Abstract
Background
Glucose-6-phosphate isomerase deficiency is a rare genetic disorder causing hereditary nonspherocytic hemolytic anemia. It is the second most common glycolytic enzymopathy in red blood cells. About 90 cases are reported worldwide, with symptoms including chronic hemolytic anemia, jaundice, splenomegaly, gallstones, cholecystitis, and in severe cases, neurological impairments, hydrops fetalis, and neonatal death.
Case presentation
This paper details the case of the first Danish patient diagnosed with glucose-6-phosphate isomerase deficiency. The patient, a 27-year-old white female, suffered from lifelong anemia of unknown origin for decades. Diagnosis was established through whole-genome sequencing, which identified two GPI missense variants: the previously documented variant p.(Thr224Met) and a newly discovered variant p.(Tyr341Cys). The pathogenicity of these variants was verified enzymatically.
Conclusions
Whole-genome sequencing stands as a potent tool for identifying hereditary anemias, ensuring optimal management strategies.
Similar content being viewed by others
Introduction
Glucose-6-phosphate isomerase (GPI) deficiency is a rare autosomal recessive disorder caused by homozygous or compound heterozygous variations in the GPI gene [1]. It is the second most common glycolytic enzymopathy in red blood cells (RBCs) after pyruvate kinase deficiency. GPI plays a crucial role in glycolysis by converting glucose-6-phosphate into fructose-6-phosphate. The resulting imbalance disrupts RBC metabolism and leads to hemolysis [2].
Most cases are diagnosed in neonatal and early childhood, causing hereditary nonspherocytic hemolytic anemia with chronic hemolysis and possible acute crises due to infections [1].
Symptoms are mild-to-severe anemia with fatigue, tachycardia, dyspnea, and pallor. Other symptoms include jaundice, splenomegaly, gallstones, and cholecystitis, and in a few severe cases, it has been shown to cause neurological deficits, hydrops fetalis, and neonatal death [1, 3].
The first report of the disease was described in 1968 by Baughan et al. [4], and since then about 90 patients have been diagnosed from a variety of ethnic groups and populations throughout the world, and over 100 different variations associated with GPI deficiency have been identified [1].
Diagnosis involves measuring GPI activity in RBCs and genetic testing with detection and confirmation of pathogenic variants of GPI [5, 6].
Treatment is primarily supportive, involving transfusions and chelation therapy, and often necessitates splenectomy [2].
In this case report, we describe the presentation and diagnosis of the first patient in Denmark diagnosed with GPI deficiency.
Case presentation
The patient is a 27-year-old woman of Venezuelan and Italian descent who was referred to our institution in 2018 owing to lifelong macrocytic hemolytic anemia.
As a newborn, she had jaundice and dilated bile ducts, leading to a diagnosis of chronic hemolytic anemia. Her gallbladder was subsequently removed. The patient was previously tested in other countries without explanation for the etiology and was initially misdiagnosed as having hereditary spherocytosis.
She experienced worsening symptoms during periods of stress, menstruation, and infections, including increased fatigue, yellowing of the eyes, dark urine, difficulty breathing, and chest pain. Episodes of jaundice decreased with a shift to a more plant-based diet, and they no longer occur during menstruation.
There is no family history of chronic hemolysis, but her grandmother had gallstones, and her grandfather was diagnosed with chronic myeloid leukemia.
Clinical findings
Physical examination revealed no pathological findings or neurological impairment. Blood tests showed moderate hemolytic anemia with macrocytosis, reticulocytosis, hyperbilirubinemia, and decreased haptoglobin, consistent with hemolysis (Table 1).
Various tests, including direct anti-globulin test, osmotic gradient ektacytometry [7, 8], PKLR sequencing, blood smear, and hemoglobin electrophoresis [9], ruled out other diagnoses such as hereditary spherocytosis, pyruvate kinase deficiency, autoimmune hemolytic diseases, glucose-6-phosphate dehydrogenase deficiency, thalassemia, and sickle-cell disease. Peripheral blood smear was unremarkable aside from mild stomatocytosis (Fig. 1).
