Abstract
Background
Several recent studies have found that Osteocalcin (OCN), a multifunctional protein secreted exclusively by osteoblasts, is beneficial to glucose metabolism and type 2 diabetes mellitus (T2DM). However, the effects of OCN on islets function especially islet ɑ cells function in patients with type 2 diabetes mellitus characterized by a bi-hormonal disease are still unclear. The purpose of this cross-sectional study was to investigate the relationship between serum OCN and the secretion of islet β cells and ɑ cells in Chinese patients with type 2 diabetes mellitus.
Methods
204 patients with T2DM were enrolled. Blood glucose (FBG, PBG0.5h, PBG1h, PBG2h, PBG3h), insulin (FINS, INS0.5h, INS1h, INS2h, INS3h), C-peptide (FCP, CP0.5h, CP1h, CP2h, CP3h), and glucagon (GLA0, GLA0.5 h, GLA1h, GLA2h, GLA3h) levels were measured on 0 h, 0.5 h, 1 h, 2 h, and 3 h after a 100 g standard bread meal load. Early postprandial secretion function of islet β cells was calculated as Δcp0.5h = CP0.5-FCP. The patients were divided into low, medium and high groups (T1, T2 and T3) according to tertiles of OCN. Comparison of parameters among three groups was studied. Correlation analysis confirmed the relationship between OCN and pancreatic secretion. Multiple regression analysis showed independent contributors to pancreatic secretion.
Main results
FBG, and PBG2h were the lowest while Δcp0.5h was the highest in the highest tertile group (respectively, p < 0.05). INS3h, area under the curve of insulin (AUCins3h) in T3 Group were significantly lower than T1 Group (respectively, p < 0.05). GLA1h in T3 group was lower than T1 group (p < 0.05), and GLA0.5 h in T3 group was lower than T2 and T1 groups (p < 0.05). Correlation analysis showed OCN was inversely correlated with Homeostatic model of insulin resistance (HOMA-IR), INS3h, AUCins3h (p < 0.05), and was still inversely correlated with FCP, GLA0.5 h, GLA1h, area under the curve of glucagon (AUCgla3h) (respectively, p < 0.05) after adjustment for body mass index (BMI) and alanine aminotransferase (ALT). The multiple regression analysis showed that OCN was independent contributor to Δcp0.5h, GLA0.5h and GLA1h (respectively, p < 0.05).
Conclusions
Higher serum OCN level is closely related to better blood glucose control, higher insulin sensitivity, increased early-phase insulin secretion of islet β cells and appropriate inhibition of postprandial glucagon secretion of islet ɑ cells in adult patients with type 2 diabetes mellitus.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a multifaceted disease and the regulation of glucose metabolism relies on the interplay of multiple hormones that act in many target organs. In 1975 Unger et al. [1] described type 2 diabetes mellitus as a bi-hormonal disease characterized by a defective insulin secretion and hyperglucagonemia with elevated blood levels of glucose. In contrast to the effects on levels of insulin, T2DM patients with hyperglycemia often show persistent fasting hyperglucagonemia and lack of suppression of glucagon levels in the postprandial state [2, 3].
Osteocalcin (OCN) is a multifunctional protein secreted exclusively by osteoblasts [4]. There are two forms of Osteocalcin in serum, carboxylated and decarboxylated. Decarboxylated OCN is an important determinant of its metabolic activity. Due to the limited detection technology, the total serum osteocalcin level is often measured in humans. A growing number of clinical studies have observed that serum osteocalcin is associated with glucose metabolism and metabolic syndrome. OCN levels were correlated negatively with the levels of glucose, hemoglobin A1c (HbA1c), insulin resistance, body mass index (BMI), visceral fat as well as atherosclerosis parameters and cardiovascular risk factors in type 2 diabetes patients, while positively with the adiponectin levels [5,6,7,8,9,10,11,12]. What is more, osteocalcin protects against the development of metabolic diseases and type 2 diabetes mellitus [13,14,15,16]. Osteocalcin also linked to the presence or absence of diabetic kidney disease, and the presence and severity of diabetic retinopathy [16,17,18,19,20]. The patients with lower circulating osteocalcin concentrations exhibited higher insulin resistance and systemic inflammation (high-sensitivity C-reactive protein [hsCRP] and interleukin-6), adipokines (leptin and adiponectin), and ectopic body fat aggregation especially in young overweight and obese women [4, 7, 9, 21, 22]. Decarboxylated OCN negatively correlated with percent trunk fat, visceral/subcutaneous fat ratio (by Computed Tomography (CT)), and fat mass as well as fasting plasma glucose and HbA1c in men and postmenopausal women with T2DM [23].
