of adipose-derived stem cells isolated with liposome magnetic beads to promote cartilage repair. J Cell Mol Med. 2021;25(9):4204–15.
Wang CC, Wang CH, Chen HC, et al. Combination of resveratrol-containing collagen with adipose stem cells for craniofacial tissue-engineering applications. Int Wound J. 2018;15(4):660–72.
Yao Z, Li J, Xiong H, et al. MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J Nanobiotechnol. 2021;19(1):169.
Li F, Wu J, Li D, et al. Engineering stem cells to produce exosomes with enhanced bone regeneration effects: an alternative strategy for gene therapy. J Nanobiotechnol. 2022;20(1):135.
Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750.
Xiong M, Zhang Q, Hu W, et al. Exosomes from adipose-derived stem cells: the emerging roles and applications in tissue regeneration of plastic and cosmetic surgery. Front Cell Dev Biol. 2020;8:574223.
Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–59.
Eirin A, Meng Y, Zhu XY, et al. The micro-RNA cargo of extracellular vesicles released by human adipose tissue-derived mesenchymal stem cells is modified by obesity. Front Cell Dev Biol. 2021;9:660851.
Qian L, Pi L, Fang BR, Meng XX. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab Invest. 2021. https://doi.org/10.1038/s41374-021-00611-8.
Chen Z, Xue S, Zhang S, Cheng K, Ye Q. Exosomes from donor-derived adipose mesenchymal stem cells prolong the survival of vascularized composite allografts. J Cell Physiol. 2021;236(8):5895–905.
Shi R, Jin Y, Hu W, et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020;318(5):C848–56.
Zhou Y, Zhao B, Zhang XL, et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 2021;12(1):257.
Park KY, Han HS, Park JW, Kwon HH, Park GH, Seo SJ. Exosomes derived from human adipose tissue-derived mesenchymal stem cells for the treatment of dupilumab-related facial redness in patients with atopic dermatitis: a report of two cases. J Cosmet Dermatol. 2021. https://doi.org/10.1111/jocd.14153.
Zhang C, Cao J, Lv W, Mou H. CircRNA_100395 Carried by exosomes from adipose-derived mesenchymal stem cells inhibits the malignant transformation of Non-small cell lung carcinoma through the miR-141-3p-LATS2 Axis. Front Cell Dev Biol. 2021;9:663147.
Conley SM, Shook JE, Zhu XY, et al. Metabolic syndrome induces release of smaller extracellular vesicles from porcine mesenchymal stem cells. Cell Transpl. 2019;28(9–10):1271–8.
Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;12:26.
Xia W, Chen H, Xie C, Hou M. Long-noncoding RNA MALAT1 sponges microRNA-92a-3p to inhibit doxorubicin-induced cardiac senescence by targeting ATG4a. Aging (Albany NY). 2020;12(9):8241–60.
Zhao Y, Tao M, Wei M, Du S, Wang H, Wang X. Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif Cells Nanomed Biotechnol. 2019;47(1):3804–13.
Bolandi Z, Mokhberian N, Eftekhary M, et al. Adipose derived mesenchymal stem cell exosomes loaded with miR-10a promote the differentiation of Th17 and Treg from naive CD4+ T cell. Life Sci. 2020;259:118218.
Li Y, Zhang J, Shi J, Liu K, Wang X, Jia Y, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12(1):221.
Heidari N, Abbasi-Kenarsari H, Namaki S, Baghaei K, Zali MR, Ghaffari Khaligh S, et al. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021;236(8):5906–20.
Yu C, Tang W, Lu R, Tao Y, Ren T, Gao Y. Human adipose-derived mesenchymal stem cells promote lymphocyte apoptosis and alleviate atherosclerosis via miR-125b-1-3p/BCL11B signal axis. Ann Palliat Med. 2021;10(2):2123–33.
Li Q, Wang Z, Xing H, Wang Y, Guo Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol Ther Nucleic Acids. 2021;23:1334–44.
Hao X, Guo Y, Wang R, Yu X, He L, Shu M. Exosomes from adipose-derived mesenchymal stem cells promote survival of fat grafts by regulating macrophage polarization via let-7c. Acta Biochim Biophys Sin (Shanghai). 2021;53(4):501–10.
Guillén MI, Compañ A, Alcaraz MJ. Evaluation of tissue-derived mesenchymal stem cells in primary human chondrocytes from patients with osteoarthritis. Methods Mol Biol. 2021;2269:221–31.
