Abstract
Background
Glucose phosphate isomerase (GPI) deficiency is a rare autosomal recessive disorder that causes hereditary nonspherocytic hemolytic anemia (HNSHA). Homozygous or compound heterozygous mutation of the GPI gene on chromosome 19q13 is the cause of GPI deficiency. Fifty-seven GPI mutations have been reported at the molecular level.
Case presentation
A 5-month-old boy was presented with repeated episodes of jaundice after birth. He suffered from moderate hemolytic anemia (hemoglobin levels ranging from 62 to 91 g/L) associated with macrocytosis, reticulocytosis, neutropenia, and hyperbilirubinemia. Whole-exome sequencing showed that he has a missense mutation c.301G > A (p.Val101Met) in exon 4 and a frameshift mutation c.812delG (p.Gly271Glufs*131) in exon 10. Mutation p.Gly271Glufs*131 is a novel frameshift null mutation in GPI deficiency.
Conclusion
In a patient with recurrent jaundice since birth, mutations in the GPI gene associated with HNSHA should be evaluated. The c.812delG (p.Gly271Glufs*131) variant may be a novel mutation of the GPI gene. Compound heterozygous mutations c.301G > A (p.Val101Met) and c.812delG (p.Gly271Glufs*131) are not relevant to neurological impairment.
Similar content being viewed by others
Background
Glucose-6-phosphate isomerase (GPI) deficiency (MIM 613470), one of hereditary nonspherocytic hemolytic anemias (HNSHA), is a rare autosomal recessive hereditary disease caused by homozygous or compound heterozygous mutations of GPI gene on chromosome 19q13 [1]. Although GPI deficiency is the second most common erythro-enzymopathy of anaerobic glycolysis after pyruvate kinase deficiency, its exact morbidity is not known yet [2]. About 90 patients have been reported, to date, from a variety of ethnic groups and populations throughout the world since the first report in 1968 by Baughan et al. [3]. GPI deficiency is characterized by mild-to-severe chronic hemolytic anemia, jaundice, splenomegaly, and an increased incidence of pigment gallstones and cholecystitis, which is mainly caused due to dysregulated catalyzation of the second step of glycolysis [4]. According to a few reports, the fate of such patients was fetal loss/hydrops fetalis or immediate neonatal death [5,6,7,8,9,10]. In addition to its essential role in carbohydrate metabolism, GPI is identical to neuroleukin, a neurotrophic factor that supports the survival of embryonic spinal neurons, skeletal neurons, and sensory neurons [4, 11]. Therefore, some patients also showed muscle weakness, mixed sensory and cerebellar ataxia, mental retardation, or epilepsy [6, 7, 9, 12,13,14,15,16,17,18,19,20,21,22,23]. GPI also functions as a tumor-secreted cytokine and an angiogenic factor, which helps in the stimulation of endothelial cell motility [24]. Diagnosis is based on the determination of the enzymatic activity of GPI in erythrocytes using a quantitative assay and confirmation by DNA sequence analysis of the GPI gene [11, 24]. Treatments for chronic hemolytic anemia include blood transfusions, splenectomy, and supportive therapy. Here, we reported a patient with compound heterozygous mutation of the GPI gene who presented chronic hemolytic anemic features and reviewed correlative literature.
Case presentation
A 5-month-old Chinese boy presented to our unit because of repeated episodes of jaundice after birth. He was noted to be markedly anemic at the age of 3 hours at the local hospital. For the next 5 months, he suffered from moderate hemolytic anemia (Hemoglobin 62–91 g/L), along with macrocytosis (mean corpuscular volume 95–116 fL), reticulocytosis (461–489 × 109 /L reticulocytes), and neutropenia (neutrophils 0.53–2.25 × 109 /L) (Table 1), and unconjugated hyperbilirubinemia (133 μmol/L). He also suffered from active bone marrow hyperplasia with erythroid hyperplasia and neutropenia (granulocytes = 17.6%, erythrocytes = 41.2%, granulocytes: erythrocytes = 0.43:1) as assessed by analyzing the blood smear and bone marrow cytomorphology. Hemolytic anemia was considered, and blood transfusion (once, at 56-day-old) and other treatments, including protein iron succinate oral solution, vitamin C, vitamin B12, and prednisone acetate, were administered at the local hospital. He was born at 38 weeks with a birth weight of 3.07 kg. His parents were not consanguineous and had no history of anemia before.