In 2007, Lee et al. [24] found that decarboxylated Osteocalcin (OCN) directly increased β-cell proliferation and insulin secretion. Also, decarboxylated OCN could improve insulin resistance and protect mice from obesity and glucose intolerance. Decarboxylated OCN stimulated β-cell function and insulin production, regulated gene expression in adipocytes (including adiponectin expression), decreased visceral fat, and protected against diet-induced obesity [13]. Decarboxylated OCN significantly augmented insulin content and enhanced human β-cell proliferation [25]. Esp−/− mice which is a gain-of-function model of osteocalcin activity, display increased insulin secretion, and reduced fat mass [14]. Decarboxylated OCN may play a regulatory role in various organs and tissues through different receptors, such as GPRC6A [26,27,28], and affect liver fat accumulation, adipose tissue distribution, gut hormones secretion, muscle motility and energy expenditure, testicular fertility, brain cognitive function and fetal brain development [4, 14, 29,30,31,32,33].
However, few studies have been reported whether osteocalcin affects islet ɑ cells, After 7 days of decarboxylated-OCN- treated (1.0 ng/mL) dispersed human islets, OCN significantly reduced ɑ-cell mass. Immunohistochemistry of islet grafts after infusing 4.5-ng/h decarboxylated-OCN showed islet β-cell co-expressed the ɑ-cell marker glucagon, which may explain the decrease in the number of ɑ cells [25]. However, serum levels and pancreas content of glucagon, particular the ratio of insulin-to-glucagon–positive cells were not affected in Gprc6aPdx1−/−mice compared with control littermates [27]. Serum glucagon level was normal, not decreased [14, 24] in Esp−/− mice that displayed severe hyperinsulinemia.
The skeleton exerted an endocrine regulation of glucose homeostasis through OCN. However, the effects of OCN on islets function especially islet ɑ cells function in patients with type 2 diabetes mellitus characterized by a bi-hormonal disease are still unclear. The purpose of this cross-sectional study was to investigate the relationship between serum OCN level and the secretion of islet β cells and ɑ cells in 204 Chinese patients with type 2 diabetes mellitus, and try to find a new way to prevent and treat type 2 diabetes mellitus.
Research design and methods
Study subjects
204 patients with T2DM hospitalized in the endocrinology wards in Jinling Hospital from September 2016 to February 2021 were enrolled. The diagnosis of T2DM was defined according to 1999 World Health Organization Criteria. Exclusion criteria were as follows: (1) taking drugs that affect the secretion of glucagon such as insulin, glucagon like peptide 1 (GLP-1)receptor agonists, and dipeptidyl peptidase-4(DPP-4) inhibitors; (2) acute complications of diabetes such as diabetic ketoacidosis; (3) severe liver and kidney dysfunction, recent acute infections and stress conditions such as cardiac insufficiency, trauma, pregnancy, or any other infectious diseases; (4) blood diseases, malignant tumors, thyroid and parathyroid diseases; (5) taking antiosteoporosis drugs (bisphosphonate, calcium, vitamin D, and vitamin K), estrogen analogs, or antineoplastic, or immune-modulating drugs within the 12 months before enrollment. All of them took a 100 g standard bread meal test. The plasma glucose, insulin, C-peptide, and glucagon levels were measured on fasting basis, and 0.5 h, 1 h, 2 h, and 3 h after a 100 g bread meal load. The patients were divided into three groups according to the tertiles of serum OCN levels, 67 cases with OCN < 14.02 ng/mL (T1 group), 68 cases with 14.02 ≤ OCN < 19.78 ng/mL (T2 group) and 69 cases with OCN ≥ 19.78 ng/mL (T3 group).
Clinical and biochemical indexes
Venous blood sample were collected in the morning following an overnight fast. Sociodemographic characteristics, physical examination information and laboratory measurements such as gender, age, durations, body mass index (BMI), serum uric acid (SUA), blood lipid, blood pressure, liver and kidney function parameters and blood glucose were collected by trained doctors. HbA1c was determined by high performance liquid chromatography (HLC-723G8 Automatic glycosylated hemoglobin Analyzer, TOSOH, Japan). Biochemical indexes were measured by automatic biochemical analyzer (Model 7600 Series Automatic Analyzer, Hitachi, Japan). Insulin and C-peptide concentrations were measured by electricity chemiluminescent immunoassay (IMMULITE 2000 XPi, Siemens, Germany) and glucagon was measured by radioimmunoassay (RIA, Millipore, Billerica, MA, USA). Serum OCN levels were evaluated by the electrochemiluminescence immunoassay method on a Roche COBAS E 801 (Roche Diagnostics Corporation, Mannheim, Germany).
