Abstract
Objective
To systematically review radiomic feature reproducibility and model validation strategies in recent studies dealing with CT and MRI radiomics of bone and soft-tissue sarcomas, thus updating a previous version of this review which included studies published up to 2020.
Methods
A literature search was conducted on EMBASE and PubMed databases for papers published between January 2021 and March 2023. Data regarding radiomic feature reproducibility and model validation strategies were extracted and analyzed.
Results
Out of 201 identified papers, 55 were included. They dealt with radiomics of bone (n = 23) or soft-tissue (n = 32) tumors. Thirty-two (out of 54 employing manual or semiautomatic segmentation, 59%) studies included a feature reproducibility analysis. Reproducibility was assessed based on intra/interobserver segmentation variability in 30 (55%) and geometrical transformations of the region of interest in 2 (4%) studies. At least one machine learning validation technique was used for model development in 34 (62%) papers, and K-fold cross-validation was employed most frequently. A clinical validation of the model was reported in 38 (69%) papers. It was performed using a separate dataset from the primary institution (internal test) in 22 (40%), an independent dataset from another institution (external test) in 14 (25%) and both in 2 (4%) studies.
Conclusions
Compared to papers published up to 2020, a clear improvement was noted with almost double publications reporting methodological aspects related to reproducibility and validation. Larger multicenter investigations including external clinical validation and the publication of databases in open-access repositories could further improve methodology and bring radiomics from a research area to the clinical stage.
Critical relevance statement
An improvement in feature reproducibility and model validation strategies has been shown in this updated systematic review on radiomics of bone and soft-tissue sarcomas, highlighting efforts to enhance methodology and bring radiomics from a research area to the clinical stage.
Key points
• 2021–2023 radiomic studies on CT and MRI of musculoskeletal sarcomas were reviewed.
• Feature reproducibility was assessed in more than half (59%) of the studies.
• Model clinical validation was performed in 69% of the studies.
• Internal (44%) and/or external (29%) test datasets were employed for clinical validation.
Graphical Abstract

Similar content being viewed by others
Introduction
The term “radiomics” indicates the extraction and analysis of large amounts of quantitative parameters, also known as radiomic features, from medical images [1]. Similar to other “omics” technologies (e.g., genomics and proteomics), the extraction of quantitative information from images obtained during standard clinical workflows may potentially enable an extensive tumor characterization, including its genotype and predictions regarding prognosis [1,2,3]. Although radiomics holds great potential to augment clinical decision-making, translation to clinical practice is very limited compared to preclinical software development [4, 5]. The translational gap is at least partially attributable to low overall methodological quality of radiomics research and reporting. This was recently highlighted in a systematic review evaluating the application of the Radiomics Quality Score [6], which was proposed by Lambin et al. in 2017 and is currently the most widespread tool to assess the comprehensiveness and adequacy of radiomic pipelines, as well as the quality of their reporting [7]. Another important initiative aiming to improve standardization and reproducibility was the Image Biomarker Standardization Initiative, which provided a stepwise consensus for different parts of execution of radiomics pipelines [8].
To bridge the gap between academic endeavors and real-life application, certain challenges of radiomics must be addressed carefully. As radiomics is based on a two-step approach consisting of data extraction and analysis [9], the main challenge of the first step (i.e., data extraction) is the reproducibility of radiomic features, which is influenced by several parameters related to image acquisition, region of interest (ROI) delineation and post-processing [10, 11]. The main challenge of the second step (i.e., data analysis) is validation of the radiomics-based models, which are built with the aim of predicting the diagnosis or outcome of interest [11]. The issues of feature reproducibility and validation strategies are well addressed as separate items in Radiomics Quality Score [7]. Additionally, they are included in international guidelines recently published to guide the translation of radiomics into clinical practice, such as criteria for development of radiomic models [12] and a checklist for evaluation of radiomics research endorsed by the European Society of Radiology and European Society of Medical Imaging Informatics [13].
In musculoskeletal oncology, radiomic studies have shown encouraging results to improve diagnosis and prognosis prediction of bone and soft-tissue sarcomas [14], which are rare cancers where quantitative imaging data may certainly aid in clinical management. Reproducibility and validation strategies in radiomics of bone and soft-tissue sarcomas were assessed in a previous systematic review including papers published up to December 2020 [14]. Reproducibility analysis and independent clinical validation were reported in 37% and 10% of the papers, respectively [14]. Particularly, the relative rarity of bone and soft-tissue sarcomas certainly contributed to preventing model validation in large datasets, thus highlighting the need for multi-center investigations or registries. Hence, the authors recommended future efforts to bring the field of radiomics from a preclinical research area to the clinical stage [14]. Since then, the number of radiomics research papers has rapidly increased. Combined with the great attention currently paid to reproducibility and validation strategies in radiomic workflows, this increase highlights the need for an update of the previous review [14] following guidelines on when and how to update systematic reviews [15]. Thus, the aim of our current study is to systematically review radiomic feature reproducibility and model validation strategies in recent studies dealing with computed tomography (CT) and magnetic resonance imaging (MRI) radiomics of bone and soft-tissue sarcomas, which have been published since 2021. The ultimate goal is to promote and facilitate a consensus on feature reproducibility and model validation in radiomic workflows.
Methods
The study was registered on the International Prospective Register of Systematic Reviews database with the registration number CRD42023395542. The methods used in the current review paralleled those employed in the previous version [14], except for the number of reviewers involved in literature search, study selection, and data extraction, namely three in the current and two in the previous reviews. Additionally, in data extraction, segmentation process and style were grouped under baseline study characteristics in the previous review [14]. Conversely, these items constituted a separate category in the current version, which also included information regarding radiomic feature types as broad categories.