Li Q, Yu H, Sun M, Yang P, Hu X, Ao Y, et al. The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 2021;125:253–66.
Liu B, Qiao G, Cao W, Li CH, Pan SH, Wang L, et al. Proteomics analyses reveal functional differences between exosomes of mesenchymal stem cells derived from the umbilical cord and those derived from the adipose tissue. Cell J. 2021;23(1):75–84.
Liu M, Yang Y, Zhao B, Yang Y, Wang J, Shen K, et al. Exosomes derived from adipose-derived mesenchymal stem cells ameliorate radiation-induced brain injury by activating the SIRT1 pathway. Front Cell Dev Biol. 2021;9:693782.
Heo JS, Kim S, Yang CE, Choi Y, Song SY, Kim HO. Human adipose mesenchymal stem cell-derived exosomes: A key player in wound healing. Tissue Eng Regen Med. 2021;18(4):537–48.
Villatoro AJ, Martín-Astorga MDC, Alcoholado C, Sánchez-Martín MDM, Becerra J. Proteomic analysis of the secretome and exosomes of feline adipose-derived mesenchymal stem cells. Animals (Basel). 2021;11(2):295.
Liang L, Zheng D, Lu C, et al. Exosomes derived from miR-301a-3p-overexpressing adipose-derived mesenchymal stem cells reverse hypoxia-induced erectile dysfunction in rat models. Stem Cell Res Ther. 2021;12(1):87.
Rozier P, Maumus M, Maria ATJ, et al. Mesenchymal stromal cells-derived extracellular vesicles alleviate systemic sclerosis via miR-29a-3p. J Autoimmun. 2021;121:102660.
Liang JX, Liao X, Li SH, et al. Antiaging properties of exosomes from adipose-derived mesenchymal stem cells in photoaged rat skin. Biomed Res Int. 2020;2020:6406395.
Shojaei S, Hashemi SM, Ghanbarian H, Sharifi K, Salehi M, Mohammadi-Yeganeh S. Delivery of miR-381-3p mimic by mesenchymal stem cell-derived exosomes inhibits triple negative breast cancer aggressiveness: an in vitro study. Stem Cell Rev Rep. 2021;17(3):1027–38.
Mohd Ali N, Yeap SK, Ho WY, et al. Adipose MSCs suppress MCF7 and MDA-MB-231 breast cancer metastasis and EMT pathways leading to dormancy via exosomal-miRNAs following co-culture interaction. Pharmaceuticals (Basel). 2020;14(1):8.
González-Cubero E, González-Fernández ML, Gutiérrez-Velasco L, Navarro-Ramírez E, Villar-Suárez V. Isolation and characterization of exosomes from adipose tissue-derived mesenchymal stem cells. J Anat. 2021;238(5):1203–17.
Cao G, Chen B, Zhang X, Chen H. Human adipose-derived mesenchymal stem cells-derived exosomal microRNA-19b promotes the healing of skin wounds through modulation of the CCL1/TGF-β signaling axis. Clin Cosmet Investig Dermatol. 2020;13:957–71.
Heo JS, Lim JY, Yoon DW, Pyo S, Kim J. Exosome and melatonin additively attenuates inflammation by transferring miR-34a, miR-124, and miR-135b. Biomed Res Int. 2020;2020:1621394.
Wang ZG, He ZY, Liang S, Yang Q, Cheng P, Chen AM. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):511.
Mizuta Y, Akahoshi T, Guo J, et al. Exosomes from adipose tissue-derived mesenchymal stem cells ameliorate histone-induced acute lung injury by activating the PI3K/Akt pathway in endothelial cells. Stem Cell Res Ther. 2020;11(1):508.
Mao HY, Liu LN, Hu YM. Mesenchymal stem cells-derived exosomal miRNA-28-3p promotes apoptosis of pulmonary endothelial cells in pulmonary embolism. Eur Rev Med Pharmacol Sci. 2020;24(20):10619–31.
Xu L, Ji H, Jiang Y, et al. Exosomes derived from circAkap7-modified adipose-derived mesenchymal stem cells protect against cerebral ischemic injury. Front Cell Dev Biol. 2020;8:569977.
Ishiy CSRA, Ormanji MS, Maquigussa E, Ribeiro RS, da Silva NA, Boim MA. Comparison of the effects of mesenchymal stem cells with their extracellular vesicles on the treatment of kidney damage induced by chronic renal artery stenosis. Stem Cells Int. 2020;2020:8814574.
