Abstract
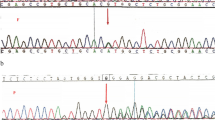
Glucose phosphate isomerase (GPI) deficiency is an autosomal recessive condition with mutations in the GPI gene on chromosome 19q13.1. Patients present with congenital non-spherocytic hemolytic anemia, and occasionally intellectual disability. In this study, we describe the clinical, hematological and biochemical parameters in the largest single-center cohort consisting of 17 GPI-deficient cases. Demographic and clinical data were noted, and red cell enzyme activity levels were estimated. Mutation analysis was done by single-stranded-conformation polymorphism, restriction-fragment length polymorphism and Sanger’s sequencing of exon 12 of the GPI gene. The male-to-female ratio was 0.7:1, median age at diagnosis was 5.0 years, 82.3% of patients had severe neonatal jaundice, and 13.3% had subtle neurological manifestations. Median Hb and MCV levels were 6.3 g/dl and 130.2 fl. Splenectomized patients required fewer transfusions. Sixteen of 17 patients had the pathogenic c.1040G > A (p.Arg347His) homozygous mutation in exon12 of the GPI gene, and one had the pathogenic c.1414C > T(p.Arg472Cys) homozygous mutation in exon 16. In summary, we report that neonatal jaundice, macrocytosis and high prevalence of p.Arg347His variant were predominant in GPI deficiency with prominent lack of neurological manifestations, and we emphasize the benefits of splenectomy and the need for genetic counseling.
Similar content being viewed by others
References
Fermo E, Vercellati C, Marcello AP, Zaninoni A, Aytac S, Cetin M, et al. Clinical and molecular spectrum of glucose-6-phosphate isomerase deficiency. Report of 12 new cases. Front Physiol. 2019;10:467.
Kanno H, Fujii H, Hirono A, Ishida Y, Ohga S, Fukumoto Y, et al. Molecular analysis of glucose phosphate isomerase deficiency associated with hereditary hemolytic anemia. Blood. 1996;88(6):2321–5.
Haller JF, Krawczyk SA, Gostilovitch L, Corkey BE, Zoeller RA. Glucose-6-phosphate isomerase deficiency results in mTOR activation, failed translocation of lipin 1α to the nucleus and hypersensitivity to glucose: Implications for the inherited glycolytic disease. Biochim Biophys Acta. 2011;1812(11):1393–402.
Repiso A, Oliva B, Vives Corrons JL, Carreras J, Climent F. Glucose phosphate isomerase deficiency: enzymatic and familial characterization of Arg346His mutation. Biochim Biophys Acta. 2005;1740(3):467–71.
Adama van Scheltema PN, Zhang A, Ball LM, Steggerda SJ, van Wijk R, Fransen van de Putte DE et al. Successful treatment of fetal hemolytic disease due to glucose phosphate isomerase deficiency (GPI) using repeated intrauterine transfusions: a case report. Clin Case Rep. 2015;3(10):862–5.
Kedar PS, Dongerdiye R, Chilwirwar P, Gupta V, Chiddarwar A, Devendra R, et al. Glucose phosphate isomerase deficiency: high prevalence of p.Arg347His mutation in indian population associated with severe hereditary non-spherocytic hemolytic anemia coupled with neurological dysfunction. Indian J Pediatr. 2019;86(8):692–9.
Beutler E, Blume KG, Kaplan JC, Löhr GW, Ramot B, Valentine WN. International committee for standardization in haematology: recommended methods for red-cell enzyme analysis. Br J Haematol. 1977;35(2):331–40.
Park H, Haller J, Smith F, Parkin N, Lythe T, Zoeller R, et al. Attenuation of hemolysis due to glucose-6-phosphate isomerase deficiency with ketogenic diet - a case report. Hemasphere. 2020;4(1):328.
Baronciani L, Zanella A, Bianchi P, Zappa M, Alfinito F, Iolascon A, et al. Study of the molecular defects in glucose phosphate isomerase-deficient patients affected by chronic hemolytic anemia. Blood. 1996;88(6):2306–10.
Xu W, Beutler E. The characterization of gene mutations for human glucose phosphate isomerase deficiency associated with chronic hemolytic anemia. J Clin Invest. 1994;94(6):2326–9.
Mojzikova R, Koralkova P, Holub D, Saxova Z, Pospisilova D, Prochazkova D, et al. Two novel mutations (p.(Ser160Pro) and p.(Arg472Cys)) causing glucose-6-phosphate isomerase deficiency are associated with erythroid dysplasia and inappropriately suppressed hepcidin. Blood Cells Mol Dis. 2018;69:23–9.
Kugler W, Breme K, Laspe P, Muirhead H, Davies C, Winkler H, et al. Molecular basis of neurological dysfunction coupled with haemolytic anaemia in human glucose-6-phosphate isomerase (GPI) deficiency. Hum Genet. 1998;103(4):450–4.
Iolascon A, Andolfo I, Barcellini W, Corcione F, Garçon L, De Franceschi L, et al. Working study group on red cells and iron of the EHA. Recommendations regarding splenectomy in hereditary hemolytic anemias. Haematologica. 2017;102(8):1304–13.
Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood. 2009;114(14):2861–8.
Lake M, Bessmer D. Haemolyitc anaemia: Enzyme deficiencies. In: McKenzie SB, Williams JL, editors. Clinical Laboratory Hematology. 2nd ed. New York: Pearson; 2009. p. 326–38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sampagar, A., Gosavi, M., Kedar, P. et al. Clinical, laboratory, and mutational profile of children with glucose phosphate isomerase deficiency: a single centre report. Int J Hematol 115, 255–262 (2022). https://doi.org/10.1007/s12185-021-03240-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03240-5