Reviewers
Literature search, study selection, and data extraction were performed independently by three musculoskeletal radiologists with 3 to 5 years of experience in radiomics and bone and soft-tissue sarcomas (S.G., C.M., D.A.). In case of disagreement, an agreement was achieved by consensus of these three readers and a fourth radiologist with 8 years of experience in artificial intelligence and radiomics (R.C.). The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were followed [16]. PRISMA checklist is provided as a supplementary table (Supplementary file 1).
Search strategy
An electronic literature search was conducted on EMBASE (Elsevier) and PubMed (MEDLINE, US National Library of Medicine and National Institutes of Health) databases for studies dealing with CT and MRI radiomics of bone and soft-tissue sarcomas, which were published between 1st January 2021 and 31st March 2023. A controlled vocabulary was adopted using medical subject headings in PubMed and the thesaurus in EMBASE. Search syntax was built by combining search terms related to two main domains, namely “musculoskeletal sarcomas” and “radiomics.” The exact search query was: (“sarcoma”/exp OR “sarcoma”) AND (“radiomics”/exp OR “radiomics” OR “texture”/exp OR “texture”). Studies were first screened by title and abstract. The full text and supplementary material of eligible studies were retrieved for further review. The references of eligible papers were also checked for additional publications to include.
Inclusion and exclusion criteria
Inclusion criteria were (i) original research studies published in peer-reviewed journals; (ii) focus on CT or MRI radiomics-based characterization of sarcomas located in bone and soft tissues for either diagnosis- or prognosis-related tasks; (iii) statement that local ethics committee approval was obtained, or ethical standards of the institutional or national research committee were followed. Exclusion criteria were (i) studies not dealing with mass characterization, such as those focused on computer-assisted diagnosis and detection systems; (ii) studies concerning retroperitoneal and visceral sarcomas or cancers other than sarcoma; (iii) animal, cadaveric or laboratory studies; (iv) papers published in languages other than English; (v) studies already included in the previous version of this review [14], such as those published online in 2020 and in a volume/issue in 2021.
Data extraction
Data were extracted to a spreadsheet with a drop-down list for all items, which were grouped into four main categories, namely baseline study characteristics, segmentation and radiomic feature type, radiomic feature reproducibility strategies, and predictive model validation strategies. Items regarding baseline study characteristics included first author’s last name, year of publication, study aim, tumor type, study design, reference standard, imaging modality, database size, and use of public data. Items concerning segmentation and radiomic feature types were segmentation process, segmentation style, and radiomic feature types as broad categories. Items regarding radiomic feature reproducibility included strategies, statistical methods, and thresholds used for reproducibility analysis. Finally, items concerning model validation included the use of machine learning validation techniques, clinical validation performed on a separate internal dataset, and clinical validation performed on an external dataset.
Results
Baseline study characteristics
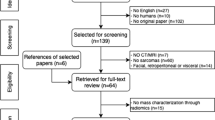
A flowchart showing the literature search process is shown in Fig. 1. After screening 201 papers and applying the eligibility criteria, 55 papers were finally included in this systematic review. Tables 1 and 2 show the characteristics of studies on radiomics of bone (n = 23) and soft-tissue (n = 32) sarcomas, respectively.
Twenty-four out of 55 studies (44%) were published in 2021, 23 (42%) in 2022, and 8 (14%) between January and March 2023. The design was prospective in 1 study (2%) and retrospective in the remaining 54 studies (98%). The investigated imaging modality was MRI (one or multiple sequences) in 43 studies (78%), CT in 9 (16%), and a combination of both in 3 (6%). The median size of the database was 120 lesions (range 25–810). In 3 studies multiple lesions for the same patient(s) were considered, thus including 142 [17], 128 [18], and 161 [19] lesions from 36, 125, and 160 patients, respectively. Public data were used only in 1 (2%) study.
Included studies aimed at predicting either diagnosis or prognosis. In diagnostic studies, classification tasks were benign vs. malignant (including intermediate malignancies such as atypical lipomatous tumor) tumor discrimination (n = 20), grading (n = 8), tumor histotype discrimination (n = 2), proliferation index Ki-67 expression (n = 1), and evaluation of marginal infiltration (n = 1). Prognostic studies aimed at predicting survival (n = 10), local and/or metastatic relapse (n = 9), response to chemotherapy or radiotherapy (n = 11), treatment complications (n = 1), and natural evolution over time before starting any treatment (n = 1). It should be noted that the aim was two- or threefold in some studies, as detailed in Tables 1 and 2. In studies focused on diagnosis-related tasks, histology was the reference standard in all cases except benign lesions diagnosed on the basis of stable imaging findings over time in four papers [17, 20,21,22]. In studies dealing with survival prediction, survival was assessed based on clinical follow-up. In studies focused on the prediction of tumor relapse, the reference standard was based on histology or clinical and imaging follow-up. In one study, the criteria for determining relapse were not specified [23]. In studies aimed at therapy response prediction, the reference standard was histology in all but one study where the response was assessed based on clinical and imaging evaluation [24]. Treatment complications were assessed based on clinical and surgical data. In the study dealing with natural evolution monitoring, radiomics was correlated to gene expression assessed using RNA sequencing [25].