On physical examination, he had a mild anemic appearance and a bodyweight of 6.1 kg. The liver was palpated about 2 cm below the right costal margin while the spleen was not palpated below the left costal margin. The chest, cardiac, abdominal, nerve, and skin were unremarkable. Cardiac ultrasonography indicated patent foramen ovale. Other tests, such as erythrocyte osmotic fragility test, hemoglobin electrophoresis, direct Coombs test, blood gas, electrolyte, chest X-ray, electrocardiogram, and ultrasonography for the brain, abdominal, and pelvic organs, showed normal results.
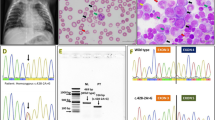
For etiology, whole-exome sequencing was performed. The outcome demonstrated that the patient had a c.301G > A (p.Val101Met) mutation in exon 4 that originated from his father and a c.812delG (p.Gly271Glufs*131) mutation in exon 10 that originated from his mother, both of which were specific compound heterozygous variants of the GPI gene. No other variants associated with hereditary hemolytic anemia were detected. The GPI variants were identified using Sanger sequencing (Fig. 1). The missense mutation c.301G > A (p.Val101Met) was previously reported in an Italian male patient (GPI Sarsina) who is homozygous and a female patient who is compound heterozygous with c.1009G > A (p.Ala337Thr) [2, 25, 26]. However, the frameshift is not reported in the ClinVar database, the Human Gene Mutation Database, and the Leiden Open Variation Database. The two variants observed in our patient were regarded as pathogenic according to the criteria by the American College of Medical Genetics and Genomics.
Sanger sequencing results of our patient and his parents. The red arrow indicates the variant locus. a A c.301G > A (p.Val101Met) hemizygous mutation in the GPI gene originated from the patient’s father. b A c.812delG (p.Gly271Glufs*131) hemizygous mutation in the GPI gene originated from the patient’s mother. P, proband; M, mother; F, father
Discussion and conclusions
Our patient was characterized by intermittent jaundice along with unconjugated hyperbilirubinemia and moderate hemolytic anemia associated with macrocytosis, reticulocytosis, and neutropenia, which were characteristics of chronic HNSHA. He did not have typical splenomegaly, which might be an advantage of his previous therapy or may appear later on. Uncertain chronic hemolytic anemia is supposed to be excluded from other erythropathies so that patients can receive treatments timely, and that reveals more potential changes. Previous reports have recommended pertinent workflows for the diagnosis of chronic hemolytic or inherited anemias [18]. Given the normal shape of erythrocytes, normal hemoglobin, and the negative results of the direct Coombs test, we assumed that our patient has an erythro-enzymopathy instead of sickle cell disease, thalassemia, or autoimmune conditions. We regret that enzymatic assays were not performed; however, the diagnosis of GPI deficiency using a biochemical method is unclear [24]. Bioinformatic analysis implied that both GPI variants were deleterious mutations. Thus, we concluded that the patient suffered from the GPI deficiency. We suggested a splenectomy and other symptomatic treatments, like fluid infusion, blood transfusions, vitamin supplementation when needed, and prevention of infections due to exogenous oxidation agents and the use of drugs, which were found to be suitable for other GPI deficiency patients [2, 27]. It is noted that transfusion-dependent patients can benefit from splenectomy [2, 4].
