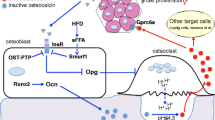
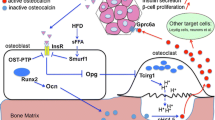
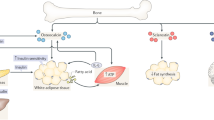
Abstract
Osteocalcin is a bone-specific protein that is regularly used in the clinical setting as a serum marker of bone turnover. Recent evidence indicates that osteocalcin plays a previously unsuspected role in the control of energy metabolism. Thus, osteocalcin-deficient mice have a profoundly deranged metabolic phenotype that includes insulin resistance, glucose intolerance and abnormal fat deposition. Additionally, osteocalcin administration in mice improves insulin sensitivity and decreases fat pad mass and serum triglyceride levels. The role of osteocalcin in human macronutrient metabolism is less clear but recent studies report positive correlations between serum osteocalcin levels and established indices of metabolic health. Herein, we review key physiological functions of osteocalcin, focussing on the roles of osteocalcin in the modulation of macronutrient metabolism, male reproductive function and foetal brain development. We consider the implications of these findings for the coordination of metabolism with development and fertility. We also consider evidence that a Class C G-protein-coupled receptor from a subgroup known to mediate nutrient-sensing acts as the osteocalcin receptor.


Similar content being viewed by others
References
Karsenty G (1998) Transcriptional regulation of osteoblast differentiation during development. Front Biosci 3:834–837
Hauschka PV, Lian JB, Gallop PM (1975) Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci USA 72:3925–3929
Price PA, Otsuka AA, Poser JW, Kristaponis J, Raman N (1976) Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci USA 73:1447–1451
Hauschka PV, Lian JB, Cole DE, Gundberg CM (1989) Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev 69:990–1047
Karsenty G, Kronenberg HM, Settembre C (2009) Genetic control of bone formation. Annu Rev Cell Dev Biol 25:629–648
Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G (1996) Increased bone formation in osteocalcin-deficient mice. Nature 382:448–452
Murshed M, Schinke T, McKee MD, Karsenty G (2004) Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol 165:625–630
Dubois-Ferrière V, Brennan TC, Dayer R, Rizzoli R, Ammann P (2011) Calcitropic hormones and IGF-I are influenced by dietary protein. Endocrinology 152:1839–1847
Rutter MM, Markoff E, Clayton L, Akeno N, Zhao G, Clemens TL, Chernausek SD (2005) Osteoblast-specific expression of insulin-like growth factor-1 in bone of transgenic mice induces insulin-like growth factor binding protein-5. Bone 36:224–231
Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G (2012) Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 50:568–575
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Ferron M, Hinoi E, Karsenty G, Ducy P (2008) Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci 105:5266–5270
Eastell R, Delmas PD, Hodgson SF, Eriksen EF, Mann KG, Riggs BL (1988) Bone formation rate in older normal women: concurrent assessment with bone histomorphometry, calcium kinetics, and biochemical markers. J Clin Endocrinol Metab 67:741–748
Brown JP, Delmas PD, Malaval L, Edouard C, Chapuy MC, Meunier PJ (1984) Serum bone Gla-protein: a specific marker for bone formation in postmenopausal osteoporosis. Lancet 1:1091–1093
Seibel MJ (2006) Biochemical markers of bone turnover part II: clinical applications in the management of osteoporosis. Clin Biochem Rev 27:123–138
Lian J, Stewart C, Puchacz E, Mackowiak S, Shalhoub V, Collart D, Zambetti G, Stein G (1989) Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci USA 86:1143–1147
Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G (1998) Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone 23:187–196
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317
Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G (2009) A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138:976–989
Mauro LJ, Olmsted EA, Skrobacz BM, Mourey RJ, Davis AR, Dixon JE (1994) Identification of a hormonally regulated protein tyrosine phosphatase associated with bone and testicular differentiation. J Biol Chem 269:30659–30667
Brennan-Speranza TC, Henneicke H, Gasparini SJ, Blankenstein KI, Heinevetter U, Cogger VC, Svistounov D, Zhang Y, Cooney GJ, Buttgereit F, Dunstan CR, Gundberg C, Zhou H, Seibel MJ (2012) Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. J Clin Invest 122:4172–4189