Segmentation and feature types
The segmentation process was performed only manually in 48 (87%) studies, semiautomatically in 5 (9%) studies, both manually and automatically (for handcrafted and deep features, respectively) in 1 study (2%), and only automatically in 1 (2%) study. Of note, in one study, manual segmentation was performed to extract handcrafted features and, in parallel, deep features were extracted from the whole images with no segmentation [26]. In three studies, tumor borders were manually delineated on one image of interest, and ROIs were then co-registered with a different MRI sequence or imaging modality [18, 24, 27]. In another study, manual segmentation was performed to include the tumor area, and an additional cubic ROI was placed in a non-tumorous area to evaluate non-tumorous radiomics [28].
The following segmentation styles were identified: 3D in 45 (82%) studies, 2D without multiple sampling in 7 (13%) studies, 2D with multiple sampling in 1 (2%) study, and multiple segmentation styles such as 3D and 2D without multiple sampling in 1 (2%) study. In the remaining study, the segmentation style was not specified [29]. Of note, a single slice showing maximum tumor extension was chosen in all studies employing 2D segmentation without multiple sampling, except in one case where it was chosen based on tumor characteristics [30] and another study where the criteria for slice selection were not specified [31].
Regarding the radiomic feature types, 48 (87%) studies included only handcrafted features, 6 (11%) studies included both handcrafted and deep features, and the remaining (2%) study included only deep features.
Feature reproducibility
Thirty-two (59%) of the 54 studies employing manual or semiautomatic segmentation process included a reproducibility analysis in their workflow. In 30 (55%) investigations [19,20,21, 23, 26, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], the reproducibility of radiomic features was assessed based on repeated segmentations performed by different readers and/or the same reader at different time points. In 2 (4%) studies [57, 58], feature reproducibility was assessed through small geometrical transformations of the ROIs mimicking multiple manual delineations. In detail, small translations of the ROI were applied in different directions, and the entity of these translations was 10% of the length of the bounding box including the tumor [57, 58]. No studies evaluated feature reproducibility based on different acquisition or post-processing techniques. The distribution of the employed feature reproducibility strategies among the included studies is shown in the bar plot in Fig. 2. Of note, in 3 studies [59,60,61], repeated segmentations were performed to assess similarity (using Dice similarity coefficient) but feature reproducibility was not evaluated. Additionally, segmentations were validated by a second experienced reader in 7 studies [17, 25, 28,29,30, 62, 63] without, however, addressing the issue of feature reproducibility.
The intraclass correlation coefficient (ICC) was the statistical method used in all papers reporting a reproducibility analysis. ICC threshold ranged between 0.7 [54] and 0.9 [20, 46] for reproducible features. Additionally, the following statistical methods were used less commonly: Bland–Altman method [54], Pearson’s correlation coefficient [52], and Spearman’s rank-order coefficient [52].
Validation techniques
At least one machine learning validation technique was used in 34 (62%) of the 55 papers. K-fold cross-validation was used in most of the studies [18, 20, 22, 24, 27, 31, 37,38,39, 44, 47, 49, 52, 54, 57, 58, 60,61,62,63,64,65,66,67,68]. The following machine learning validation techniques were used less commonly: bootstrapping [34, 46], leave-one-out cross-validation [17, 28], and nested cross-validation [43, 55, 56, 69]. In one study, both K-fold cross-validation and nested cross-validation techniques were employed [50]. Figure 3 provides an overview of these machine learning validation techniques.
Overview of machine learning validation techniques. In k-fold cross-validation (a), the data is split into k equally sized partitions, and each is used in turn to validate a model trained on the remaining. The process for leave-one-out cross-validation (b) is the same, but k equals the total sample size. In nested cross-validation (c), an outer and an inner loops of k-fold cross-validation are performed. Typically, the inner loop is used for model tuning, and the outer one to assess its accuracy. Bootstrapping (d) is based on a different principle: random sampling from the original dataset is performed, with replacement. As a result, the produced samples may include multiple (or even no) instances of each original case
Clinical validation
A clinical validation of the radiomics-based prediction model was reported in 38 (69%) of the 55 studies. In 22 (40%) studies, it was performed on a separate set of data from the primary institution, namely the internal test dataset, which was chosen randomly [19, 20, 24, 28, 29, 31, 32, 37, 41, 42, 45, 47, 52, 53, 59, 65, 66, 68], based on temporal criteria [61, 69, 70] or different acquisition scanners [62]. Of note, in a multi-center study, patients were split into training and test cohorts randomly rather than following geographical criteria [68]. Thus, this was considered as an internal test dataset. In 14 (25%) studies [26, 36, 38, 39, 43, 44, 48,49,50,51, 56, 63, 64, 67], clinical validation was performed on an independent set of data from an external institution, namely the external test dataset. In 2 (4%) studies [22, 33], both internal and external test datasets were used for clinical validation. The distribution of the employed clinical validation strategies among the included studies is shown in the bar plot in Fig. 4. Radiomic feature reproducibility and model validation strategies of the included studies are summarized in Table 3, along with the same information extracted from the previous version of this review [14] for comparison.
Discussion
This systematic review addressed the issues of feature reproducibility and validation strategies in CT and MRI radiomics of bone and soft-tissue sarcomas, as these are two main challenges hampering the generalizability of radiomic models and preventing their clinical implementation. Among papers published between January 2021 and March 2023, more than half reported a reproducibility analysis of radiomic features (59%) and a clinical validation of the predictive model against an internal test dataset, an external test dataset, or both (69% overall, among which 29% also or exclusively external). These assessments almost doubled compared to the previous version of this review including papers published up to December 2020, where they amounted to 37% and 39%, respectively [14]. Hence, although the percentage of investigations without any reproducibility and/or validation assessment is still considerable, significant efforts have been made to include them in radiomics studies to facilitate generalizability and thus clinical transferability. In particular, external clinical validation is crucial to ensure clinical translation of imaging biomarkers and should be encouraged.