Chen Y, Li J, Ma B, et al. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging (Albany NY). 2020;12(18):18274–96.
Weiss C, Kornicka-Grabowska K, Mularczyk M, Siwinska N, Marycz K. Extracellular microvesicles (MV’s) isolated from 5-azacytidine-and-resveratrol-treated cells improve viability and ameliorate endoplasmic reticulum stress in metabolic syndrome derived mesenchymal stem cells. Stem Cell Rev Rep. 2020;16(6):1343–55.
Chen L, Wang Y, Li S, et al. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics. 2020;10(20):9425–42.
Bari E, Roato I, Perale G, et al. Biohybrid bovine bone matrix for controlled release of mesenchymal stem/stromal cell lyosecretome: a device for bone regeneration. Int J Mol Sci. 2021;22(8):4064.
Hoang DH, Nguyen TD, Nguyen HP, et al. Differential wound healing capacity of mesenchymal stem cell-derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum- and xeno-free condition. Front Mol Biosci. 2020;7:119.
Zhu M, Liu X, Li W, Wang L. Exosomes derived from mmu_circ_0000623-modified ADSCs prevent liver fibrosis via activating autophagy. Hum Exp Toxicol. 2020;39(12):1619–27.
Zhang Y, Han F, Gu L, et al. Adipose mesenchymal stem cell exosomes promote wound healing through accelerated keratinocyte migration and proliferation by activating the AKT/HIF-1α axis. J Mol Histol. 2020;51(4):375–83.
Wang Y, Chu Y, Li K, et al. Exosomes secreted by adipose-derived mesenchymal stem cells foster metastasis and osteosarcoma proliferation by increasing COLGALT2 expression. Front Cell Dev Biol. 2020;8:353.
Ha DH, Kim SD, Lee J, et al. Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul Toxicol Pharmacol. 2020;115:104686.
Cunnane EM, Lorentz KL, Ramaswamy AK, et al. Extracellular vesicles enhance the remodeling of cell-free silk vascular scaffolds in rat aortae. ACS Appl Mater Interfaces. 2020;12(24):26955–65.
Gao F, Zuo B, Wang Y, Li S, Yang J, Sun D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020;255:117719.
Zhang L, Wang Q, Su H, Cheng J. Exosomes from adipose derived mesenchymal stem cells alleviate diabetic osteoporosis in rats through suppressing NLRP3 inflammasome activation in osteoclasts. J Biosci Bioeng. 2021;131(6):671–8.
Sharif S, Ghahremani MH, Soleimani M. Differentiation induction and proliferation inhibition by a cell-free approach for delivery of exogenous miRNAs to neuroblastoma cells using mesenchymal stem cells. Cell J. 2021;22(4):556–64.
Xing X, Han S, Cheng G, Ni Y, Li Z, Li Z. Proteomic analysis of exosomes from adipose-derived mesenchymal stem cells: a novel therapeutic strategy for tissue injury. Biomed Res Int. 2020;2020:6094562.
Shin KO, Ha DH, Kim JO, et al. Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de Novo synthesis of ceramides in atopic dermatitis. Cells. 2020;9(3):680.
Liu K, Shi H, Peng Z, Wu X, Li W, Lu X. Exosomes from adipose mesenchymal stem cells overexpressing stanniocalcin-1 promote reendothelialization after carotid endarterium mechanical injury. Stem Cell Rev Rep. 2021. https://doi.org/10.1007/s12015-021-10180-4.
Gandolfi MG, Gardin C, Zamparini F, et al. Mineral-doped poly(L-lactide) acid scaffolds enriched with exosomes improve osteogenic commitment of human adipose-derived mesenchymal stem cells. Nanomaterials (Basel). 2020;10(3):432.
Xing X, Li Z, Yang X, et al. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging (Albany NY). 2020;12(4):3880–98.
Zhao S, Qi W, Zheng J, et al. Exosomes derived from adipose mesenchymal stem cells restore functional endometrium in a rat model of intrauterine adhesions. Reprod Sci. 2020;27(6):1266–75.
Lou G, Chen L, Xia C, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39(1):4.
Thankam FG, Chandra I, Diaz C, et al. Matrix regeneration proteins in the hypoxia-triggered exosomes of shoulder tenocytes and adipose-derived mesenchymal stem cells. Mol Cell Biochem. 2020;465(1–2):75–87.