Standard bread meal test
All subjects were fasted at least 8 h before this testing. All patients stopped all hypoglycemic drugs in the morning, and received a 100 g standard bread meal test (bread made of 100 g dry wheat white flour containing carbohydrate equals 75 g glucose. To avoid the gastrointestinal side effects of taking large amounts of glucose orally, we used the standard 100 g steamed bread meal test instead of the 75 g oral glucose tolerance test.) [34, 35] and a simultaneous insulin-C-peptide-glucagon releasing test in 3 h. Concentrations of serum glucose (FBG, PBG0.5h, PBG1h, PBG2h, PBG3h), insulin (FINS, INS0.5 h, INS1h, INS2h, INS3h), C-peptide (FCP, CP0.5h, CP1h, CP2h, CP3h) and glucagon (GLA0, GLA0.5h, GLA1h, GLA2h, GLA3h) levels were recorded at 0 h, 0.5 h, 1 h, 2 h and 3 h after meal. Areas under the curve of glucose (AUCglu3h), glucagon (AUCgla3h),C-peptide (AUCcp3h) and insulin (AUCins3h) in 3 h were calculated with trapezoidal methods.
Evaluation secretion function of pancreatic alpha cell and beta cell
Homeostatic model of insulin resistance (HOMA-IR) was calculated using the formula: [fasting insulin mIU/L) × fasting plasma glucose (mmol/L)]/22.5. Homeostasis model of β cell function (HOMA-β) = 20 × fasting insulin (mIU/L)/(fasting plasma glucose(mmol/L)-3.5); insulin and C peptide at all time points, AUC for insulin and C-peptide in 3 h (AUCins3h, AUCcp3h) and HOMA-β were used to assess the pancreatic beta cell function. Early postprandial secretion function of islet β cells was calculated as Δcp0.5h = CP0.5-FCP. Glucagon levels (GLA0, GLA0.5 h, GLA1h, GLA2h, GLA3h) on fasting basis, 0.5 h, 1 h, 2 h and 3 h after the bread meal, and AUC for glucagon in 3 h (AUCgla3h) were used to evaluate the pancreatic alpha cell function.
Statistical analyses
Statistical analysis was performed by SPSS22.0. Data were expressed as median and range for skewed distribution and mean ± standard deviation for normal distribution. One-way ANOVA or Kruskal–Wallis test was applied to compare differences among groups. Spearman’s coefficient determined the relationships between variables. After adjustments for confounding factors, partial correlation coefficient was done to further clarify the relationship between OCN and islet function. Multiple regression analysis showed the independent factors to islets function. P < 0.05 was identified as statistical significance.
Results
General clinical characteristics of all the included cases
204 patients with type 2 diabetes were included in the study. There were 140 men and 64 women, 31% of women and 69% of men. The average age and diabetes duration of all the participants were 55.06 ± 14.0 years, and 6.0 (2.0,12.75) years. The BMI of all patients was 25.28 (23.20,27.16).
Differences in serum OCN stratified by sex, BMI, age, diabetes duration, HbA1C
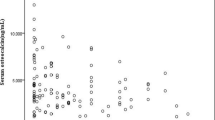
Divided by sex (Male group and female group), age (youth group (18–44 years old), middle-aged group (45–59 years old), and elderly group (≥ 60 years old)) and diabetes duration (diabetes duration ≤ 5 years and diabetes duration > 5 years), there was no difference in osteocalcin levels between the groups (respectively, p > 0.05). Grouped by BMI, serum OCN in BMI < 24 kg/m2 Group was significant higher than 24 kg/m2 ≤ BMI < 28 kg/m2 group and BMI ≥ 28 kg/m2 group (respectively, p < 0.05) (Fig. 1A). A significantly higher level of serum OCN was observed in the HbA1c ≤ 8 group than it in HbA1c > 8 group (p < 0.05) (Fig. 1B).