CT and MRI radiomics of bone and soft-tissue sarcomas have progressively gained attention in musculoskeletal oncology to solve several diagnosis- or outcome-related tasks. In the previous version of this review [14], a rapid increase in research papers was observed and almost half of them (n = 23) were published in 2020. Since then, the number of new publications has remained almost unchanged every year, with 24 papers in 2021, 23 in 2022, and 8 in the first trimester of 2023. Most included studies (98%) were retrospective, similarly to the previous review [14]. Although prospective studies could provide the highest level of evidence supporting the clinical validity and usefulness of radiomic biomarkers [7], bone and soft-tissue sarcomas are low prevalent [71, 72] and retrospective design allows including relatively large amounts of data already available in radiology departments. The median size of the database was 120 lesions, having doubled compared to the previous review [14]. Of note, the use of public data was described only in one study dealing with soft-tissue sarcomas (2%) [46], even less than the previous review where it was reported in three cases [14]. Specifically, a public dataset available on The Cancer Imaging Archive was employed (https://www.cancerimagingarchive.net) [73]. Public datasets are essential to allow research groups from around the world to test and compare different radiomic models using common data. Hence, the use of public data should be promoted through new publicly available imaging databases in the future.
Segmentations included the entire tumor volume (3D) in most studies (84%) and, less frequently, single slices (2D) with or without multiple sampling. The segmentation process was performed manually in most studies (89%) and semiautomatically less frequently, as also observed in the previous review [14]. In addition, a fully automatic segmentation was used in two investigations (4%, one of which employing both automatic and manual segmentations). Furthermore, while most studies included only handcrafted features, deep features were employed in 13% of the studies (either alone or together with handcrafted features). In contrast to handcrafted features based on predefined mathematical formulas, deep features are obtained inside the layers of convolutional neural networks [74]. Future investigations focusing on deep features and convolutional neural networks with the use of very large datasets will better highlight the potential value of deep learning methods in radiomic workflows.
Radiomic feature reproducibility was evaluated in more than half of the studies (59%) employing manual or semiautomatic segmentation, which increased by approximately three-quarters compared to the previous version of this review [14]. This methodological assessment allows for identifying robust features and avoiding biases related to non-reliable, noisy features [75]. Inter- and intra-observer variability related to multiple ROI delineations by different readers or the same reader at different time points was the focus of reproducibility analysis in most studies. Less frequently, ROI perturbations obtained through geometrical transformations were used to mimic multiple delineations and evaluate feature reproducibility. No study assessed the influence of image acquisition parameters or post-processing techniques on feature reproducibility. Thus, this latter domain deserves further investigation, which could be facilitated by prospective design in future studies. Finally, ICC was the statistical method of choice in all studies including a reproducibility analysis, with threshold values ranging from 0.7 to 0.9, which were in line with recent guidelines for performing and assessing ICC [76].
At least one machine learning validation technique was used in more than half (62%) of the papers and K-fold cross-validation was performed most commonly, similarly to the previous review [14]. These resampling strategies are extremely useful with relatively limited data samples to reduce overfitting and better estimate the radiomic model performance on new data [77, 78]. Besides, a clinical validation of the radiomic model should be performed through real testing against unseen data [79]. We found that clinical validation was reported in 69% of studies. In detail, it was performed against unseen separate data from the primary institution (internal test dataset) and unseen independent data from a different institution (external test dataset) in 44% and 29% of the studies, respectively. Of note, two studies (4%) included both internal and external test datasets for clinical validation. The number of radiomic papers reporting clinical validation increased compared to the previous review [14] and, particularly, the number of those including an external test dataset tripled. Although the percentage of studies without any clinical validation is not negligible and future efforts are required, this may suggest that we are on the right track to bridge the gap between research concepts and clinical application in radiomics of bone and soft-tissue sarcomas.
Some limitations of this study need to be considered. First, this review focused on feature reproducibility and model validation strategies employed in bone and soft-tissue sarcoma studies to facilitate achieving a consensus on these aspects in radiomic workflows. However, this consensus has still to be reached. Second, this study is limited to a systematic review and no meta-analysis was performed, as radiomic papers dealing with bone and soft-tissue sarcomas are heterogenous in terms of objectives and subgroups of sarcoma with relatively small sample size per each objective and subgroup. Additionally, most studies assessed reproducibility as a feature-reduction method in radiomic pipelines based on an ICC threshold, without reporting ICC values for all features. Finally, in studies reporting a clinical validation, different metrics were used for model performance estimation. All these reasons prevented us from including reproducibility and validation methods in a meta-analysis.
Limitations notwithstanding, feature reproducibility and validation strategies were systematically reviewed in radiomic studies dealing with bone and soft-tissue sarcomas and published between January 2021 and March 2023. Compared to a previous review addressing the same issues in studies published up to December 2020 [14], a clear improvement was noted with almost double publications reporting methodological aspects related to reproducibility and validation. Larger investigations involving multiple institutions and the publication of new databases in freely available repositories should be promoted to further improve the methodology of radiomic studies and bring them a from preclinical research area to the clinical stage.
Availability of data and materials
Data supporting the results can be obtained upon request to the corresponding author.
