Abstract
Background
Vitamins are essential micronutrients with antioxidant potential that may provide a complementary treatment for patients with chronic diseases. Our aim was to assess the effect of vitamin supplementation on the antioxidant status and glycemic index of type 2 diabetes mellitus patients.
Methods
We performed a systematic review with meta-analyses. Electronic searches were conducted in PubMed, Scopus, and Web of Science (December 2017). Randomized controlled trials evaluating the effect of any vitamin or vitamin complex supplementation on antioxidant status as primary outcome were included. The outcomes considered were: reduction of malondialdehyde (MDA); augmentation of glutathione peroxidase (GPx); changes in total antioxidant capacity (TAC), enhance in superoxide dismutase enzyme—SOD, and thiobarbituric acid reactive substances (TBARS). Outcomes of glycemic control were also evaluated. Pairwise meta-analyses were performed using software Review Manager 5.3.
Results
Thirty trials fulfilled the inclusion criteria, but only 12 could be included in the meta-analyses of antioxidant outcomes. The most commonly studied vitamins were B, C, D and E. Vitamin E was related to significant reduction of blood glucose as well as glycated hemoglobin compared to placebo, while both vitamins C and E were mainly associated with reducing MDA and TBARS and elevating GPx, SOD and TAC, compared to placebo. However, outcome reports in this field are still inconsistent (e.g. because of a lack of standard measures).
Conclusions
Supplementation of vitamin E may be a valuable strategy for controlling diabetes complications and enhancing antioxidant capacity. The effects of other micronutrients should be further investigated in larger and well-designed trials to properly place these complementary therapies in clinical practice.
Similar content being viewed by others
Background
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by an increase in blood glucose concentration (fasting blood glucose ≥ 126 mg/dL). There are currently 425 million people with diabetes worldwide, and this number is expected to reach 629 million by 2045, with type 2 diabetes (T2DM) being the most expressive form of the disease [1, 2]. The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) consensus statement on the management of T2DM recommend life-style changes (healthy diet and physical activity) in combination with metformin at the time of diagnosis, and the addition of other medication in patients who do not achieve the desired glycemic control [1]. Lowering glycated hemoglobin (HbA1c) to below 7% has been shown to be one of the primary endpoints in reducing microvascular complications of DM and possibly macrovascular disease [3].
Current evidence has demonstrated that oxidative stress plays an important role in the pathogenesis of chronic diseases such as DM [4, 5] and may diminish the antioxidative defense system of the body, increasing the oxidative load [6]. Some studies have shown that individuals with low concentrations of antioxidants are at increased risk of diabetes complications [5, 7, 8] and that T2DM is associated with endothelial dysfunction [9]. These conditions may develop into macro and microvascular diseases such as retinopathy, nephropathy, lower extremity amputations, coronary artery and cardiovascular diseases [10,11,12], which are the main causes of morbidity and mortality worldwide [2].
The damaging effects of oxidative stress are mainly caused by the production of free radicals of oxygen and reactive oxygen species (ROS), but these substances can be modified by enzymatic or non-enzymatic antioxidants such as superoxide dismutase, vitamins, minerals, and polyphenols [13]. A previous study described how the supplementation with multivitamins in a population with a high prevalence of micronutrient deficiency significantly decreased cerebrovascular disease mortality [14]. Other researchers have analyzed the antioxidant properties of natural products through chemical or biological methods. They have suggested that the consumption of food rich in antioxidants can retard or prevent the occurrence of disease [15, 16]. Nevertheless, previous systematic reviews and individual randomized controlled trials (RCTs) that have measured the effect of vitamin supplementation on antioxidant status and glycemic control of diabetic patients have provided conflicting results, so that the benefit, or otherwise, of such supplementation remains uncertain [17,18,19,20,21].
Thus, we aimed to conduct a systematic review and pairwise meta-analyses to gather current evidence on the effects of any vitamin supplementation on antioxidant status in T2DM patients, in order to elucidate its real benefits.
Methods
We conducted and reported this systematic review and meta-analyses according to the Cochrane Recommendations and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [22, 23]. Two independent reviewers performed all the steps and discrepancies were solved by consensus with a third author.