The level of serum osteocalcin was the highest in normal weight BMI < 24 kg/m2 group, and the levels of serum osteocalcin in BMI ≥ 28 kg/m2 group and 24 kg/m2 ≤ BMI < 28 kg/m2 group were significantly lower than that in BMI < 24 kg/m2 group (respectively, P < 0.05) (A). The serum osteocalcin level in patients with HbA1C > 8% was lower than that in patients with HbA1C ≤ 8% (P < 0.05) (B). OCN Osteocalcin, BMI body mass index, HbA1c hemoglobin A1c
Comparison of baseline clinical parameters of patients with T2DM among tertiles of OCN levels
As Table 1 shows, BMI was lower in T3 group (OCN ≥ 19.78 ng/mL) than group T1 (OCN < 14.02 ng/mL) (25.97 (22.66, 26.69) vs. 26.03 (24.65, 28.08) p < 0.05). Serum uric acid (SUA) is lower in T2 group (14.02 ≤ OCN < 19.78 ng/mL) than group T1 (299.08 ± 76.35 vs 340.91 ± 104.92 p < 0.05). There were no significant differences in gender composition, age, diabetes duration, systolic blood pressure (SBP), diastolic blood pressure (DBP), alanine aminotransferase (ALT), aspartate aminotransferase AST, urea nitrogen (UN), creatinine (Cr), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), HbA1c among three groups (respectively, p > 0.05) (Table 1).
Comparison of glucose metabolism and islet alpha cell and beta cell secretory function among tertiles of OCN levels
Glucose metabolism
As Table 1 shows, FBG was lower in T3 group than group T2 (7.57 ± 3.11 vs. 9.30 ± 5.16; p < 0.05). PBG2h and AUCglu3h was lower in T3 group than group T2 and group T1 (15.02 ± 3.82 vs. 16.71 ± 5.36 vs. 16.64 ± 4.05; 38.11 ± 11.22 vs. 43.66 ± 13.00 vs. 42.60 ± 10.56, p < 0.05) (Fig. 2A). There was no significant difference in HOMA-IR among three groups.
Comparison of glucose metabolism and islet alpha cell and beta cell secretory function among tertiles of OCN levels. PBG2h was lower in T3 group than group T2 and group T1 (15.02 ± 3.82 vs. 16.71 ± 5.36 vs. 16.64 ± 4.05, p < 0.05) (A). AUCins3h in T3 Group was significantly lower than T1 Group (53.45 (29.05, 93.65) vs. 78.48 (44.12, 123.72), p < 0.05) (B). Δcp0.5 h in T3 Group was significantly higher than T1Group and T2Group (0.53 (0.14, 1.06) vs. 0.36 (− 0.01, 0.80) vs. 0.24 (0.06, 0.62), p < 0.05) (C). GLA0.5 h in T3 group was lower than T2 and T1 groups (35.38 ± 17.90 vs. 41.51 ± 15.82 vs. 43.76 ± 19.09, p < 0.05) (D). Compared with the T1 group, GLA1h in the T3 group was lower (32.12 ± 12.80 vs. 38.32 ± 17.03, p < 0.05) (E). OCN Osteocalcin, PBG2h postprandial 2 h blood glucose, GLA0.5h postprandial 0.5 h glucagon, GLA1h postprandial 1 h glucagon, AUCins3h area under the curve of insulin, Δcp0.5h early postprandial secretion function of islet β cells
Beta cell secretory function
There were marked differences in the FINS, INS3h, FCP, AUCins3h, Δcp0.5h among three groups (respectively, p < 0.05). Make pairwise comparison of those indexes and discover that the INS3h, FCP, AUCins3h in T3 Group were significantly lower than T1 Group (18.1 (11.7, 35.12) vs. 27.0 (15.90, 48.2); 1.26 (0.76,2.29) vs. 1.85 (1.20,2.74); 53.45 (29.05,93.65) vs. 78.48 (44.12,123.72), p < 0.05), while Δcp0.5h in T3 Group were significantly higher than T1 Group and T2 Group (0.53 (0.14,1.06) vs. 0.36 (− 0.01,0.80) vs. 0.24 (0.06,0.62), p < 0.05). There were no significant differences in HOMA-β, INS0.5h, INS1h, INS2h, CP0.5h, CP1h, CP2h, CP3h, AUCcp3h among three groups. (Table 1 and Fig. 2B, C).
Alpha cell secretory function
There were significant differences in GLA0.5h and GLA1h in different tertiles of OCN after a 100 g bread meal load (respectively, p < 0.05). Compared with the T1 group, GLA1h in the T3 group was lower (32.12 ± 12.80 vs. 38.32 ± 17.03, p < 0.05). GLA0.5h in T3 group was lower than T2 and T1 groups (35.38 ± 17.90 vs. 41.51 ± 15.82 vs. 43.76 ± 19.09, p < 0.05). There were no significant differences in GLA0, GLA2h, GLA3h, AUCgla3h among three groups. (Table 1and Fig. 2D, E).