Heo JS, Choi Y, Kim HO. Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cells Int. 2019;2019:7921760.
Wang X, Shah FA, Vazirisani F, et al. Exosomes influence the behavior of human mesenchymal stem cells on titanium surfaces. Biomaterials. 2020;230:119571.
Xu H, Wang Z, Liu L, Zhang B, Li B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem. 2020;121(3):2089–102.
Yuan R, Dai X, Li Y, Li C, Liu L. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol Med Rep. 2021;24(5):758.
Liu L, Zhang H, Mao H, Li X, Hu Y. Exosomal miR-320d derived from adipose tissue-derived MSCs inhibits apoptosis in cardiomyocytes with atrial fibrillation (AF). Artif Cells Nanomed Biotechnol. 2019;47(1):3976–84.
Xiao S, Xiao C, Miao Y, et al. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Ther. 2021;12(1):255.
Bonafede R, Brandi J, Manfredi M, et al. The anti-apoptotic effect of ASC-exosomes in an in vitro ALS model and their proteomic analysis. Cells. 2019;8(9):1087.
Shimoda A, Sawada SI, Sasaki Y, Akiyoshi K. Exosome surface glycans reflect osteogenic differentiation of mesenchymal stem cells: profiling by an evanescent field fluorescence-assisted lectin array system. Sci Rep. 2019;9(1):11497.
Chen B, Cai J, Wei Y, et al. Exosomes are comparable to source adipose stem cells in fat graft retention with up-regulating early inflammation and angiogenesis. Plast Reconstr Surg. 2019;144(5):816e–27e.
Chen S, Tang Y, Liu Y, et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52(5):e12669.
Deng S, Zhou X, Ge Z, et al. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114:105564.
Wang X, Zhu Y, Wu C, Liu W, He Y, Yang Q. Adipose-derived mesenchymal stem cells-derived exosomes carry microRNA-671 to alleviate myocardial infarction through inactivating the TGFBR2/Smad2 axis. Inflammation. 2021. https://doi.org/10.1007/s10753-021-01460-9.
Eguchi S, Takefuji M, Sakaguchi T, et al. Cardiomyocytes capture stem cell-derived, anti-apoptotic microRNA-214 via clathrin-mediated endocytosis in acute myocardial infarction. J Biol Chem. 2019;294(31):11665–74.
Chen J, Ren S, Duscher D, et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J Cell Physiol. 2019;234(12):23097–110.
Zhang C, Wang P, Mohammed A, et al. Function of adipose-derived mesenchymal stem cells in monocrotaline-induced pulmonary arterial hypertension through miR-191 via regulation of BMPR2. Biomed Res Int. 2019;2019(16):2858750.
Liu Z, Xu Y, Wan Y, Gao J, Chu Y, Li J. Exosomes from adipose-derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell Death Discov. 2019;5:79.
Villatoro AJ, Alcoholado C, Martín-Astorga MC, Fernández V, Cifuentes M, Becerra J. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Vet Immunol Immunopathol. 2019;208:6–15.
Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. 2019;109:59–68. Erratum in: Int J Biochem Cell Biol. 2020;126:105805.
Wang J, Wu H, Peng Y, et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnol. 2021;19(1):202.
Ma T, Fu B, Yang X, Xiao Y, Pan M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120(6):10847–54.
Wang C, Wang M, Xu T, et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9(1):65–76.
Fathollahi A, Hashemi SM, Haji Molla Hoseini M, Yeganeh F. In vitro analysis of immunomodulatory effects of mesenchymal stem cell- and tumor cell -derived exosomes on recall antigen-specific responses. Int Immunopharmacol. 2019;67:302–10.
Ren L, Song ZJ, Cai QW, et al. Adipose mesenchymal stem cell-derived exosomes ameliorate hypoxia/serum deprivation-induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis in vitro. Biochem Biophys Res Commun. 2019;508(1):138–44.
Safwat A, Sabry D, Ragiae A, Amer E, Mahmoud RH, Shamardan RM. Adipose mesenchymal stem cells-derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J Circ Biomark. 2018;7:1849454418807827.
Domenis R, Cifù A, Quaglia S, et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep. 2018;8(1):13325.
Huang X, Ding J, Li Y, et al. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp Cell Res. 2018;371(1):269–77.
Huang B, Lu J, Ding C, Zou Q, Wang W, Li H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res Ther. 2018;9(1):216.
Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018;119(11):9433–43.
Li M, Lei H, Xu Y, et al. Exosomes derived from mesenchymal stem cells exert therapeutic effect in a rat model of cavernous nerves injury. Andrology. 2018;6(6):927–35.
Cho BS, Kim JO, Ha DH, Yi YW. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res Ther. 2018;9(1):187.
Bucan V, Vaslaitis D, Peck CT, Strauß S, Vogt PM, Radtke C. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol Neurobiol. 2019;56(3):1812–24.
Liu X, Wang S, Wu S, et al. Exosomes secreted by adipose-derived mesenchymal stem cells regulate type I collagen metabolism in fibroblasts from women with stress urinary incontinence. Stem Cell Res Ther. 2018;9(1):159.
Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, Silvestre A, Alcaraz MJ. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem. 2018;47(1):11–25.
Shen T, Zheng QQ, Shen J, et al. Effects of adipose-derived mesenchymal stem cell exosomes on corneal stromal fibroblast viability and extracellular matrix synthesis. Chin Med J (Engl). 2018;131(6):704–712. Erratum in: Chin Med J (Engl). 2021;134(11):1375.
Han YD, Bai Y, Yan XL, et al. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018;497(1):305–12.
Xue C, Shen Y, Li X, et al. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cells Dev. 2018;27(7):456–65.
Li W, Liu Y, Zhang P, et al. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl Mater Interfaces. 2018;10(6):5240–54.
Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, Castejón MA, Alcaraz MJ. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxid Med Cell Longev. 2017;2017:7197598.
Zhu LL, Huang X, Yu W, Chen H, Chen Y, Dai YT. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia. 2018;50(2):e12871.
Zhu F, Chong Lee Shin OLS, et al. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget. 2017;8(41):70707–26.
Wang L, Hu L, Zhou X, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7(1):13321. Erratum in: Sci Rep. 2018;8(1):7066. Erratum in: Sci Rep. 2021;11(1):3245.
Casado S, Lobo MDVT, Paíno CL. Dynamics of plasma membrane surface related to the release of extracellular vesicles by mesenchymal stem cells in culture. Sci Rep. 2017;7(1):6767.
Shimoda A, Tahara Y, Sawada SI, Sasaki Y, Akiyoshi K. Glycan profiling analysis using evanescent-field fluorescence-assisted lectin array: Importance of sugar recognition for cellular uptake of exosomes from mesenchymal stem cells. Biochem Biophys Res Commun. 2017;491(3):701–7.
Lv JW, Wen W, Jiang C, et al. Inhibition of microRNA-214 promotes epithelial–mesenchymal transition process and induces interstitial cystitis in postmenopausal women by upregulating Mfn2. Exp Mol Med. 2017;49(7):e357.
Cui X, He Z, Liang Z, Chen Z, Wang H, Zhang J. Exosomes from adipose-derived mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/β-Catenin signaling pathway. J Cardiovasc Pharmacol. 2017;70(4):225–31.
Qu Y, Zhang Q, Cai X, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21(10):2491–502.
Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H. Priming Aadipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng Part A. 2017;23(21–22):1212–20.
Wang K, Jiang Z, Webster KA, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem Cells Transl Med. 2017;6(1):209–22.
Reza AMMT, Choi YJ, Yasuda H, Kim JH. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci Rep. 2016;6:38498.
Chen KH, Chen CH, Wallace CG, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7(46):74537–56.
Yu B, Shao H, Su C, et al. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci Rep. 2016;6:34562.
Hu L, Wang J, Zhou X, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016; 6:32993. Erratum in: Sci Rep. 2020;10(1):6693.
Matula Z, Németh A, Lőrincz P, et al. The role of extracellular vesicle and tunneling nanotube-mediated intercellular cross-talk between mesenchymal stem cells and human peripheral T cells. Stem Cells Dev. 2016;25(23):1818–32.
Villatoro AJ, Martín-Astorga MDC, Alcoholado C, Cárdenas C, Fariñas F, Becerra J, Visser R. Altered proteomic profile of adipose tissue-derived mesenchymal stem cell exosomes from cats with severe chronic gingivostomatitis. Animals (Basel). 2021;11(8):2466.
Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129(11):2182–9.
Lin KC, Yip HK, Shao PL, et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–85.
Wei JJ, Chen YF, Xue CL, et al. Protection of nerve injury with exosome extracted from mesenchymal stem Cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38(1):33–6. https://doi.org/10.3881/j.issn.1000-503X.2016.01.006.