Abbreviations
- CT:
-
Computed tomography
- ICC:
-
Intraclass correlation coefficient
- MRI:
-
Magnetic resonance imaging
- ROI:
-
Region of interest
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses
References
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577. https://doi.org/10.1148/radiol.2015151169
Gitto S, Cuocolo R, Albano D et al (2020) MRI radiomics-based machine-learning classification of bone chondrosarcoma. Eur J Radiol 128:109043. https://doi.org/10.1016/j.ejrad.2020.109043
Gitto S, Interlenghi M, Cuocolo R et al (2023) MRI radiomics-based machine learning for classification of deep-seated lipoma and atypical lipomatous tumor of the extremities. Radiol Med 128:989–998. https://doi.org/10.1007/s11547-023-01657-y
Kocak B, Baessler B, Cuocolo R et al (2023) Trends and statistics of artificial intelligence and radiomics research in Radiology, Nuclear Medicine, and Medical Imaging: bibliometric analysis. Eur Radiol 33:7542–7555. https://doi.org/10.1007/s00330-023-09772-0
van Leeuwen KG, Schalekamp S, Rutten MJCM, van Ginneken B, de Rooij M (2021) Artificial intelligence in radiology: 100 commercially available products and their scientific evidence. Eur Radiol 31:3797–3804. https://doi.org/10.1007/s00330-021-07892-z
Spadarella G, Stanzione A, Akinci D’Antonoli T et al (2022) Systematic review of the radiomics quality score applications: an EuSoMII Radiomics Auditing Group Initiative. Eur Radiol 33:1884–1894. https://doi.org/10.1007/s00330-022-09187-3
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762. https://doi.org/10.1038/nrclinonc.2017.141
Zwanenburg A, Vallières M, Abdalah MA et al (2020) The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 295:328–338. https://doi.org/10.1148/radiol.2020191145
Kocak B, Durmaz ES, Erdim C, Ates E, Kaya OK, Kilickesmez O (2020) Radiomics of Renal Masses: Systematic Review of Reproducibility and Validation Strategies. AJR Am J Roentgenol 214:129–136. https://doi.org/10.2214/AJR.19.21709
Varghese BA, Cen SY, Hwang DH, Duddalwar VA (2019) Texture Analysis of Imaging: What Radiologists Need to Know. AJR Am J Roentgenol 212:520–528. https://doi.org/10.2214/AJR.18.20624
Traverso A, Wee L, Dekker A, Gillies R (2018) Repeatability and Reproducibility of Radiomic Features: A Systematic Review. Int J Radiat Oncol Biol Phys 102:1143–1158. https://doi.org/10.1016/j.ijrobp.2018.05.053
Huang EP, O’Connor JPB, McShane LM et al (2023) Criteria for the translation of radiomics into clinically useful tests. Nat Rev Clin Oncol 20:69–82. https://doi.org/10.1038/s41571-022-00707-0
Kocak B, Baessler B, Bakas S et al (2023) CheckList for EvaluAtion of Radiomics research (CLEAR): a step-by-step reporting guideline for authors and reviewers endorsed by ESR and EuSoMII. Insights Imaging 14:75. https://doi.org/10.1186/s13244-023-01415-8
Gitto S, Cuocolo R, Albano D et al (2021) CT and MRI radiomics of bone and soft-tissue sarcomas: a systematic review of reproducibility and validation strategies. Insights Imaging 12:68. https://doi.org/10.1186/s13244-021-01008-3
Garner P, Hopewell S, Chandler J et al (2016) When and how to update systematic reviews: consensus and checklist. BMJ 354:i3507. https://doi.org/10.1136/bmj.i3507
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 6:n71. https://doi.org/10.1136/bmj.n71
Ristow I, Madesta F, Well L et al (2022) Evaluation of magnetic resonance imaging-based radiomics characteristics for differentiation of benign and malignant peripheral nerve sheath tumors in neurofibromatosis type 1. Neuro Oncol 24:1790–1798. https://doi.org/10.1093/neuonc/noac100
Fields BKK, Demirjian NL, Hwang DH et al (2021) Whole-tumor 3D volumetric MRI-based radiomics approach for distinguishing between benign and malignant soft tissue tumors. Eur Radiol 31:8522–8535. https://doi.org/10.1007/s00330-021-07914-w
Hu P, Chen L, Zhou Z (2021) Machine Learning in the Differentiation of Soft Tissue Neoplasms: Comparison of Fat-Suppressed T2WI and Apparent Diffusion Coefficient (ADC) Features-Based Models. J Digit Imaging 34:1146–1155. https://doi.org/10.1007/s10278-021-00513-7
Cilengir AH, Evrimler S, Serel TA, Uluc E, Tosun O (2023) The diagnostic value of magnetic resonance imaging-based texture analysis in differentiating enchondroma and chondrosarcoma. Skeletal Radiol 52:1039–1049. https://doi.org/10.1007/s00256-022-04242-y
Ozturk M, Polat AV, Selcuk MB (2021) Whole-lesion ADC histogram analysis versus single-slice ADC measurement for the differentiation of benign and malignant soft tissue tumors. Eur J Radiol 143:109934. https://doi.org/10.1016/j.ejrad.2021.109934
Chianca V, Cuocolo R, Gitto S et al (2021) Radiomic Machine Learning Classifiers in Spine Bone Tumors: A Multi-Software. Multi-Scanner Study. Eur J Radiol 137:109586. https://doi.org/10.1016/j.ejrad.2021.109586