Search strategies and inclusion criteria
We searched for relevant articles in the databases PubMed, Scopus and Web of Science, without any time limit (updated December 18th, 2017). In addition, we conducted a manual search on the reference lists of the retrieved articles, reviews and trial registration databases to identify registers missed by the electronic search. Complete search strategies are presented in Additional file 1: Search strategies.
We included RCTs assessing adult patients (over 18 years old) of any gender with any stage of T2DM and evaluating plasmatic antioxidant parameters or oxidative stress. Patients received vitamins (types A and/or B complex and/or C and/or D and/or E or variants administered alone or in combination with other vitamins, micronutrients or minerals) irrespective of form, dosage, duration or route of administration compared with placebo or no treatment or other vitamins (active control).
Two researchers independently screened titles and abstracts of the articles retrieved by the systematic review to identify irrelevant records. In a second stage, full text articles were evaluated to identify any of the following exclusion criteria: non-randomized controlled trials (type of studies); interventions other than vitamins; individuals aged under 18 years; different populations or other type of diabetes (prediabetes, type 1 diabetes mellitus, gestational diabetes mellitus); outcomes measure other than antioxidant-related; trials published in non-roman characters.
Data extraction and quality assessment
The following data were independently extracted from the included studies by two researchers: baseline characteristics (authors’ names, year of publication, study design, country, sample size, gender, age, patients’ condition, trial duration); methodological aspects; and clinical outcomes of interest. For primary outcomes, studies should report alterations in plasma antioxidant parameters or oxidative stress, such: vitamin levels, antioxidant enzyme levels [superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT)], oxidative stress biomarkers (e.g. harmful products MDA (malondialdehyde) and thiobarbituric acid reactive substances (TBARS) or changes in plasma total antioxidant capacity (TAC). Other changes in anthropometric and glycemic parameters such as fasting blood glucose and HbA1c reduction, regarded as the ‘core outcome set’ for diabetes control, were also collected, when available.
Two different instruments, the Jadad score [24] and the Cochrane Collaboration’s tool for assessing the Risk of Bias [22], were used to evaluate the included studies’ methodological aspects, such as proper randomization, blinding, account for patients withdrawals and dropouts and other bias that may affect data interpretation.
Statistical analyses
Pairwise meta-analyses of the included RCTs were performed for the main outcome measures whenever the number of studies for each outcome of interest allowed. These analyses were conducted using the software Review Manager version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
For each meta-analysis we used the random effects model and the inverse variance (IV) method to interpolate the mean differences (MD) or standardized (std.) mean differences (SMD) of each study from baseline. Results are reported with a 95% confidence interval (CI). A p value less than 0.05 (two-tailed) was considered indicative of a statistically significant difference between groups. The between-trial heterogeneity was assessed using the inconsistency index value (I2) (I2 > 50% indicates high and significant heterogeneity) [22]. We also conducted sensitivity analyses to test the robustness of the results in order to evaluate the impact of any study on data heterogeneity. The analysis consisted of the hypothetical sequential removal of studies from the meta-analysis. When possible, subgroup analyses were also performed.
Results
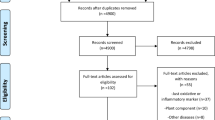
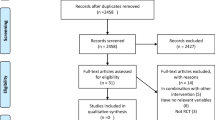
The systematic search conducted in the three databases retrieved 1570 records and 196 were excluded as duplicates. During the study’s title and abstract reading process (screening), 1243 records were excluded and 104 were considered for full-text appraisal, of which 25 articles were suitable for final analyses. Six articles were added from manual searches, finally yielding 31 articles representing 30 RCTs [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] (Fig. 1). The main characteristics of the included studies are provided in Table 1.
All studies involved patients diagnosed with T2DM (n = 1430) and were conducted mainly in Iran (n = 9 trials) [25, 32,33,34, 38, 41, 43, 44, 48, 51]; followed by the United Kingdom (n = 4) [42, 49, 52, 53] and the United States of America (n = 3) [28, 29, 47]. Evaluated treatments comprised: vitamin B (n = 1 study) [25], vitamin C (n = 10) [26, 27, 29, 34, 37, 38, 40,41,42, 50]; vitamin D (n = 7) [30, 35, 43, 44, 48, 49, 53, 55]; vitamin E (n = 12) [28, 32,33,34, 36, 39, 45,46,47, 51, 52, 54], and a mixture of vitamins B, C and E (n = 1) [31]. In four of these trials (13.3%), vitamins were delivered by food fortification (oil or yogurt) [32, 44, 48, 51]. A placebo or negative control was the main comparator in 29 studies (96.7%), while eight trials (26.7%) included head-to-head comparisons. Duration of treatment ranged from two to 24 weeks and patients’ age ranged from 46 to 72 years.