Lou G, Song X, Yang F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122.
Ko SF, Yip HK, Zhen YY, et al. Adipose-derived mesenchymal stem cell exosomes suppress hepatocellular carcinoma growth in a rat model: Apparent diffusion coefficient, natural killer T-cell responses, and histopathological features. Stem Cells Int. 2015;2015:853506.
Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):127.
Blazquez R, Sanchez-Margallo FM, de la Rosa O, et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol. 2014;5:556.
Goudarzi F, Kiani A, Moradi M, Haghshenas B, Hashemnia M, Karami A, Mohammadalipour A. Intraprostatic injection of exosomes isolated from adipose-derived mesenchymal stem cells for the treatment of chronic non-bacterial prostatitis. J Tissue Eng Regen Med. 2021;15(12):1144–54.
Franquesa M, Hoogduijn MJ, Ripoll E, et al. Update on controls for isolation and quantification methodology of extracellular vesicles derived from adipose tissue mesenchymal stem cells. Front Immunol. 2014;5:525.
Eirin A, Riester SM, Zhu XY, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64.
Katsuda T, Oki K, Ochiya T. Potential application of extracellular vesicles of human adipose tissue-derived mesenchymal stem cells in Alzheimer’s disease therapeutics. Methods Mol Biol. 2015;1212:171–81.
Yang T, Zhao F, Zhou L, et al. Therapeutic potential of adipose-derived mesenchymal stem cell exosomes in tissue-engineered bladders. J Tissue Eng. 2021;12:20417314211001544.
Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383(1–2):13–20.
Katsuda T, Tsuchiya R, Kosaka N, et al. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197.
Kwon HH, Yang SH, Lee J, et al. Combination treatment with human adipose tissue stem cell-derived exosomes and fractional CO2 laser for acne scars: a 12-week prospective, double-blind, randomized, split-face study. Acta Derm Venereol. 2020;100(18):adv00310.
Cho BS, Lee J, Won Y, et al. Skin brightening efficacy of exosomes derived from human adipose tissue-derived stem/stromal cells: a prospective, split-face, randomized placebo-controlled study. Cosmetics. 2020;7(4):90.
Wang J, Yi Y, Zhu Y, et al. Effects of adipose-derived stem cell released exosomes on wound healing in diabetic mice. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020;34(1):124–31. https://doi.org/10.7507/1002-1892.201903058 (in Chinese).
Shiekh PA, Singh A, Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials. 2020;249:120020.
Liu K, Chen C, Zhang H, Chen Y, Zhou S. Adipose stem cell-derived exosomes in combination with hyaluronic acid accelerate wound healing through enhancing re-epithelialization and vascularization. Br J Dermatol. 2019;181(4):854–6.
He L, Zhu C, Jia J, et al. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway. Biosci Rep. 2020;40(5):BSR20192549.
Lv Q, Deng J, Chen Y, Wang Y, Liu B, Liu J. Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol Pharm. 2020;17(5):1723–33.
Li X, Xie X, Lian W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1–14.
Wang M, Wang C, Chen M, et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano. 2019;13(9):10279–93.
Shafei S, Khanmohammadi M, Heidari R, et al. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: an in vivo study. J Biomed Mater Res A. 2020;108(3):545–56.
Cooper DR, Wang C, Patel R, et al. Human adipose-derived stem cell conditioned media and exosomes containing MALAT1 promote human dermal fibroblast migration and ischemic wound healing. Adv Wound Care (New Rochelle). 2018;7(9):299–308.
Ren S, Chen J, Duscher D, et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res Ther. 2019;10(1):47.
Yang C, Luo L, Bai X, et al. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch Biochem Biophys. 2020;681:108259.
Choi EW, Seo MK, Woo EY, Kim SH, Park EJ, Kim S. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp Dermatol. 2018;27(10):1170–2.
Zhang W, Bai X, Zhao B, et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018;370(2):333–42.
Zhu M, Liu Y, Qin H, et al. Osteogenically-induced exosomes stimulate osteogenesis of human adipose-derived stem cells. Cell Tissue Bank. 2021;22(1):77–91.
Zhu CT, Li T, Hu YH, Zou M, Guo Q, Qu XW. Exosomes secreted by mice adipose-derived stem cells after low-level laser irradiation treatment reduce apoptosis of osteocyte induced by hypoxia. Eur Rev Med Pharmacol Sci. 2017;21(24):5562–70.