The GPI gene is located on chromosome 19q13.1, contains 18 exons, and its cDNA of 1.9 kb codes for 558 amino acids [4]. To date, 57 GPI mutations have been reported at the molecular level (The Human Gene Mutation Database, http://www.hgmd.cf.ac.uk, accessed on 12 Feb 2022), which includes 53 missense/nonsense, one splicing, and 3 small deletions [28]. Although our patient is the 8th case of GPI deficiency in China since the first report in 1992 by Zhao et al. [29], our patient is only the 4th case where genetic confirmation was performed (Table 2). Our patient has a missense mutation c.301G > A (p.Val101Met) and a frameshift mutation c.812delG (p.Gly271Glufs*131). The fact that the mutation p.Val101Met was identified in two different ethnic groups implied that either the origin of the mutation is very old or that the same mutation arose in more than one individual. A study of the GPI polymorphisms may be helpful in elucidating whether this mutation has a single origin. A targeted next-generation sequencing clinical panel of GPI genes can expedite molecular diagnosis rather than using Sanger sequencing in such cases [24]. The mutation p.Val101Met was first found in the Chinese population. Further knowledge regarding the GPI polymorphisms may help to draw further conclusions.
GPI plays an important role in physiological activities in addition to its essential role in the energy pathway, and it is present in all living organisms and expressed in all tissues [20]. The enzyme is homodimeric, traditionally termed large and small domains [30]. Several missense mutations in the GPI gene induce protein abnormalities that influence the enzyme catalytic activity [30]. Previous studies have analyzed the amino acid substitution p.Val101Met. It is located in the α7 helix of its big domain, where the longer side chain of methionine might decrease the local packing efficiency and a protein destabilization through a clash with the phenyl group of F85 between α6 and α7 [31]. Alternatively, it also potentially disrupts the active site architecture by altering the critical interactions between α6, α7, and α15 and the 3/10 helix between residues 270 and 274, which is one of the active sites [30]. The neurological symptoms were absent in the three patients, suggesting that the neurotrophic activity of the GPI enzyme is not affected by the amino acid substitution p.Val101Met. Compared to patient GPI Sarsina, our patient has the frameshift mutation c.812delG (p.Gly271Glufs*131), which disrupts the open reading frame of the GPI mRNA transcript, predicting the formation of a truncated polypeptide, altering active site architecture (residues 270–274), and lacking about 28% of the COOH terminal amino acid sequence and the active site 519. As a result, this abnormal polypeptide may not be compatible with dimerization, suggesting that our patient can be considered functionally hemizygous for the missense mutation (p.Val101Met) present in the other chromosome. In other words, this frameshift mutation can be assessed as potentially pathogenic.
The current report describes the clinical features and the molecular etiology of a Chinese patient with GPI deficiency, a very rare cause of HNSHA. The patient was compound heterozygous for the novel GPI frameshift mutation p.Gly271Glufs*131 and missense mutation p.Val101Met. Molecular structural analysis suggested that both variants affect the active site of the enzyme but do not interfere with its neurotrophic properties.
Availability of data and materials
The variant data that support the findings of this study have been deposited in ClinVar with the SCV accession codes, SCV002549741 and SCV002549742.
Abbreviations
- GPI:
-
Glucose-6-phosphate isomerase
- HNSHA:
-
Hereditary nonspherocytic hemolytic anemia
References
Manco L, Bento C, Victor BL, Pereira J, Relvas L, Brito RM, et al. Hereditary nonspherocytic hemolytic anemia caused by red cell glucose-6-phosphate isomerase (GPI) deficiency in two Portuguese patients: clinical features and molecular study. Blood Cells Mol Dis. 2016;60:18–23.
Fermo E, Vercellati C, Marcello AP, Zaninoni A, Aytac S, Cetin M, et al. Clinical and molecular Spectrum of Glucose-6-phosphate isomerase deficiency. Report of 12 New Cases. Front Physiol. 2019;10:467.
Baughan MA, Valentine WN, Paglia DE, Ways PO, Simons ER, DeMarsh QB. Hereditary hemolytic anemia associated with glucosephosphate isomerase (GPI) deficiency--a new enzyme defect of human erythrocytes. Blood. 1968;32(2):236–49.
Kugler W, Lakomek M. Glucose-6-phosphate isomerase deficiency. Baillieres Best Pract Res Clin Haematol. 2000;13(1):89–101.
Whitelaw AG, Rogers PA, Hopkinson DA, Gordon H, Emerson PM, Darley JH, et al. Congenital haemolytic anaemia resulting from glucose phosphate isomerase deficiency: genetics, clinical picture, and prenatal diagnosis. J Med Genet. 1979;16(3):189–96.