Similar to a previous report, the patient's ektacytometry (Fig. 2) curve was right-shifted and did not support a diagnosis of hereditary spherocytosis [10].
Ektacytometry. Osmotic gradient ektacytometry was performed on ethylenediaminetetraacetic acid–blood on a LoRRca ektacytometer (RR Mechatronics, Zwaag, Netherlands) within 48 h of venipuncture according to manufacturer’s instructions. The patient’s ektacytometry curve was right shifted (Omin = 161 mOsm/kg, Omax = 338 mOsm/kg) but with normal maximal deformability (EImax = 0.605)
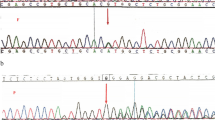
Whole-genome sequencing (WGS) was performed at the Danish National Genome Center (https://eng.ngc.dk) according to their standard procedures, and variants were subsequently filtered using a custom anemia in silico gene panel (Additional file 1). WGS revealed two likely disease-causing variants in the GPI gene (NM_000175.5, NP_000166.2). The first detected missense variant (c.671C > T, p.(Thr224Met)) has an overall allele frequency in the background population of 0.0039% (gnomAD v3.1.2) and alters a moderately to highly conserved amino acid.
In silico analysis (REVEL [11]) indicated an increased likelihood that the variant is pathogenic (score: 0.852), and it has been documented in patients with GPI deficiency in both homozygous (Kanno et al., 1996 [12]) and compound heterozygous forms (Xu et al., 1994 [13]). The variant has also been shown to co-segregate with the disease in a family [12]. The variant was classified as pathogenic according to American College of Medical Genetics and Genomics (ACMG) guidelines [14].
The second detected missense variant (c.1022A > G, p.(Tyr341Cys)) in GPI has an overall allele frequency of 0.0026% (gnomAD v3.1.2) and modifies a highly conserved amino acid. Despite its absence in prior literature or functional studies, in silico analysis (REVEL) suggested a high probability of pathogenicity (score: 0.987), leading to its classification as likely pathogenic according to ACMG and ClinGen guidelines [15].
Enzymatic assays showed a GPI activity of 7 U/g Hb (normal range 32–72 U/g Hb) and elevated hexokinase (HK) activity of 3.4 U/g Hb (normal range 0.8–1.5 U/g Hb). The strongly decreased GPI activity supports the diagnosis of GPI deficiency. The absence of available relatives for testing impeded our ability to clearly discern the distinct impacts of the two GPI variants. Ideally, we would have preferred this to comprehensively authenticate the novel Tyr431Cys variant. The HK activity is measured as a reference to evaluate mean red cell age.
Treatment and follow-up
Currently, the patient’s therapeutic intervention consists only of folic acid. Although her hemoglobin has been steadily low, blood transfusions or splenectomy have not been necessary. Activators of the glycolytic pathway such as mitapivat, etovapivat, and AG946 are either approved or under clinical investigation for other hereditary anemias. However, these activators target pyruvate kinase (PK), which acts downstream of GPI in the glycolytic pathway. Presuming that PK activation would have an impact, this could potentially reduce hemolysis by enhancing the availability of adenosine triphosphate (ATP) in GPI-deficient RBCs. Nevertheless, PK activators are also known to decrease the level of the glycolytic intermediate 2,3-Diphosphoglycerate (2,3-DPG). This in turn elevates the oxygen affinity of hemoglobin, which subsequently diminishes oxygen delivery to tissues.