Correlation between serum OCN and metrics of glucose metabolism
Spearman’s correlation test (Table 2) revealed that serum OCN was inversely correlated with BMI, ALT, PBG2h (rs = − 0.190, − 0.212, − 0.141, respectively, p < 0.05). Serum OCN was also inversely correlated with FINS, FCP, AUCins3h (rs = − 0.138, − 0.198, − 0.146, respectively, p < 0.05). Meanwhile, Serum OCN was inversely correlated with alpha cell secretory function indexes such as GLA0.5h, GLA1h, AUCgla3h (rs = − 0.217, − 0.151, − 0.150, respectively, p < 0.05). The test also explored that serum OCN was inversely correlated with HOMA-IR (r = − 0.153, p < 0.05) (Table 2). Relationship between serum OCN and FCP, GLA0.5h, GLA1h, AUCgla3h (r = − 0.160, − 0.211, − 0.153, − 0.151, respectively, p < 0.05) remained significant after adjustment for age, sex, the disease durations, BMI and serum UN, serum Cr, SUA, ALT, AST by partial correlation analysis (Table 3).
Multiple regression analysis showing relationship between the serum OCN and pancreatic secretion
To further determine which variables were independently associated with pancreatic secretion, multiple regression analysis was performed in all patients with type 2 diabetes. The results showed that: (1) OCN and UN were independent contributors to Δcp0.5h (β = 0.035, − 0.203, respectively, p < 0.05), and the other variables such as sex, age, BMI, duration of diabetes, SBP, DBP, ALT, AST, Cr, SUA, TG, TC, HDL, LDL, HbA1c, FBG and PBG0.5h failed to enter the final model; (2) OCN, age and ALT were independent contributors to GLA0.5h (β = − 0.430, 0.239, 0.274, respectively, p < 0.05),and the other variables such as sex, BMI, duration of diabetes, SBP, DBP, AST, UN, Cr, SUA, TG, TC, HDL, LDL, HbA1c, FBG and PBG0.5h failed to enter the final model; (3) OCN was independent contributor to GLA1h (β = − 0.305, p < 0.05), and the other variables such as sex, age, BMI, duration of diabetes, SBP, DBP, ALT, AST, UN, Cr, SUA, TG, TC, HDL, LDL, HbA1c, FBG,PBG0.5h and PBG1h failed to enter the final model (Table 4).
Discussion
A growing number of clinical studies have observed that osteocalcin is associated with blood glucose control and metabolic syndrome [5, 7]. Our study firstly explored the relationship between serum osteocalcin and islet function, especially glucagon secretion in islet ɑ cells.
In the present cross-sectional study most of the patients were overweight or obese type 2 diabetes, and insulin resistance is significant in this population. There were no significant differences in serum osteocalcin levels among groups by sex, age and duration of diabetes. This was different from previous studies that serum osteocalcin may be affected by gender and age [9, 36], and may be related to the difference of the selected population.
Serum osteocalcin in normal weight patients with type 2 diabetes was significantly higher than in overweight or obese patients with type 2 diabetes. The serum osteocalcin level was significantly negatively correlated with BMI and HOMA-IR. The relationship between BMI and OCN has been confirmed by multiple studies in different populations. A meta-analysis of 28 cross-sectional studies found an overall significant inverse association between serum osteocalcin and BMI in healthy adults, especially in metabolic syndrome patients [6]. Moreover, osteocalcin concentration in obese and overweight people is lower than normal controls, this is in agreement with other studies on postmenopausal women [5, 22]. A mean of 16.8% weight loss led to increases in OCN levels, and an 8.7% weight loss plus regular exercise led to increased circulating OCN that paralleled reduced fat mass [37].
Similar to previous studies [20, 38, 39], we also found a significant positive association between serum osteocalcin levels and glycemic control. Patients with Type 2 diabetes mellitus with higher serum osteocalcin level had better blood glucose control (HbA1C ≤ 8%), especially lower fasting blood glucose and 2 h postprandial blood glucose. In the highest serum OCN level group the area under the blood glucose curve of standard steamed bread meal experiment in 3 h decreased significantly.