Schröter W, Eber SW, Bardosi A, Gahr M, Gabriel M, Sitzmann FC. Generalised glucosephosphate isomerase (GPI) deficiency causing haemolytic anaemia, neuromuscular symptoms and impairment of granulocytic function: a new syndrome due to a new stable GPI variant with diminished specific activity (GPI Homburg). Eur J Pediatr. 1985;144(4):301–5.
Eber SW, Gahr M, Lakomek M, Prindull G, Schröter W. Clinical symptoms and biochemical properties of three new glucosephosphate isomerase variants. Blut. 1986;53(1):21–8.
Ravindranath Y, Paglia DE, Warrier I, Valentine W, Nakatani M, Brockway RA. Glucose phosphate isomerase deficiency as a cause of hydrops fetalis. N Engl J Med. 1987;316(5):258–61.
Shalev O, Shalev RS, Forman L, Beutler E. GPI Mount Scopus--a variant of glucosephosphate isomerase deficiency. Ann Hematol. 1993;67(4):197–200.
Adama van Scheltema PN, Zhang A, Ball LM, Steggerda SJ, van Wijk R, van de Putte Fransen DE, et al. Successful treatment of fetal hemolytic disease due to glucose phosphate isomerase deficiency (GPI) using repeated intrauterine transfusions: a case report. Clin Case Rep. 2015;3(10):862–5.
Burger NCM, van Wijk R, Bresters D, Schell EA. A novel mutation of glucose phosphate isomerase (GPI) causing severe neonatal Anemia due to GPI deficiency. J Pediatr Hematol Oncol. 2019;41(3):e186–9.
Helleman PW, Van Biervliet JP. Haematological studies in a new variant of glucosephosphate isomerase deficiency (GPI Utrecht). Helv Paediatr Acta. 1976;30(6):525–36.
Kahn A, Buc HA, Girot R, Cottreau D, Griscelli C. Molecular and functional anomalies in two new mutant glucose-phosphate-insomerase variants with enzyme deficiency and chronic hemolysis. Hum Genet. 1978;40(3):293–304.
Zanella A, Izzo C, Rebulla P, Perroni L, Mariani M, Canestri G, et al. The first stable variant of erythrocyte glucose-phosphate isomerase associated with severe hemolytic anemia. Am J Hematol. 1980;9(1):1–11.
Beutler E, West C, Britton HA, Harris J, Forman L. Glucosephosphate isomerase (GPI) deficiency mutations associated with hereditary nonspherocytic hemolytic anemia (HNSHA). Blood Cells Mol Dis. 1997;23(3):402–9.
Kugler W, Breme K, Laspe P, Muirhead H, Davies C, Winkler H, et al. Molecular basis of neurological dysfunction coupled with haemolytic anaemia in human glucose-6-phosphate isomerase (GPI) deficiency. Hum Genet. 1998;103(4):450–4.
Puliyel M, Gallagher PG, Berdoukas V, Glader B, Coates T. Glucose phosphate isomerase deficiency in 2 patients with novel mutations presenting as severe neurologic abnormalities and transfusion dependent hemolytic anemia. Blood. 2013;122(21):947.
Jamwal M, Aggarwal A, Das A, Maitra A, Sharma P, Krishnan S, et al. Next-generation sequencing unravels homozygous mutation in glucose-6-phosphate isomerase, GPIc.1040G>a (p.Arg347His) causing hemolysis in an Indian infant. Clin Chim Acta Int J Clin Chem. 2017;468:81–4.
Huang K, Wu RH, Yao JF, Zhang WH, Fang BL, Ma JY, et al. Glucose phosphate isomerase gene mutation causing severe hemolytic anemia with residual neurological symptoms. Zhonghua Er Ke Za Zhi Chin J Pediatr. 2019;57(2):153–5.
Kedar PS, Dongerdiye R, Chilwirwar P, Gupta V, Chiddarwar A, Devendra R, et al. Glucose phosphate isomerase deficiency: high prevalence of p.Arg347His mutation in Indian population associated with severe hereditary non-Spherocytic hemolytic Anemia coupled with neurological dysfunction. Indian J Pediatr. 2019;86(8):692–9.