Buitenhuis HC, Soute BAM, Vermeer C (1990) Comparison of the vitamins K1, K2 and K3 as cofactors for the hepatic vitamin K-dependent carboxylase. Biochim Biophys Acta 1034:170–175
Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y (2001) Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50:1126–1133
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953
Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF (2001) Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A 98:2005–2010
Kajimura D, Lee HW, Riley Kyle RJ, Arteaga-Solis E, Ferron M, Zhou B, Clarke CJ, Hannun YA, DePinho RA, Guo EX, Mann JJ, Karsenty G (2013) Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab 17:901–915
Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G (2010) Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142:296–308
Schafer AL, Sellmeyer DE, Schwartz AV, Rosen CJ, Vittinghoff E, Palermo L, Bilezikian JP, Shoback DM, Black DM (2011) Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1–84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study). J Clin Endocrinol Metab 96:E1982–E1989
Bozec A, Bakiri L, Jimenez M, Rosen ED, Catalá-Lehnen P, Schinke T, Schett G, Amling M, Wagner EF (2013) Osteoblast-specific expression of Fra-2/AP-1 controls Adiponectin/Osteocalcin expression and affects metabolism. J Cell Sci 126(23):5432–5440
Ituarte EA, Halstead LR, Iida-Klein A, Ituarte HG, Hahn TJ (1989) Glucose transport system in UMR-106-01 osteoblastic osteosarcoma cells: regulation by insulin. Calcif Tissue Int 45:27–33
Kream BE, Smith MD, Canalis E, Raisz LG (1985) Characterization of the effect of insulin on collagen synthesis in fetal rat bone. Endocrinology 116:296–302
Zhang W, Shen X, Wan C, Zhao Q, Zhang L, Zhou Q, Deng L (2012) Effects of insulin and insulin-like growth factor 1 on osteoblast proliferation and differentiation: differential signalling via Akt and ERK. Cell Biochem Funct 30:297–302
Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere M-C, Aja S, Hussain MA, Brüning JC, Clemens TL (2010) Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142:309–319
Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM (2002) Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419:316–321
Medema RH, Kops GJPL, Bos JL, Burgering BMT (2000) AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404:782–787
Kode A, Mosialou I, Silva BC, Joshi S, Ferron M, Rached MT, Kousteni S (2012) FoxO1 protein cooperates with ATF4 protein in osteoblasts to control glucose homeostasis. J Biol Chem 287:8757–8768
Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC (2007) Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting β-catenin from T cell Factor- to forkhead box O-mediated transcription. J Biol Chem 282:27298–27305
Iyer S, Ambrogini E, Bartell SM, Han L, Roberson PK, de Cabo R, Jilka RL, Weinstein RS, O’Brien CA, Manolagas SC, Almeida M (2013) FOXOs attenuate bone formation by suppressing Wnt signaling. J Clin Invest 123:3409–3419
Poser JW, Price PA (1979) A method for decarboxylation of gamma-carboxyglutamic acid in proteins. properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem 254:431–436
Lacombe J, Karsenty G, Ferron M (2013) In vivo analysis of the contribution of bone resorption to the control of glucose metabolism in mice. Mol Metabol 2:498–504
Wei J, Ferron M, Clarke CJ, Hannun YA, Jiang H, Blaner WS, Karsenty G (2014) Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest 124:1–13
Levinger I, Jerums G, Stepto NK, Parker L, Serpiello FR, McConell GK, Anderson M, Hare DL, Byrnes E, Ebeling PR, Seeman E (2014) The effect of acute exercise on undercarboxylated osteocalcin and insulin sensitivity in obese men. J Bone Miner Res. doi:10.1002/jbmr.2285
Hill HS, Grams J, Walton RG, Liu J, Moellering DR, Garvey WT (2014) Carboxylated and uncarboxylated forms of osteocalcin directly modulate the glucose transport system and inflammation in adipocytes. Horm Metab Res 46:341–347
Barnes PJ (2006) How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol 148:245–254
Buttgereit F, Burmester GR, Straub RH, Seibel MJ, Zhou H (2011) Exogenous and endogenous glucocorticoids in rheumatic diseases. Arthritis Rheum 63:1–9
Buttgereit F, Doering G, Schaeffler A, Witte S, Sierakowski S, Gromnica-Ihle E, Jeka S, Krueger K, Szechinski J, Alten R (2008) Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet 371:205–214