In the highest serum OCN levels group, fasting insulin, postprandial plasma insulin at the third hour and area under 3-h insulin curve of 100 g standard bread experiment were significantly reduced, as well as FCP. Therefore, this reduced fasting, postprandial and total insulin secretion, alleviated hyperinsulinemia in overweight or obese type 2 diabetes. Conversely, early pancreatic beta cell secretion, Δcp0.5h, was significantly increased in the highest OCN group, and multiple regression analysis showed that serum OCN was independent contributors to Δcp0.5h. This is consistent with the results of previous studies [4, 25, 26, 28, 40, 41]. Osteocalcin treatment increased insulin release, and OCN level was negatively correlated with fasting plasma glucose, HbA1c, insulin resistance, BMI and body fat. In our study Osteocalcin alleviated insulin resistance, reduced hyperinsulinemia and enhanced insulin action in type 2 diabetes patients. Excitingly, Osteocalcin increases early postprandial insulin secretion, which contributes to the stability of postprandial blood glucose. More importantly, our research found that patients with Type 2 diabetes mellitus with the highest OCN levels had lowest serum glucagon at postprandial 0.5 h and 1 h. Moreover, osteocalcin was significantly negatively correlated with glucagon secretion at postprandial 0.5 h and 1 h, and especially the total glucagon secretion of 3 h in 100 g bread meal experiment. In particular, multiple regression analysis showed the serum OCN was independent contributor to GLA0.5h and GLA1h. Insulin resistance attenuates the inhibitory effect of insulin on islet ɑ cells secretion and cause hyperglucagonemia, while hyperglucagonemia is an important part of the pathogenesis of insulin resistance in type 2 diabetes [42,43,44,45,46]. Failure to adequately suppress glucagon could lead to the impaired glucose regulation in pre-diabetes and type 2 diabetes [46, 47]. Inappropriate glucagon secretion after meal, which causes a large amount of liver glucose output even after meals, is an important cause of hyperglycemia in type 2 diabetes mellitus, and appropriately suppression of postprandial glucagon secretion is beneficial to postprandial blood glucose control [45, 46]. During last few decades, inhibition of glucagon secretion or its action to improve insulin sensitivity and decrease insulin resistance is one of numerous therapeutic approaches to treat type 2 diabetes mellitus [48]. But agents that inhibit postprandial glucagon secretion or antagonize glucagon action or inhibit glucagon receptor GCGRs have obvious side effects, such as hepatic steatosis, hyperlipidemia, and hyperaminoacidemia, could lead to more serious metabolic disorders and even serious cardiovascular diseases [49,50,51]. Inhibition of early postprandial glucagon secretion is especially important for blood glucose stabilization [46]. Our study found serum osteocalcin levels were significantly associated with early postprandial glucagon inhibition, such as GLA0.5h and GLA1h. Therefore, this may provide a new appropriate direction for the treatment of type 2 diabetes mellitus. To our best knowledge, for the first time this study evaluated the association of bone formation marker Osteocalcin with the islet ɑ cells secreting glucagon in adult patients with type 2 diabetes mellitus. Whether pancreatic islet ɑ cells also have osteocalcin receptors, and whether the mechanism that osteocalcin acts on pancreatic islet ɑ cells is as the same as it acts on pancreatic islet β cells, still need more in-depth researches.
Our study still has certain limitations. This is only a cross-sectional observational study. The number of cases included is not enough, and all participants are inpatients, so there may be deviations in the study results. Prospective studies can be carried out in the later recruiting a wider population to further clarify the effects of serum osteocalcin on islet α cells secretion function and treatment of diabetes, further more cytological experiments will be done to explore the specific action mechanism of osteocalcin on islet α cells.
Conclusion
In summary, this research has showed higher serum OCN level is closely related to better blood glucose control, higher insulin sensitivity, increased early-phase insulin secretion of islet β cells and appropriate inhibition of postprandial glucagon secretion of islet ɑ cells in adult patients with type 2 diabetes mellitus.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1(7897):14–6.
Li XC, Zhuo JL. Current insights and new perspectives on the roles of hyperglucagonemia in non-insulin-dependent type 2 diabetes. Curr Hypertens Rep. 2013;15(5):522–30.
Demant M, Bagger JI, Suppli MP, et al. Determinants of fasting hyperglucagonemia in patients with type 2 diabetes and nondiabetic control subjects. Metab Syndr Relat Disord. 2018;16(10):53–536.
Liu JM, Rosen CJ, Ducy P, et al. Regulation of glucose handling by the skeleton: insights from mouse and human studies. Diabetes. 2016;65(11):3225–32.
Wang L, Li T, Liu J, et al. Association between glycosylated hemoglobin A1c and bone biochemical markers in type 2 diabetic postmenopausal women: a cross-sectional study. BMC Endocr Disord. 2019;19(1):31.