Giraudo C, Fichera G, Del Fiore P et al (2022) Tumor cellularity beyond the visible in soft tissue sarcomas: Results of an ADC-based, single center, and preliminary radiomics study. Front Oncol 12:879553. https://doi.org/10.3389/fonc.2022.879553
Buizza G, Paganelli C, D’Ippolito E et al (2021) Radiomics and Dosiomics for Predicting Local Control after Carbon-Ion Radiotherapy in Skull-Base Chordoma. Cancers (Basel) 13:339. https://doi.org/10.3390/cancers13020339
Crombé A, Bertolo F, Fadli D et al (2023) Distinct patterns of the natural evolution of soft tissue sarcomas on pre-treatment MRIs captured with delta-radiomics correlate with gene expression profiles. Eur Radiol 33:1205–1218. https://doi.org/10.1007/s00330-022-09104-8
Liu S, Sun W, Yang S et al (2022) Deep learning radiomic nomogram to predict recurrence in soft tissue sarcoma: a multi-institutional study. Eur Radiol 32:793–805. https://doi.org/10.1007/s00330-021-08221-0
Fields BKK, Demirjian NL, Cen SY et al (2023) Predicting Soft Tissue Sarcoma Response to Neoadjuvant Chemotherapy Using an MRI-Based Delta-Radiomics Approach. Mol Imaging Biol 25:776–787. https://doi.org/10.1007/s11307-023-01803-y
Xu L, Yang P, Hu K et al (2021) Prediction of neoadjuvant chemotherapy response in high-grade osteosarcoma: added value of non-tumorous bone radiomics using CT images. Quant Imaging Med Surg 11:1184–1195. https://doi.org/10.21037/qims-20-681
Yang Y, Ma X, Wang Y, Ding X (2022) Prognosis prediction of extremity and trunk wall soft-tissue sarcomas treated with surgical resection with radiomic analysis based on random survival forest. Updates Surg 74:355–365. https://doi.org/10.1007/s13304-021-01074-8
Chang H, Kang Y, Ahn JM, Lee E, Lee JW, Kang HS (2022) Texture analysis of magnetic resonance image to differentiate benign from malignant myxoid soft tissue tumors: A retrospective comparative study. PLoS One 17:e0267569. https://doi.org/10.1371/journal.pone.0267569
Zhong J, Zhang C, Hu Y et al (2022) Automated prediction of the neoadjuvant chemotherapy response in osteosarcoma with deep learning and an MRI-based radiomics nomogram. Eur Radiol 32:6196–6206. https://doi.org/10.1007/s00330-022-08735-1
Yue Z, Wang X, Wang Y, Wang H, Jiang W (2022) Clinical-Radiomics Nomogram from T1W, T1CE, and T2FS MRI for Improving Diagnosis of Soft-Tissue Sarcoma. Mol Imaging Biol 24:995–1006. https://doi.org/10.1007/s11307-022-01751-z
Hu Y, Wang H, Yue Z et al (2023) A contrast-enhanced MRI-based nomogram to identify lung metastasis in soft-tissue sarcoma: A multi-centre study. Med Phys 50:2961–2970. https://doi.org/10.1002/mp.16136
Cay N, Mendi BAR, Batur H, Erdogan F (2022) Discrimination of lipoma from atypical lipomatous tumor/well-differentiated liposarcoma using magnetic resonance imaging radiomics combined with machine learning. Jpn J Radiol 40:951–960. https://doi.org/10.1007/s11604-022-01278-x
Fadli D, Kind M, Michot A, Le Loarer F, Crombe A (2022) Natural Changes in Radiological and Radiomics Features on MRIs of Soft-Tissue Sarcomas Naïve of Treatment: Correlations With Histology and Patients’ Outcomes. J Magn Reson Imaging 56:77–96. https://doi.org/10.1002/jmri.28021
Liang H, Yang S, Zou H et al (2022) Deep Learning Radiomics Nomogram to Predict Lung Metastasis in Soft-Tissue Sarcoma: A Multi-Center Study. Front Oncol 12:897676. https://doi.org/10.3389/fonc.2022.897676
Luo Z, Li J, Liao Y, Liu R, Shen X, Chen W (2022) Radiomics Analysis of Multiparametric MRI for Prediction of Synchronous Lung Metastases in Osteosarcoma. Front Oncol 12:802234. https://doi.org/10.3389/fonc.2022.802234
Gitto S, Cuocolo R, van Langevelde K et al (2022) MRI radiomics-based machine learning classification of atypical cartilaginous tumour and grade II chondrosarcoma of long bones. EBioMedicine 75:103757. https://doi.org/10.1016/j.ebiom.2021.103757
Yang Y, Zhang L, Wang T et al (2023) MRI Fat-Saturated T2-Weighted Radiomics Model for Identifying the Ki-67 Index of Soft Tissue Sarcomas. J Magn Reson Imaging 58:534–545. https://doi.org/10.1002/jmri.28518
Miao L, Cao Y, Zuo L et al (2022) Predicting pathological complete response of neoadjuvant radiotherapy and targeted therapy for soft tissue sarcoma by whole-tumor texture analysis of multisequence MRI imaging. Eur Radiol 33:3984–3994. https://doi.org/10.1007/s00330-022-09362-6
Sun W, Liu S, Guo J et al (2021) A CT-based radiomics nomogram for distinguishing between benign and malignant bone tumours. Cancer Imaging 21:20. https://doi.org/10.1186/s40644-021-00387-6
Zhang L, Ge Y, Gao Q et al (2021) Machine Learning-Based Radiomics Nomogram With Dynamic Contrast-Enhanced MRI of the Osteosarcoma for Evaluation of Efficacy of Neoadjuvant Chemotherapy. Front Oncol 11:758921. https://doi.org/10.3389/fonc.2021.758921
Peeken JC, Asadpour R, Specht K et al (2021) MRI-based delta-radiomics predicts pathologic complete response in high-grade soft-tissue sarcoma patients treated with neoadjuvant therapy. Radiother Oncol 164:73–82. https://doi.org/10.1016/j.radonc.2021.08.023