Overall, the methodological quality of included trials was low to moderate, with a mean Jadad Score of 2.7 (range 1–5). All studies scored on randomization, but only 20% of them properly described how randomization was achieved. Almost all trials (90%) accounted for patient’ withdrawals or dropouts, and half of the studies was double-blinded. However, only two trials described the blinding methods. The Risk of Bias assessment (see Additional file 1: Quality assessment), established that trials were of low risk of bias (> 75%) in the domains of randomization, incomplete outcome data and selective reporting. Allocation concealment was considered unclear in 25 trials (86.2%), and studies often failed to provide details regarding blinding of participants or outcome measures. Overall, 70% of trials were funded by industries or reported conflict of interest.
Considering the primary outcomes of interest related to antioxidant status, 12 RCTs (encompassing 13 articles) were able to be included in the meta- analyses (all of them compared vitamin to placebo) [25, 28, 33, 36, 38, 39, 41, 43,44,45, 48, 51, 54]. Not all studies were statistically evaluated since outcomes were not comparable (e.g. because of lack of raw data). Gathering evidence, especially on antioxidant potential, was hampered by the lack of standardization of outcome reporting in the clinical trials (e.g. inconsistent reporting, using different measures, scales and units).
Meta-analyses were obtained for augmentation of GPx levels (Units/gram of Hemoglobin—U/g Hb), reduction in plasma MDA (nmol/L) and TBARS (µmol/L) reductions, and favorable changes in TAC (mmol/L) and SOD (U/g Hb). In these cases, no subgroup analyses were performed due to the limited number of studies. Overall, results were statistically different from placebo to favored the use of vitamins with values of MD 9.40 (95% CI [7.79; 11.00]) for GPx (p < 0.001) and MD − 0.53 (95% CI [− 0.81; − 0.25]) for MDA (p < 0.001), with I2 values of 44 and 47%, respectively. Vitamins were also superior to placebo in reducing TBARS with an overall effect size of SMD − 4.84 (95% CI [− 6.01; − 3.67]) (p < 0.001; I2 = 54%) and in increasing TAC (SMD 0.38 [0.11; 0.65]; p = 0.006) and SOD levels (SMD 0.64 [0.11; 1.17]; p = 0.02). These positive results came mostly from studies where the interventions were vitamin E (n = 7 trials) [28, 33, 36, 39, 45, 51, 54]; vitamin C (n = 2) [38, 41] and vitamin D (n = 2) [44, 48] (see Fig. 2).
Forest plots for the outcomes of antioxidant status. a Augmentation of GPx level (U/g Hb). b Reduction of MDA (nmol/L). c Reduction of TBARS (µmol/L). d Changes in TAC (mmol/L). e Changes in SOD (U/g). Statistical method: Mean difference (MD) and Std. Mean Difference (SMD), IV, Random, 95% confidence interval
The meta-analyses of the glycemic control parameters (17 included trials obtained from 18 articles) are shown in Figs. 3, 4 [28,29,30, 33, 35,36,37,38,39, 43, 44, 46,47,48,49, 52, 53, 55]. No statistical differences were observed in subgroup analyses comparing vitamins C or D with placebo. However, for both outcomes of mean change in blood glucose (mg/dL) and reduction of HbA1c (as a percentage), the effects of vitamin E were significantly better than the control (values of MD − 13.89 (95% CI [− 19.89; − 7.89]) and MD − 0.47 (95% CI [− 0.69; − 0.26]), respectively).
The moderate to high heterogeneity of some meta-analyses (I2 ranging from 15 to 71%) was caused by more than one study and can be considered acceptable in this context. Sensitivity analyses were conducted with all the meta-analyses (data not shown) and despite the sequential hypothetical removal of studies with reduction in the heterogeneity, the results remained unchanged.