Kord-Varkaneh H, Djafarian K, Khorshidi M, et al. Association between serum osteocalcin and body mass index: a systematic review and meta-analysis. Endocrine. 2017;58(1):24–32.
Pittas AG, Harris SS, Eliades M, et al. Association between Serum Osteocalcin and Markers of Metabolic Phenotype. J Clin Endocrinol Metab. 2009;94(3):827–32.
Polgreen LE, Jacobs DR, Nathan BM, et al. Association of osteocalcin with obesity, insulin resistance, and cardiovascular risk factors in young adults. Obesity. 2012;20(11):2194–201.
Rui X, Xu B, Su J, et al. Differential pattern for regulating insulin secretion, insulin resistance, and lipid metabolism by osteocalcin in male and female T2DM patients. Med Sci Monit. 2014;20:711–9.
Tan A, Gao Y, Yang X, et al. Low serum osteocalcin level is a potential marker for metabolic syndrome: results from a Chinese male population survey. Metabolism. 2011;60(8):1186–92.
Darwish L, Nguyen MM, Saleem M, et al. Lower serum osteocalcin concentrations in patients with type 2 diabetes and relationships with vascular risk factors among patients with coronary artery disease. J Diabetes Complicat. 2019;33(5):390–7.
Kanazawa I, Yamaguchi T, Yamamoto M, et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94(1):45–9.
Ferron M, McKee MD, Levine RL, et al. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50(2):568–75.
Yeap BB. Osteocalcin: an endocrine link between bone and glucose metabolism. Expert Rev Endocrinol Metab. 2011;6(2):177–85.
Desentis-Desentis MF, Rivas-Carrillo JD, Sánchez-Enríquez S. Protective role of osteocalcin in diabetes pathogenesis. J Bone Miner Metab. 2020;38(6):765–71.
Oosterwerff MM, van Schoor NM, Lips P, et al. Osteocalcin as a predictor of the metabolic syndrome in older persons: a population-based study. Clin Endocrinol. 2013;78(2):242–7.
Liu C, Wo J, Zhao Q, et al. Association between serum total osteocalcin level and type 2 diabetes mellitus: a systematic review and meta-analysis. Horm Metab Res. 2015;47(11):813.
Massera D, Biggs ML, Walker MD, et al. Biochemical markers of bone turnover and risk of incident diabetes in older women: the cardiovascular health study. Diabetes Care. 2018;41(9):1901–8.
An Y, Liu S, Wang W, et al. Low serum levels of bone turnover markers are associated with the presence and severity of diabetic retinopathy in patients with type 2 diabetes mellitus. J Diabetes. 2021;13(2):111–23.
Ye X, Yu R, Jiang F, et al. Osteocalcin and risks of incident diabetes and diabetic kidney disease: a 4.6-year prospective cohort study. Diabetes Care. 2022;5(4):830–6.
Liao M, Huang L, Mao Y, et al. Serum osteocalcin is associated with inflammatory factors in metabolic syndrome: a population-based study in Chinese males. Mediators Inflamm. 2015;2015: 683739.
Lucey AJ, Paschos GK, Thorsdottir I, et al. Young overweight and obese women with lower circulating osteocalcin concentrations exhibit higher insulin resistance and concentrations of C-reactive protein. Nutr Res. 2013;33(1):67–75.
Kanazawa I, Yamaguchi T, Yamauchi M, et al. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. 2011;22(1):187–94.
Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69.
Sabek OM, Nishimoto SK, Fraga D, et al. Osteocalcin effect on human beta-cells mass and function. Endocrinology. 2015;156(9):3137–46.
Pi M, Kapoor K, Ye R, et al. Evidence for osteocalcin binding and activation of GPRC6A in beta-cells. Endocrinology. 2016;157(5):1866–80.
Wei J, Hanna T, Suda N, et al. Osteocalcin promotes beta-cell proliferation during development and adulthood through Gprc6a. Diabetes. 2014;63(3):1021–31.
Diaz-Franco MC, Franco-Diaz DLR, Villafan-Bernal JR. OsteocalcinGPRC6A: an update of its clinical and biological multiorganic interactions (Review). Mol Med Rep. 2019;19(1):15–22.
Li J, Zhang H, Yang C, et al. An overview of osteocalcin progress. J Bone Miner Metab. 2016;34(4):367–79.
Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Rev Endocr Metab Disord. 2015;16(2):93–8.