Discussion
GPI deficiency is the second most common glycolytic enzymopathy in RBCs after PK deficiency, but its exact frequency is unknown, and it is likely underdiagnosed owing to the lack of awareness and availability of testing. Diagnosis of GPI deficiency can be challenging, and genetic testing has become an important tool in the diagnostic process. Enzymatic assays are scarcely available and most often require fresh blood shipment to highly specialized laboratories. Contrarily, next-generation sequencing (NGS)-based methods such as WGS are becoming increasingly available for hemolytic anemia owing to a decrease in cost and do not require special handling.
Lack of knowledge about rare anemias as well as cumbersome testing can delay or prevent diagnosis of rare anemias. Furthermore, the clinical presentation of chronic hemolytic anemia can be similar, requiring multiple tests to exclude other diagnoses [6].
This patient exhibited consistently low hemoglobin, moderate hemolysis, intermittent jaundice, and hyperbilirubinemia, but no splenomegaly. It remains uncertain whether a splenectomy may become necessary in the future. Splenectomy has shown to reduce hemolysis and dependence on transfusion [16].
The implementation and advancement of next-generation sequencing have improved routine diagnostic workup of hereditary anemias. It can certainly aid in diagnosing and thereby correctly managing GPI deficiency as well as other ultrarare hereditary anemias. However, it is important to note that genetic testing may not always identify the specific variant responsible for GPI deficiency, and for novel variants, estimation of pathogenicity is usually based on prediction. Therefore, additional testing, such as enzymatic assays, in this case, remains necessary [17].
No curative or targeted treatments exist for this disorder. Regular blood transfusions may be necessary for patients, including monitoring for iron overload. Promising new treatment options, including activators of the glycolytic pathway, are currently being clinically investigated for numerous other hereditary anemias, but to date, no clinical studies have targeted GPI deficiency. Establishing diagnosis and mapping patients with rare anemias is pivotal for research into these debilitating diseases and a prerequisite for clinical trials on novel treatment options.
Conclusion
This case report underscores the significance of diagnosing and managing GPI deficiency. The complexity and rarity of this condition often lead to misdiagnosis, emphasizing the value of advanced diagnostic tools such as WGS. While supportive care is the current approach, potential treatments targeting the glycolytic pathway offer hope. Establishing accurate diagnosis and understanding of rare anemias remains crucial for advancing research and exploring innovative therapies.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Kugler W, Lakomek M. Glucose-6-phosphate isomerase deficiency. Best Pract Res Clin Haematol. 2000;13(1):89–101. https://doi.org/10.1053/beha.1999.0059.
Koralkova P, Van Solinge WW, Van Wijk R. Rare hereditary red blood cell enzymopathies associated with hemolytic anemia - pathophysiology, clinical aspects, and laboratory diagnosis. Int J Lab Hematol. 2014;36(3):388–97. https://doi.org/10.1111/ijlh.12223.
van Scheltema PN, Zhang A, Ball LM, et al. Successful treatment of fetal hemolytic disease due to glucose phosphate isomerase deficiency ( GPI ) using repeated intrauterine transfusions: a case report. Clin Case Rep. 2015;3(10):862–5. https://doi.org/10.1002/ccr3.358.
Baughan MA, Valentine WN, Paglia DE, Ways PO, Simons ER, Demarsh QB. Hereditary hemolytic anemia associated with glucosephosphate isomerase (GPI) deficiency-a new enzyme defect of human erythrocytes. Blood. 1968;32:236.
Zu Y, Wang H, Lin W, Zou C. Hereditary nonspherocytic hemolytic anemia caused by glucose-6-phosphate isomerase (GPI) deficiency in a Chinese patient: a case report. BMC Pediatr. 2022;22(1):461. https://doi.org/10.1186/s12887-022-03522-9.
Fermo E, Vercellati C, Marcello AP, et al. Clinical and molecular spectrum of glucose-6-phosphate isomerase deficiency report of 12 new cases. Front Physiol. 2019. https://doi.org/10.3389/fphys.2019.00467.
Van WR, Fermo E, Glenthøj A, et al. Improving the EMA binding test by using commercially available fluorescent beads. Published Online. 2020. https://doi.org/10.3389/fphys.2020.569289.
