Abstract
Objectives
To evaluate the performance of a deep learning radiomic nomogram (DLRN) model at predicting tumor relapse in patients with soft tissue sarcomas (STS) who underwent surgical resection.
Methods
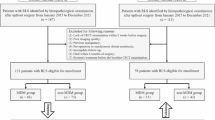
In total, 282 patients who underwent MRI and resection for STS at three independent centers were retrospectively enrolled. In addition, 113 of the 282 patients received additional contrast-enhanced MRI scans. We separated the participants into a development cohort and an external test cohort. The development cohort consisted of patients from one center and the external test cohort consisted of patients from two other centers. Two MRI-based DLRNs for prediction of tumor relapse after resection of STS were established. We universally tested the DLRNs and compared them with other prediction models constructed by using widespread adopted predictors (i.e., staging systems and Ki67) instead of radiomics features.
Results
The DLRN1 model incorporated plain MRI-based radiomics signature into the clinical data, and the DLRN2 model integrated radiomics signature extracted from plain and contrast-enhanced MRI with the clinical predictors. Across both study sets, the two MRI-based DLRNs had relatively better prognostic capability (C index ≥ 0.721 and median AUC ≥ 0.746; p < 0.05 compared with most other models and predictors) and less opportunity for prediction error (integrated Brier score ≤ 0.159). The decision curve analysis indicates that the DLRNs have greater benefits than staging systems, Ki67, and other models. We selected appropriate cutoff values for the DLRNs to divide STS recurrence into three risk strata (low, medium, and high) and calculated those groups’ cumulative risk rates.
Conclusion
The DLRNs were shown to be a reliable and externally validated tool for predicting STS recurrence by comparing with other prediction models.
Key Points
• The prediction of a high recurrence rate of STS before emergence of local recurrence can help to determine whether more active treatment should be implemented.
• Two MRI-based DLRNs for prediction of tumor relapse were shown to be a reliable and externally validated tool for predicting STS recurrence.
• We used the DLRNs to divide STS recurrence into three risk strata (low, medium, and high) to facilitate more targeted postoperative management in the clinic.






Similar content being viewed by others
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- C index:
-
Concordance index
- CNNs:
-
Convolutional neural networks
- DL:
-
Deep learning
- DLRN model:
-
Deep learning radiomic nomogram model
- FNCLCC:
-
French Federation Nationale des Centres de Lutte Contre le Cancer
- IBS:
-
Integrated Brier score
- MSKCC:
-
Memorial Sloan-Kettering Cancer Center
- NCI:
-
United States National Cancer Institute
- RFS time:
-
Relapse-free survival time
- SNR:
-
The signal-to-noise ratio
- STSs:
-
Soft tissue sarcomas
References
Guerrero WM, Deneve JL (2016) Local recurrence of extremity soft tissue sarcoma. Surg Clin North Am 96(5):1157–1174
Hansen T, Katenkamp K, Brodhun M, Katenkamp D (2006) Low-grade fibrosarcoma--report on 39 not otherwise specified cases and comparison with defined low-grade fibrosarcoma types. Histopathology. 49(2):152–160
Tan MC, Brennan MF, Kuk D et al (2016) Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surg 263(3):593–600
Snow SN, Gordon EM, Larson PO, Bagheri MM, Bentz ML, Sable DB (2004) Dermatofibrosarcoma protuberans: a report on 29 patients treated by Mohs micrographic surgery with long-term follow-up and review of the literature. Cancer. 101(1):28–38
Abatzoglou S, Turcotte RE, Adoubali A, Isler MH, Roberge D (2010) Local recurrence after initial multidisciplinary management of soft tissue sarcoma: is there a way out? Clin Orthop Relat Res 468(11):3012–3018
Gronchi A, Lo Vullo S, Colombo C et al (2010) Extremity soft tissue sarcoma in a series of patients treated at a single institution local control directly impacts survival. Ann Surg 251(3):506–511
Koshy M, Rich SE, Mohiuddin MM (2010) Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: a SEER analysis. Int J Radiat Oncol Biol Phys 77(1):203–209
Italiano A, Le Cesne A, Mendiboure J et al (2014) Prognostic factors and impact of adjuvant treatments on local and metastatic relapse of soft-tissue sarcoma patients in the competing risks setting. Cancer. 120(21):3361–3369
Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A et al (2014) Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: a cohort study of 922 consecutive patients. Acta Orthop 85(3):323–332
Callegaro D, Miceli R, Mariani L, Raut CP, Gronchi A (2017) Soft tissue sarcoma nomograms and their incorporation into practice. Cancer. 123(15):2802–2820
Sekimizu M, Ogura K, Yasunaga H et al (2019) Development of nomograms for prognostication of patients with primary soft tissue sarcomas of the trunk and extremity: report from the Bone and Soft Tissue Tumor Registry in Japan. BMC Cancer 19(1):657
Cahlon O, Brennan MF, Jia X, Qin LX, Singer S, Alektiar KM (2012) A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg 255(2):343–347
Roberts CC, Kransdorf MJ, Beaman FD et al (2016) ACR appropriateness criteria follow-up of malignant or aggressive musculoskeletal tumors. J Am Coll Radiol 13(4):389–400
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology. 278(2):563–577
Kermany DS, Goldbaum M, Cai W et al (2018) Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 172(5):1122–1131 e9
Peng H, Dong D, Fang MJ et al (2019) Prognostic value of deep learning PET/CT-based radiomics: potential role for future individual induction chemotherapy in advanced nasopharyngeal carcinoma. Clin Cancer Res 25(14):4271–4279
Zhang X, Jiang L, Yang D, Yan J, Lu X (2019) Urine sediment recognition method based on multi-view deep residual learning in microscopic image. J Med Syst 43(11):325
Orlhac F, Frouin F, Nioche C, Ayache N, Buvat I (2019) Validation of a method to compensate multicenter effects affecting CT radiomics. Radiology. 291(1):53–59
Orlhac F, Lecler A, Savatovski J et al (2021) How can we combat multicenter variability in MR radiomics? Validation of a correction procedure. Eur Radiol 31(4):2272–2280
Schröder MS, Culhane AC, Quackenbush J, Haibe-Kains B (2011) survcomp: an R/Bioconductor package for performance assessment and comparison of survival models. Bioinformatics 27(22):3206–3208
Cohen ME, Ko CY, Bilimoria KY et al (2013) Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg 217(2):336
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10(21):7252–7259
Labarre D, Aziza R, Filleron T et al (2009) Detection of local recurrences of limb soft tissue sarcomas: is magnetic resonance imaging (MRI) relevant? Eur J Radiol 72(1):50–53
Crombe A, Marcellin PJ, Buy X et al (2019) Soft-tissue sarcomas: assessment of MRI features correlating with histologic grade and patient outcome. Radiology. 291(3):710–721
Tagliafico AS, Bignotti B, Rossi F, Valdora F, Martinoli C (2019) Local recurrence of soft tissue sarcoma: a radiomic analysis. Radiol Oncol 53(3):300–306
Peeken JC, Bernhofer M, Spraker MB et al (2019) CT-based radiomic features predict tumor grading and have prognostic value in patients with soft tissue sarcomas treated with neoadjuvant radiation therapy. Radiother Oncol 135:187–196
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77(21):e104–e1e7
Toulmonde M, Bonvalot S, Meeus P et al (2014) Retroperitoneal sarcomas: patterns of care at diagnosis, prognostic factors and focus on main histological subtypes: a multicenter analysis of the French Sarcoma Group. Ann Oncol 25(3):735–742
Funding
This study was funded by the Project Grant No. ZR2020MH286 supported by the Shandong Provincial Natural Science Foundation. This study was funded by the Clinical Medicine +X Project of the Affiliated Hospital of Qingdao University (Grant No. QDFY+X2021015). This work was supported by the Medicine and Health Technology Development Program of Shandong Province (Grant No. 2019WS373).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Hexiang Wang.
Conflict of interest
The authors of this article declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Shunli Liu has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• multi-center study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1576 kb)
Rights and permissions
About this article
Cite this article
Liu, S., Sun, W., Yang, S. et al. Deep learning radiomic nomogram to predict recurrence in soft tissue sarcoma: a multi-institutional study. Eur Radiol 32, 793–805 (2022). https://doi.org/10.1007/s00330-021-08221-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08221-0