Oury F, Sumara G, Sumara O, et al. Endocrine Regulation of Male Fertility by the Skeleton. Cell. 2011;144(5):796–809.
Oury F, Ferron M, Huizhen W, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest. 2013;123(6):2421–33.
Brennan-Speranza TC, Conigrave AD. Osteocalcin: an osteoblast-derived polypeptide hormone that modulates whole body energy metabolism. Calcif Tissue Int. 2015;96(1):1–10.
Yuan-Quan L, Hospital JP. Standard bread meal test in clinical diagnosis of Diabetes mellitus application. Clin Lab J (Electronic Edition), 2012.
Kempf K, Martin S, Haastert B, et al. Diagnostic accuracy of a standardized carbohydrate-rich breakfast compared to an oral glucose tolerance test in occupational medicine. Dtsch Med Wochenschr. 2013;138:1297–303.
Bouillon R, Bex M, Van Herck E, et al. Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab. 1995;80(4):1194–202.
Fernandez-Real JM, Izquierdo M, Ortega F, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J Clin Endocrinol Metab. 2009;94(1):237–45.
Hwang Y, Jeong I, Ahn KJ, et al. The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced β-cell function in middle-aged male subjects. Diab Metab Res Rev. 2009;25(14):768–72.
Kanazawa I. Osteocalcin as a hormone regulating glucose metabolism. World J Diabetes. 2015;6(18):1345–54.
Bilotta FL, Arcidiacono B, Messineo S, et al. Insulin and osteocalcin: further evidence for a mutual cross-talk. Endocrine. 2018;59(3):622–32.
Kanazawa I, Yamaguchi T, Tada Y, et al. Serum osteocalcin level is positively associated with insulin sensitivity and secretion in patients with type 2 diabetes. Bone. 2011;48(4):720–5.
Hamasaki H, Morimitsu S. Association of glucagon with obesity, glycemic control and renal function in adults with type 2 diabetes mellitus. Can J Diabetes. 2021;45(3):249–54.
Ferrannini E, Muscelli E, Natali A, et al. Association of fasting glucagon and proinsulin concentrations with insulin resistance. Diabetologia. 2007;50(11):2342–7.
Ojha A, Ojha U, Mohammed R, et al. Current perspective on the role of insulin and glucagon in the pathogenesis and treatment of type 2 diabetes mellitus. Clin Pharmacol Adv Appl. 2019;11:57–65.
Bozadjieva KN, Lubaczeuski C, Blandino-Rosano M, et al. Glucagon resistance and decreased susceptibility to diabetes in a model of chronic hyperglucagonemia. Diabetes. 2021;70(2):477–91.
Faerch K, Vistisen D, Pacini G, et al. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes. 2016;65(11):3473–81.
Shah P, Vella A, Basu A, et al. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85(11):4053–9.
Campbell JE, Drucker DJ. Islet alpha cells and glucagon–critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11(6):329–38.
Guzman CB, Zhang XM, Liu R, et al. Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(11):1521–8.
Kazda CM, Frias J, Foga I, et al. Treatment with the glucagon receptor antagonist LY2409021 increases ambulatory blood pressure in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(8):1071–7.
Morgan ES, Tai LJ, Pham NC, et al. Antisense inhibition of glucagon receptor by IONIS-GCGRRx improves type 2 diabetes without increase in hepatic glycogen content in patients with type 2 diabetes on stable metformin therapy. Diabetes Care. 2019;42(4):585–93.
Acknowledgements
The authors are very thankful to forward their gratitude to the data collectors, supervisors, and study subjects for their cooperation.
Funding
This work was supported by the National Natural Science Foundation of China (81873174); Key Research and Development Plan Project of Jiangsu Province - Social Development Projects (BE2020701).
Author information
Authors and Affiliations
Contributions
HL, JL, WW and JS conceived and designed the research. JL, XY, ZF and PZ conducted the research and collected data. HL, WW and PZ analyzed data and performed the statistical analysis. HL wrote the manuscript. BL and JS critically revised the manuscript for key intellectual content. HL is responsible for the integrity of the work. HL, JL and WW contributed equally to this paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Approval was obtained from the ethics committee of Southern Medical University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Competing interests
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lei, H., Liu, J., Wang, W. et al. Association between osteocalcin, a pivotal marker of bone metabolism, and secretory function of islet beta cells and alpha cells in Chinese patients with type 2 diabetes mellitus: an observational study. Diabetol Metab Syndr 14, 160 (2022). https://doi.org/10.1186/s13098-022-00932-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00932-8