Glenthøj A, Brieghel C, Nardo-Marino A, et al. Facilitating EMA binding test performance using fluorescent beads combined with next-generation sequencing. Published online. 2021. https://doi.org/10.1002/jha2.277.
Nygaard M, Petersen J, Bjerrum OW. Haemoglobinopathia Ypsilanti-A rare, but important differential diagnosis to polycythaemia vera. Leuk Res Rep. 2013. https://doi.org/10.1016/j.lrr.2013.09.002.
Zaninoni A, Fermo E, Vercellati C, et al. Use of laser assisted optical rotational cell analyzer (LoRRca MaxSis) in the diagnosis of RBC membrane disorders, enzyme defects, and congenital dyserythropoietic anemias: a monocentric study on 202 patients. Front Physiol. 2018. https://doi.org/10.3389/FPHYS.2018.00451/FULL.
Ioannidis NM, Rothstein JH, Pejaver V, et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016;99(4):877–85. https://doi.org/10.1016/j.ajhg.2016.08.016.
Kanno H, Fujii H, Hirono A, et al. Molecular analysis of glucose phosphate isomerase deficiency associated with hereditary hemolytic anemia. Blood. 1996;88(6):2321–5. https://doi.org/10.1182/BLOOD.V88.6.2321.BLOODJOURNAL8862321.
Xu W, Beutler E. The characterization of gene mutations for human glucose phosphate isomerase deficiency associated with chronic hemolytic anemia. J Clin Investig. 1994;94(6):2326–9. https://doi.org/10.1172/JCI117597.
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Gen Med. 2015. https://doi.org/10.1038/gim.2015.30.
Pejaver V, Byrne AB, Feng BJ, et al. Calibration of computational tools for missense variant pathogenicity classification and ClinGen recommendations for PP3/BP4 criteria. Am J Hum Genet. 2022. https://doi.org/10.1016/j.ajhg.2022.10.013.
Whitelaw AGL, Rogers PA, Hopkinson DA, et al. Congenital haemolytic anaemia resulting from glucose phosphate isomerase deficiency: genetics, clinical picture, and prenatal diagnosis. J Med Genet. 1979;16(3):189–96. https://doi.org/10.1136/jmg.16.3.189.
Van El CG, Cornel MC, Borry P, et al. Whole-genome sequencing in health care recommendations of the European Society of Human Genetics on behalf of the ESHG public and professional policy committee. Eur J Hum Genet. 2013;10(17):580–4. https://doi.org/10.1038/ejhg.2013.46.
Acknowledgements
Not applicable.
Funding
Open access funding provided by Copenhagen University Sources of Funding: Rigshospitalet’s Research Foundation.
Author information
Authors and Affiliations
Contributions
JP and AG planned this study. RvW performed functional analyses. AØR performed genetic variant filtering. SH, AG, and JP analyzed data and wrote the first draft of the manuscript. SH and AG prepared all the figures. All authors contributed to the final approved version of this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Regional Ethics Committee of the Capital Region of Denmark (H-21064560).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare the following conflicts of interest: AG: consultant for Agios Pharmaceuticals, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Novo Nordisk A/S, Pharmacosmos UK Ltd, and Vertex Pharmaceuticals. Received research support from Agios Pharmaceuticals, Inc., Novo Nordisk A/S, Saniona, and Sanofi. RvW: consultant for Agios Pharmaceuticals. Research funding from Agios Pharmaceuticals and Pfizer. All other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Custom in silico gene panel used for patients suspected of hereditary anemia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Holme, S., van Wijk, R., Rasmussen, A.Ø. et al. Glucose phosphate isomerase deficiency demasked by whole-genome sequencing: a case report. J Med Case Reports 18, 130 (2024). https://doi.org/10.1186/s13256-024-04466-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-024-04466-7