Wang C, Hu Q, Song W, Yu W, He Y. Adipose stem cell-derived exosomes decrease fatty infiltration and enhance rotator cuff healing in a rabbit model of chronic tears. Am J Sports Med. 2020;48(6):1456–64.
Shahir M, Mahmoud Hashemi S, Asadirad A, et al. Effect of mesenchymal stem cell-derived exosomes on the induction of mouse tolerogenic dendritic cells. J Cell Physiol. 2020;235(10):7043–55.
Yang S, Guo S, Tong S, Sun X. Exosomal miR-130a-3p regulates osteogenic differentiation of human adipose-derived stem cells through mediating SIRT7/Wnt/β-catenin axis. Cell Prolif. 2020;53(10):e12890.
Du W, Su L, Zhang N, Wang H. Exosomes derived from preadipocytes improve osteogenic differentiation, potentially via reduced miR-223 expression. Mol Med Rep. 2019;19(2):951–8.
Hong Y, Sun Y, Rong X, Li D, Lu Y, Ji Y. Exosomes from adipose-derived stem cells attenuate UVB-induced apoptosis, ROS, and the Ca2+ level in HLEC cells. Exp Cell Res. 2020;396(2):112321.
Bari E, Perteghella S, Catenacci L, et al. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine (London). 2019;14(6):753–65.
Patel RS, Carter G, El Bassit G, et al. Adipose-derived stem cells from lean and obese humans show depot specific differences in their stem cell markers, exosome contents and senescence: role of protein kinase C delta (PKCδ) in adipose stem cell niche. Stem Cell Investig. 2016;3:2.
Zhang Z, Huang G, Mao G, Hu S. Characterization of exosomal long non-coding RNAs in chondrogenic differentiation of human adipose-derived stem cells. Mol Cell Biochem. 2021;476(3):1411–20.
Zhao C, Chen JY, Peng WM, Yuan B, Bi Q, Xu YJ. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol Med Rep. 2020;21(4):1881–9.
Li D, Luo H, Ruan H, et al. Isolation and identification of exosomes from feline plasma, urine and adipose-derived mesenchymal stem cells. BMC Vet Res. 2021;17(1):272.
Xiong J, Liu Z, Wu M, Sun M, Xia Y, Wang Y. Comparison of proangiogenic effects of adipose-derived stem cells and foreskin fibroblast exosomes on artificial dermis prefabricated flaps. Stem Cells Int. 2020;2020:5293850.
Liu CY, Yin G, Sun YD, et al. Effect of exosomes from adipose-derived stem cells on the apoptosis of Schwann cells in peripheral nerve injury. CNS Neurosci Ther. 2020;26(2):189–96.
Ching RC, Wiberg M, Kingham PJ. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res Ther. 2018;9(1):266.
Tian XL, Jiang B, Yan H. Effect of adipose-derived mesenchymal stem cell exosomes on the proliferation and migration of keratinocytes and the underlying mechanisms. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23(1):68–73. https://doi.org/10.3969/j.issn.2095-4344.1527.
Pu CM, Liu CW, Liang CJ, et al. Adipose-derived stem cells protect skin flaps against ischemia/reperfusion injury via interleukin-6 expression. J Invest Dermatol. 2017;137(6):1353–62.
Zhang J, Yi Y, Yang S, Zhu Y, Hu X. Effects of adipose-derived stem cell released exosomes on proliferation, migration, and tube-like differentiation of human umbilical vein endothelial cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32(10):1351–7. https://doi.org/10.7507/1002-1892.201804016 (in Chinese).
Xu F, Xiang Q, Huang J, et al. Exosomal miR-423-5p mediates the proangiogenic activity of human adipose-derived stem cells by targeting Sufu. Stem Cell Res Ther. 2019;10(1):106.
Qin DY, Zhang YH, Li XY, Yang WX. Proteomics analysis of exosomes from adipose-derived stem cells in skin damage repair. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24(13):2011–9. https://doi.org/10.3969/j.issn.2095-4344.2064.
Gu YB, Zhang DJ, Yuan W, Song LJ. Exosomes from adipose-derived stem cells can promote cavernous nerve regeneration in rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24(31):4979–85. https://doi.org/10.3969/j.issn.2095-4344.2121.