Discussion
Our study is the first systematic review with meta-analysis to evaluate the available evidence of vitamin supplementation in T2DM patients for the improvement of antioxidant status in different ways (GPx, SOD and TAC levels augmentation and reduction in MDA and TBARS products). Previous studies have focused on glycemic control, insulin resistance and changes in endothelial functions [18, 20, 21, 56].
Our results revealed that supplementation of certain vitamins in T2DM, especially vitamin E, can produce a significant impact on the parameters of antioxidant status and glycemic control, which may positively benefit patients. Vitamin C was more related to changes in antioxidant status, while little evidence was found for the effect of other vitamins (e.g. D or B).
The beneficial effects of vitamin E may be explained by the reduction of the damaging effects of free radicals on the structural and functional components of cells and vessel walls [56, 57]. It is believed that diabetes is associated with increased oxidative stress because of increased blood concentrations of thiobarbituric acid reactive substances and serum malondialdehyde, the end products of lipid peroxidation [58]. The adverse physiological effects which result include increased leakiness of cell membranes where the structural integrity of membranes has been altered; inactivation of membrane bound enzymes and surface receptors and the involvement of oxidized LDL (LDL-ox). When total antioxidant status (TAC) is high enough to combat the oxidative stress, the MDA and TBARS levels are in the normal limits and vice versa. Antioxidants decrease the oxidative damage directly by reacting with free radicals or indirectly by inhibiting the activity or expression of free radicals [59, 60].
Non-enzymatic antioxidants such as vitamins C and E and glutathione interrupt free radical chain reactions. The combination of these vitamins appears to be promising. Although only one RCT evaluating a mixed vitamin complex was found in our systematic review [31], a previous study reported that antioxidant combinations might be an appropriate formula for the management of diabetes [61]. A 3-month study on the supplementation of vitamins C and E showed that patients’ blood glucose decreased while SOD and glutathione levels increased [62]. Moreover, the long-term use of dietary supplements, including multivitamin or mineral complexes showed benefits in C-reactive protein, HDL cholesterol, triacylglycerides, serum homocysteine, blood pressure and incidence of diabetes [14, 63,64,65]. However, vitamin C alone did not present a greater profile than vitamin E.
In the literature, vitamin D is related to gene expression control which may trigger a biological response to oxidative stress, such as inhibiting nitric oxide synthase (iNOS) or increasing glutatione levels [66]. The antioxidant effect of vitamin D is among the most recent non-calcemic roles suggested for this compound [67]. There is evidence from both humans and animal models suggesting that vitamin D may play an important role in modifying the risk of diabetes [66, 68]. Low vitamin D status is associated with future macrovascular events in patients with T2DM. This association may be the result of the link between vitamin D status and the renin-angiotensin system, endothelial function, blood pressure, or even chronic inflammation [20, 69, 70]. However, our results were constrained in defining a vitamin D antioxidant profile, since few RCTs involving this micronutrient were included [43, 44].
Some trials [32, 44, 48, 51] did not use direct drug supplementation but incorporated the vitamin in food (e.g. oil, yogurt), which may have affected final results. Moreover, because the total daily dosage of vitamin intake and treatment duration varied among the studies, effects on antioxidant and glycemic profiles may have been underestimated. Regimens for vitamin C varied from 500 to 3000 mg/day; for vitamin E they ranged from 400 to 1600 IU/day and for vitamin D doses were of 500 to 200,000 IU/day. Longer period trials with reasonable lower daily doses may increase the intracellular concentration of vitamins and result in an adequate effect that should then be evaluated.
In spite of the encouraging results reported above, the small number of studies properly reporting data prevented a fully satisfactory assessment of the outcomes related to antioxidant status. Moreover, methodological aspects of the included trials demonstrated low to moderate quality, especially concerning accurate description of randomization and blinding. The moderate to high heterogeneity in some meta-analyses can be explained by the differences in the intrinsic characteristics of studies, the conduct and design of trials with low quality, the small sample sizes of some studies, patient’s conditions with possible comorbidities, different pharmacological treatments, and differences in outcome measures.