Gounarides JS, Korach-André M, Killary K, Argentieri G, Turner O, Laurent D (2008) Effect of dexamethasone on glucose tolerance and fat metabolism in a diet-induced obesity mouse model. Endocrinology 149:758–766
de Oliveira C, de Mattos A, Biz C, Oyama L, Ribeiro E, Oller do Nascimento C (2011) High-fat diet and glucocorticoid treatment cause hyperglycemia associated with adiponectin receptor alterations. Lipids Health Dis 10:11
Henneicke H, Herrmann M, Kalak R, Brennan-Speranza TC, Heinevetter U, Bertollo N, Day RE, Huscher D, Buttgereit F, Dunstan CR, Seibel MJ, Zhou H (2011) Corticosterone Selectively Targets endo-cortical surfaces by an osteoblast-dependent mechanism. Bone 49:733–742
Oury F, Ferron M, Huizhen W, Confavreux C, Xu L, Lacombe J, Srinivas P, Chamouni A, Lugani F, Lejeune H, Kumar TR, Plotton I, Karsenty G (2013) Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest 123:2421–2433
Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith Charles E, Hermo L, Suarez S, Roth Bryan L, Ducy P, Karsenty G (2011) Endocrine regulation of male fertility by the skeleton. Cell 144:796–809
Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, Christenson L, Li B, Zhang J, Jackson PD, Faber P, Brunden KR, Harrington JJ, Quarles LD (2008) GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One 3:e3858
Conigrave AD, Hampson DR (2010) Broad-spectrum amino acid-sensing class C G-protein coupled receptors: molecular mechanisms, physiological significance and options for drug development. Pharmacol Ther 127:252–260
Bouschet T, Martin S, Henley JM (2012) Regulation of calcium sensing receptor trafficking by RAMPs. Adv Exp Med Biol 744:39–48
Conigrave AD, Quinn SJ, Brown EM (2000) L-Amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci 97:4814–4819
Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD (2005) Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 280:40201–40209
Pi M, Wu Y, Quarles LD (2011) GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res 26:1680–1683
Jacobsen SE, Norskov-Lauritsen L, Thomsen AR, Smajilovic S, Wellendorph P, Larsson NH, Lehmann A, Bhatia VK, Brauner-Osborne H (2013) Delineation of the GPRC6A receptor signaling pathways using a mammalian cell line stably expressing the receptor. J Pharmacol Exp Ther 347:298–309
Wei J, Hanna T, Suda N, Karsenty G, Ducy P (2014) Osteocalcin promotes β-cell proliferation during development and adulthood through GPRC6A. Diabetes 63:1021–1031
Wellendorph P, Johansen LD, Jensen AA, Casanova E, Gassmann M, Deprez P, Clément-Lacroix P, Bettler B, Bräuner-Osborne H (2009) No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J Mol Endocrinol 42:215–223
Smajilovic S, Clemmensen C, Johansen L, Wellendorph P, Holst J, Thams P, Ogo E, Bräuner-Osborne H (2013) The l-α-amino acid receptor GPRC6A is expressed in the islets of Langerhans but is not involved in l-arginine-induced insulin release. Amino Acids 44:383–390
Clemmensen C, Smajilovic S, Madsen AN, Klein AB, Holst B, Bräuner-Osborne H (2013) Increased susceptibility to diet-induced obesity in GPRC6A receptor knockout mice. J Endocrinol 217:151–160
Clemmensen C, Pehmoller C, Klein AB, Ratner C, Wojtaszewski JF, Brauner-Osborne H (2013) Enhanced voluntary wheel running in GPRC6A receptor knockout mice. Physiol Behav 118:144–151
Desjardins C (1978) Endocrine regulation of reproductive development and function in the male. J Anim Sci 47(Suppl 2):56–79
Dorrington JH, Armstrong DT (1979) Effects of FSH on gonadal functions. Recent Prog Horm Res 35:301–342
Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, Huang YY, Lee H, Srinivas P, Gao XB, Suyama S, Langer T, Mann JJ, Horvath TL, Bonnin A, Karsenty G (2013) Maternal and offspring pools of osteocalcin influence brain development and functions. Cell 155:228–241
Kapustin AN, Shanahan CM (2011) Osteocalcin: a novel vascular metabolic and osteoinductive factor? Arterioscler Thromb Vasc Biol 31:2169–2171
Shanahan CM, Cary NRB, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME (1999) Medial localization of mineralization-regulating proteins in association with Mönckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation 100:2168–2176
Proudfoot D, Davies JD, Skepper JN, Weissberg PL, Shanahan CM (2002) Acetylated low-density lipoprotein stimulates human vascular smooth muscle cell calcification by promoting osteoblastic differentiation and inhibiting phagocytosis. Circulation 106:3044–3050
Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM (2003) Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol 23:489–494
Idelevich A, Rais Y, Monsonego-Ornan E (2011) Bone Gla protein increases HIF-1α-dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscl Thromb Vasc Biol 31:e55–e71
Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, Cantrell DA (2012) PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med 209:2441–2453
Araldi E, Schipani E (2010) Hypoxia, HIFs and bone development. Bone 47:190–196
Kanazawa I, Yamaguchi T, Tada Y, Yamauchi M, Yano S, Sugimoto T (2011) Serum osteocalcin level is positively associated with insulin sensitivity and secretion in patients with type 2 diabetes. Bone 48:720–725
Inaba M, Nishizawa Y, Mita K, Kumeda Y, Emoto M, Kawagishi T, Ishimura E, Nakatsuka K, Shioi A, Morii H (1999) Poor glycemic control impairs the response of biochemical parameters of bone formation and resorption to exogenous 1,25-dihydroxyvitamin D3 in patients with type 2 diabetes. Osteoporos Int 9:525–531
Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B (2009) Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 94:827–832
Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellstrom D (2009) Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res 24:785–791
Gravenstein KS, Napora JK, Short RG, Ramachandran R, Carlson OD, Metter EJ, Ferrucci L, Egan JM, Chia CW (2011) Cross-sectional evidence of a signaling pathway from bone homeostasis to glucose metabolism. J Clin Endocrinol Metab 96:E884–E890
Sayinalp S, Gedik O, Koray Z (1995) Increasing serum osteocalcin after glycemic control in diabetic men. Calcif Tissue Int 57:422–425
Lu C, Ivaska KK, Alen M, Wang Q, Tormakangas T, Xu L, Wiklund P, Mikkola TM, Pekkala S, Tian H, Vaananen HK, Cheng S (2012) Serum osteocalcin is not associated with glucose but is inversely associated with leptin across generations of nondiabetic women. J Clin Endocrinol Metab 97:4106–4114
Osorio J (2012) Metabolism: no role for osteocalcin in glucose metabolism-challenging a paradigm? Nat Rev Endocrinol 8:626
von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U (2004) Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism 53:918–921
Garcia-Martin A, Cortes-Berdonces M, Luque-Fernandez I, Rozas-Moreno P, Quesada-Charneco M, Munoz-Torres M (2011) Osteocalcin as a marker of metabolic risk in healthy postmenopausal women. Menopause 18:537–541
Yeap BB, Chubb SA, Flicker L, McCaul KA, Ebeling PR, Beilby JP, Norman PE (2010) Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur J Endocrinol 163:265–272
Levinger I, Zebaze R, Jerums G, Hare DL, Selig S, Seeman E (2011) The effect of acute exercise on undercarboxylated osteocalcin in obese men. Osteoporos Int 22:1621–1626
Schafer AL, Sellmeyer DE, Schwartz AV, Rosen CJ, Vittinghoff E, Palermo L, Bilezikian JP, Shoback DM, Black DM (2011) Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1–84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study). J Clin Endocrinol Metab 96:E1982–E1989
Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, Sugimoto T (2011) Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int 22:187–194
Pollock NK, Bernard PJ, Gower BA, Gundberg CM, Wenger K, Misra S, Bassali RW, Davis CL (2011) Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with beta-cell function. J Clin Endocrinol Metab 96:E1092–E1099
Iki M, Tamaki J, Fujita Y, Kouda K, Yura A, Kadowaki E, Sato Y, Moon JS, Tomioka K, Okamoto N, Kurumatani N (2012) Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos Int 23:761–770
Hwang YC, Jeong IK, Ahn KJ, Chung HY (2009) The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metabol Res Rev 25:768–772
Confavreux CB, Borel O, Lee F, Vaz G, Guyard M, Fadat C, Carlier MC, Chapurlat R, Karsenty G (2012) Osteoid osteoma is an osteocalcinoma affecting glucose metabolism. Osteoporos Int 23:1645–1650
Conflict of Interest
T.C. Brennan-Speranza, A.D. Conigrave declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brennan-Speranza, T.C., Conigrave, A.D. Osteocalcin: An Osteoblast-Derived Polypeptide Hormone that Modulates Whole Body Energy Metabolism. Calcif Tissue Int 96, 1–10 (2015). https://doi.org/10.1007/s00223-014-9931-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9931-y