Chen H, Zhang X, Wang X et al (2021) MRI-based radiomics signature for pretreatment prediction of pathological response to neoadjuvant chemotherapy in osteosarcoma: a multicenter study. Eur Radiol 31:7913–7924. https://doi.org/10.1007/s00330-021-07748-6
Pan J, Zhang K, Le H et al (2021) Radiomics Nomograms Based on Non-enhanced MRI and Clinical Risk Factors for the Differentiation of Chondrosarcoma from Enchondroma. J Magn Reson Imaging 54:1314–1323. https://doi.org/10.1002/jmri.27690
Chen S, Li N, Tang Y et al (2021) Radiomics Analysis of Fat-Saturated T2-Weighted MRI Sequences for the Prediction of Prognosis in Soft Tissue Sarcoma of the Extremities and Trunk Treated With Neoadjuvant Radiotherapy. Front Oncol 11:710649. https://doi.org/10.3389/fonc.2021.710649
Pereira HM, Leite Duarte ME, Ribeiro Damasceno I, de Oliveira Moura Santos LA, Nogueira-Barbosa MH (2021) Machine learning-based CT radiomics features for the prediction of pulmonary metastasis in osteosarcoma. Br J Radiol 94:20201391. https://doi.org/10.1259/bjr.20201391
Yan R, Hao D, Li J et al (2021) Magnetic Resonance Imaging-Based Radiomics Nomogram for Prediction of the Histopathological Grade of Soft Tissue Sarcomas: A Two-Center Study. J Magn Reson Imaging 53:1683–1696. https://doi.org/10.1002/jmri.27532
Gitto S, Cuocolo R, Annovazzi A et al (2021) CT radiomics-based machine learning classification of atypical cartilaginous tumours and appendicular chondrosarcomas. EBioMedicine 68:103407. https://doi.org/10.1016/j.ebiom.2021.103407
Peeken JC, Neumann J, Asadpour R et al (2021) Prognostic Assessment in High-Grade Soft-Tissue Sarcoma Patients: A Comparison of Semantic Image Analysis and Radiomics. Cancers (Basel) 13:1929. https://doi.org/10.3390/cancers13081929
Liu J, Lian T, Chen H et al (2021) Pretreatment Prediction of Relapse Risk in Patients with Osteosarcoma Using Radiomics Nomogram Based on CT: A Retrospective Multicenter Study. Biomed Res Int 2021:6674471. https://doi.org/10.1155/2021/6674471
Sudjai N, Siriwanarangsun P, Lektrakul N et al (2023) Tumor-to-bone distance and radiomic features on MRI distinguish intramuscular lipomas from well-differentiated liposarcomas. J Orthop Surg Res 18:255. https://doi.org/10.1186/s13018-023-03718-4
Li X, Lan M, Wang X et al (2023) Development and validation of a MRI-based combined radiomics nomogram for differentiation in chondrosarcoma. Front Oncol 13:1090229. https://doi.org/10.3389/fonc.2023.1090229
White LM, Atinga A, Naraghi AM et al (2023) T2-weighted MRI radiomics in high-grade intramedullary osteosarcoma: predictive accuracy in assessing histologic response to chemotherapy, overall survival, and disease-free survival. Skeletal Radiol 52:553–564. https://doi.org/10.1007/s00256-022-04098-2
Yang Y, Zhou Y, Zhou C, Zhang X, Ma X (2022) MRI-Based Computer-Aided Diagnostic Model to Predict Tumor Grading and Clinical Outcomes in Patients With Soft Tissue Sarcoma. J Magn Reson Imaging 56:1733–1745. https://doi.org/10.1002/jmri.28160
Yang Y, Zhou Y, Zhou C, Ma X (2022) Novel computer aided diagnostic models on multimodality medical images to differentiate well differentiated liposarcomas from lipomas approached by deep learning methods. Orphanet J Rare Dis 17:158. https://doi.org/10.1186/s13023-022-02304-x
Gitto S, Corino VDA, Annovazzi A et al (2022) 3D vs. 2D MRI radiomics in skeletal Ewing sarcoma: Feature reproducibility and preliminary machine learning analysis on neoadjuvant chemotherapy response prediction. Front Oncol 12:1016123. https://doi.org/10.3389/fonc.2022.1016123
Gitto S, Bologna M, Corino VDA et al (2022) Diffusion-weighted MRI radiomics of spine bone tumors: feature stability and machine learning-based classification performance. Radiol Med 127:518–525. https://doi.org/10.1007/s11547-022-01468-7
Tang Y, Cui J, Zhu J, Fan G (2022) Differentiation Between Lipomas and Atypical Lipomatous Tumors of the Extremities Using Radiomics. J Magn Reson Imaging 56:1746–1754. https://doi.org/10.1002/jmri.28167
Lee S, Jung J-Y, Nam Y et al (2023) Diagnosis of Marginal Infiltration in Soft Tissue Sarcoma by Radiomics Approach Using T2-Weighted Dixon Sequence. J Magn Reson Imaging 57:752–760. https://doi.org/10.1002/jmri.28331
Lee SE, Jung J-Y, Nam Y et al (2021) Radiomics of diffusion-weighted MRI compared to conventional measurement of apparent diffusion-coefficient for differentiation between benign and malignant soft tissue tumors. Sci Rep 11:15276. https://doi.org/10.1038/s41598-021-94826-w
Cao J, Wang X, Qiao Y et al (2023) Differentiation of benign and malignant spinal schwannoma using guided attention inference networks on multi-source MRI: comparison with radiomics method and radiologist-based clinical assessment. Acta Radiol 64:1184–1193. https://doi.org/10.1177/02841851221119375
Fradet G, Ayde R, Bottois H et al (2022) Prediction of lipomatous soft tissue malignancy on MRI: comparison between machine learning applied to radiomics and deep learning. Eur Radiol Exp 6:41. https://doi.org/10.1186/s41747-022-00295-9