Huang P, Tang L, Wang AP, Liu LJ, Xiong J, Xiao YY, et al. Glucose-6-phosphate isomerase deficiency caused by GPI gene mutation: a case report and literature review - CNKI. J Clin Pediatr. 2020;38(10):785–8.
See WQ, So C-CJ, Cheuk DK-L, van Wijk R, Ha S-Y. Congenital hemolytic Anemia because of glucose phosphate isomerase deficiency: identification of 2 novel missense mutations in the GPI gene. J Pediatr Hematol Oncol. 2020;42(7):e696–7.
Park H, Haller J, Smith F, Parkin N, Lythe T, Zoeller RA, et al. Attenuation of hemolysis due to Glucose-6-phosphate isomerase deficiency with ketogenic diet – a case report. HemaSphere. 2020;4(1):e328.
Kedar PS, Gupta V, Dongerdiye R, Chiddarwar A, Warang P, Madkaikar MR. Molecular diagnosis of unexplained haemolytic anaemia using targeted next-generation sequencing panel revealed (p.Ala337Thr) novel mutation in GPI gene in two Indian patients. J Clin Pathol. 2019;72(1):81–5.
Zanella A, Rebulla P, Izzo C, Zanuso F, Kahane I, Molinari E, et al. A new mutant erythrocyte glucosephosphate isomerase (GPI) associated with GSH abnormality. Am J Hematol. 1978;5(1):11–23.
Baronciani L, Zanella A, Bianchi P, Zappa M, Alfinito F, Iolascon A, et al. Study of the molecular defects in glucose phosphate isomerase-deficient patients affected by chronic hemolytic anemia. Blood. 1996;88(6):2306–10.
Rossi F, Ruggiero S, Gallo M, Simeone G, Matarese SMR, Nobili B. Amoxicillin-induced hemolytic anemia in a child with glucose 6-phosphate isomerase deficiency. Ann Pharmacother. 2010;44(7–8):1327–9.
The human gene mutation database. D.N. Cooper, E.V. Ball, P.D. Stenson, A.D. Phillips, K. Evans, S. Heywood, et al. 2022. http://www.hgmd.cf.ac.uk/ac/index.php. Accessed 12 Feb 2022.
Zhao XM, Hu YM, Geng LZ, Hu YH, Zheng P, Wu ZL, et al. Xiantianxing putaotanglinsuanyigoumei quefa suozhi rongxuexing pinxue yili [glucose phosphate isomerase gene mutation causing severe hemolytic anemia]. Zhonghua Xue Ye Xue Za Zhi Chin J Hematol. 1992;13(5):266–7.
Read J, Pearce J, Li X, Muirhead H, Chirgwin J, Davies C. The crystal structure of human phosphoglucose isomerase at 1.6 a resolution: implications for catalytic mechanism, cytokine activity and haemolytic anaemia. J Mol Biol. 2001;309(2):447–63.
Lin H, Kao Y, Chen S, Meng M. Effects of inherited mutations on catalytic activity and structural stability of human glucose-6-phosphate isomerase expressed in Escherichia coli. Biochim Biophys Acta. 2009;1794(2):315–23.
Acknowledgements
Not applicable.
Funding
The writing of the current manuscript was supported by the National Natural Science Foundation (81670786), Key R&D Projects of Zhejiang Provincial Department of Science and Technology (2021C03094), and Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents (2014). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Ethics declarations
Ethics approval and consent to participate
The use of patient and patient’s parents data was approved by the Institutional Ethics Committee of Children’s Hospital, Zhejiang University School of Medicine, and written informed consents were obtained from the patient’s parents.
Consent for publication
The written consent form for publication has been obtained from the guardians of the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zu, Y., Wang, H., Lin, W. et al. Hereditary nonspherocytic hemolytic anemia caused by glucose-6-phosphate isomerase (GPI) deficiency in a Chinese patient: a case report. BMC Pediatr 22, 461 (2022). https://doi.org/10.1186/s12887-022-03522-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03522-9