Yin G, Liu C, Lin Y, Xie Z, Hou C, Lin H. Effect of exosomes from adipose-derived stem cells on peripheral nerve regeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32(12):1592–6. https://doi.org/10.7507/1002-1892.201707051 (in Chinese).
Yang Y, Cai Y, Zhang Y, Liu J, Xu Z. Exosomes secreted by adipose-derived stem cells contribute to angiogenesis of brain microvascular endothelial cells following oxygen-glucose deprivation in vitro through microRNA-181b/TRPM7 axis. J Mol Neurosci. 2018;65(1):74–83.
Wu ZY, Zhang HJ, Zhou ZH, et al. The effect of inhibiting exosomes derived from adipose-derived stem cells via the TGF-β1/Smad pathway on the fibrosis of keloid fibroblasts. Gland Surg. 2021;10(3):1046–56.
Zhao B, Zhang X, Zhang YL, et al. Human exosomes accelerate cutaneous wound healing by promoting collagen synthesis in a diabetic mouse model. Stem Cells Dev. 2021. https://doi.org/10.1089/scd.2021.0100.
Hu X, Yi Y, Zhu Y, et al. Effect of adipose-derived stem cell derived exosomes on angiogenesis after skin flap transplantation in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019;33(12):1560–5. https://doi.org/10.7507/1002-1892.201904023 (in Chinese).
Du L, Li G, Yang Y, et al. Exosomes from microRNA-199-3p-modified adipose-derived stem cells promote proliferation and migration of endothelial tip cells by downregulation of semaphorin 3A. Int J Clin Exp Pathol. 2018;11(10):4879–88.
Liu H, Zhang M, Shi M, et al. Adipose-derived mesenchymal stromal cell-derived exosomes promote tendon healing by activating both SMAD1/5/9 and SMAD2/3. Stem Cell Res Ther. 2021;12(1):338.
Fu G, Lu L, Pan Z, Fan A, Yin F. Adipose-derived stem cell exosomes facilitate rotator cuff repair by mediating tendon-derived stem cells. Regen Med. 2021;16(4):359–72.
Huang H, Xu Z, Qi Y, et al. Exosomes from SIRT1-overexpressing ADSCs restore cardiac function by improving angiogenic function of EPCs. Mol Ther Nucleic Acids. 2020;21:737–50.
An Y, Zhao J, Nie F, et al. Exosomes from adipose-derived stem cells (ADSCs) overexpressing miR-21 promote vascularization of endothelial cells. Sci Rep. 2019;9(1):12861.
Yang S, Guo S, Tong S, Sun X. Promoting osteogenic differentiation of human adipose-derived stem cells by altering the expression of exosomal miRNA. Stem Cells Int. 2019;2019:1351860.
Wu J, Yang Q, Wu S, et al. Adipose-derived stem cell exosomes promoted hair regeneration. Tissue Eng Regen Med. 2021;18(4):685–91.
Gurunathan S, Kang MH, Kim JH. Diverse effects of exosomes on COVID-19: a perspective of progress from transmission to therapeutic developments. Front Immunol. 2021;12:716407.
Yousefi Dehbidi M, Goodarzi N, Azhdari MH, Doroudian M. Mesenchymal stem cells and their derived exosomes to combat Covid-19. Rev Med Virol. 2021. https://doi.org/10.1002/rmv.2281.
Grangier A, Branchu J, Volatron J, et al. Technological advances towards extracellular vesicles mass production. Adv Drug Deliv Rev. 2021. https://doi.org/10.1016/j.addr.2021.113843.
Patel DB, Gray KM, Santharam Y, et al. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng Transl Med. 2017;2(2):170–9.
Mathieu M, Névo N, Jouve M, et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12(1):4389.
Acknowledgements
None.
Funding
Medical and Health Innovation Project of Chinese Academy of Medical Sciences (2017-I2M-1-007, 2021-I2M-1-052).
Author information
Authors and Affiliations
Contributions
CJG and HTY participated in conceptualization and supervision and wrote the original draft; LRQ and SHY had contributed to methodology, investigation and data analysis; JHY took part in writing, reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. Jianguo Chen and Ruiquan Liu contributed equally to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, J., Liu, R., Huang, T. et al. Adipose stem cells-released extracellular vesicles as a next-generation cargo delivery vehicles: a survey of minimal information implementation, mass production and functional modification. Stem Cell Res Ther 13, 182 (2022). https://doi.org/10.1186/s13287-022-02849-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-02849-5