Bouhamama A, Leporq B, Khaled W et al (2022) Prediction of Histologic Neoadjuvant Chemotherapy Response in Osteosarcoma Using Pretherapeutic MRI Radiomics. Radiol Imaging Cancer 4:e210107. https://doi.org/10.1148/rycan.210107
Yin P, Sun C, Wang S et al (2021) Clinical-Deep Neural Network and Clinical-Radiomics Nomograms for Predicting the Intraoperative Massive Blood Loss of Pelvic and Sacral Tumors. Front Oncol 11:752672. https://doi.org/10.3389/fonc.2021.752672
Yin P, Zhi X, Sun C et al (2021) Radiomics Models for the Preoperative Prediction of Pelvic and Sacral Tumor Types: A Single-Center Retrospective Study of 795 Cases. Front Oncol 11:709659. https://doi.org/10.3389/fonc.2021.709659
Navarro F, Dapper H, Asadpour R et al (2021) Development and External Validation of Deep-Learning-Based Tumor Grading Models in Soft-Tissue Sarcoma Patients Using MR Imaging. Cancers (Basel) 13:2866. https://doi.org/10.3390/cancers13122866
Zhang M, Tong E, Hamrick F et al (2021) Machine-Learning Approach to Differentiation of Benign and Malignant Peripheral Nerve Sheath Tumors: A Multicenter Study. Neurosurgery 89:509–517. https://doi.org/10.1093/neuros/nyab212
Yamazawa E, Takahashi S, Shin M et al (2022) MRI-Based Radiomics Differentiates Skull Base Chordoma and Chondrosarcoma: A Preliminary Study. Cancers (Basel) 14:3264. https://doi.org/10.3390/cancers14133264
Deng X-Y, Chen H-Y, Yu J-N et al (2021) Diagnostic Value of CT- and MRI-Based Texture Analysis and Imaging Findings for Grading Cartilaginous Tumors in Long Bones. Front Oncol 11:700204. https://doi.org/10.3389/fonc.2021.700204
Gronchi A, Miah AB, Dei Tos AP et al (2021) Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 32:1348–1365. https://doi.org/10.1016/j.annonc.2021.07.006
Strauss SJ, Frezza AM, Abecassis N et al (2021) Bone sarcomas: ESMO–EURACAN–GENTURIS–ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 32:1520–1536. https://doi.org/10.1016/j.annonc.2021.08.1995
Vallières M, Freeman CR, Skamene SR, El Naqa I (2015) A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol 60:5471–5496. https://doi.org/10.1088/0031-9155/60/14/5471
Zhou X, Wang H, Feng C et al (2022) Emerging Applications of Deep Learning in Bone Tumors: Current Advances and Challenges. Front Oncol 12:908873. https://doi.org/10.3389/fonc.2022.908873
Gitto S, Cuocolo R, Emili I et al (2021) Effects of Interobserver Variability on 2D and 3D CT- and MRI-Based Texture Feature Reproducibility of Cartilaginous Bone Tumors. J Digit Imaging 34:820–832. https://doi.org/10.1007/s10278-021-00498-3
Koo TK, Li MY (2016) A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 15:155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Cuocolo R, Caruso M, Perillo T, Ugga L, Petretta M (2020) Machine Learning in oncology: A clinical appraisal. Cancer Lett 481:55–62. https://doi.org/10.1016/j.canlet.2020.03.032
Parmar C, Barry JD, Hosny A, Quackenbush J, Aerts HJ (2018) Data Analysis Strategies in Medical Imaging. Clin Cancer Res 24:3492–3499. https://doi.org/10.1158/1078-0432.CCR-18-0385
Fanciullo C, Gitto S, Carlicchi E, Albano D, Messina C (2022) Radiomics of Musculoskeletal Sarcomas: A Narrative Review. J Imaging 8:45. https://doi.org/10.3390/jimaging8020045
González-Viguera J, Reynés-Llompart G, Lozano A (2021) Outcomes and computed tomography radiomic features extraction in soft tissue sarcomas treated with neoadjuvant radiation therapy. Rep Pract Oncol Radiother 26:804–813. https://doi.org/10.5603/RPOR.a2021.0092
Funding
Investigator Grant awarded by Fondazione AIRC per la Ricerca sul Cancro for the project “RADIOmics-based machine-learning classification of BOne and Soft Tissue Tumors (RADIO-BOSTT)” (L.M. Sconfienza). The funding source provided financial support without any influence on the study design, on the acquisition and analysis of data, and on the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Search and collection of papers: S.G. and D.A. Extraction of data: S.G. and C.M. Analysis of data: S.G., R.C., and M.H. Paper draft: S.G. and R.C. Draft revision: M.H., C.M., D.A., P.O., E.K., M.M., P.vO., and L.M.S. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
L.M. Sconfienza is a member of the Insights into Imaging Advisory Editorial Board. He has not taken part in the review or selection process of this article. The remaining authors declare that they have no competing interests related to this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA 2020 Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gitto, S., Cuocolo, R., Huisman, M. et al. CT and MRI radiomics of bone and soft-tissue sarcomas: an updated systematic review of reproducibility and validation strategies. Insights Imaging 15, 54 (2024). https://doi.org/10.1186/s13244-024-01614-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-024-01614-x