The marked heterogeneity in the outcomes reporting of oxidative stress and antioxidant capacity might be due the lack of standardization in the selection or measurement of the outcome in clinical trials. Different measures and units are usually employed (e.g., enzyme levels (catalase, superoxide dismutase); FRAP—ferric reducing ability of plasma assay; ORAC—oxygen radical absorbance capacity assay; TAS—total antioxidant status, among others) [71, 72]. This can be justified in part because of the range of substances and antioxidant components in the organism, together with the difficulty in measuring them all at once. The issue of lack of outcome standardization is common to different areas, but has been associated with a bad reporting practice—outcome switching, and hamper comparisons between interventions [73]. The development of a core outcome set for antioxidant status in chronic diseases is important for study design and could minimize bias. Measures such as TAC, TBARS and MDA could be employed as standard.
Our study has some limitations. We included RCTs with differences in methodological design and population characteristics (e.g. age, gender, disease stage and comorbidities, diabetes treatments, study duration) and none of them were sufficiently powerful due to the relatively small number of participants. There was some difficulty in finding and gathering trials of the same vitamin or vitamin complex assessing similar outcomes. We were able to statistically analyze three vitamins (C, D and E), but other micronutrients and vitamin combinations (especially vitamins C and E) should be better investigated. Subgroup meta-analyses were poorly obtained.
We strongly recommend that further well-designed, large-scale, long-term head-to-head controlled trials and meta-analyses be carried out to demonstrate the effects of individual or multivitamin supplementations on T2DM, since previous results are promising.
Conclusions
The consumption of vitamin E (alone or in combination) promotes health benefits since it affects plasma antioxidant capacity and the concentration of enzymes and reduces MDA and TBARS levels. T2DM patients have a high risk of experiencing micro and macrovascular complications, and daily vitamin supplementation provides an alternative strategy for metabolic control, in addition to the combination of diet, exercise and medication. These substances may represent a step forward in disease management. Further studies should be conducted to strengthen this evidence, especially for defining doses and regimen of vitamin E, and support its use in daily practice.
Abbreviations
- ADA:
-
American Diabetes Association
- DM:
-
diabetes mellitus
- EASD:
-
European Association for the Study of Diabetes
- T2DM:
-
type 2 diabetes mellitus
- HbA1c:
-
glycated hemoglobin
- CAT:
-
catalase
- MDA:
-
malondialdehyde
- GPx:
-
glutathione peroxidase
- TAC:
-
total antioxidant capacity
- SOD:
-
superoxide dismutase enzyme
- TBARS:
-
thiobarbituric acid reactive substances
- ROS:
-
reactive oxygen species
- RCT:
-
randomized controlled trials
References
American Diabetes Association. Standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Suppl 1):S1–2.
International Diabetes Federation. IDF diabetes atlas. 8th ed. Brussels: IDF; 2017.
Cobitz AR, Ambery P. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(5):e58.
Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5.
Ceriello A, Testa R, Genovese S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr Metab Cardiovasc Dis. 2016;26(4):285–92.
Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015;13(10):423–44.
Sankhla M, Sharma TK, Mathur K, Rathor JS, Butolia V, Gadhok AK, et al. Relationship of oxidative stress with obesity and its role in obesity induced metabolic syndrome. Clin Lab. 2012;58(5–6):385–92.
Kositsawat J, Freeman VL. Vitamin C and A1c relationship in the National Health and Nutrition Examination Survey (NHANES) 2003–2006. J Am Coll Nutr. 2011;30(6):477–83.
Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev. 2001;22(1):36–52.
Hink U, Tsilimingas N, Wendt M, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus: therapeutic implications. Treat Endocrinol. 2003;2(5):293–304.
Duckworth WC. Hyperglycemia and cardiovascular disease. Curr Atheroscler Rep. 2001;3(5):383–91.
Sasaki S, Inoguchi T. The role of oxidative stress in the pathogenesis of diabetic vascular complications. Diabetes Metab J. 2012;36(4):255–61.
Lamb RE, Goldstein BJ. Modulating an oxidative-inflammatory cascade: potential new treatment strategy for improving glucose metabolism, insulin resistance, and vascular function. Int J Clin Pract. 2008;62(7):1087–95.
Mark SD, Wang W, Fraumeni JF Jr., Li JY, Taylor PR, Wang GQ, et al. Lowered risks of hypertension and cerebrovascular disease after vitamin/mineral supplementation: the Linxian Nutrition Intervention Trial. Am J Epidemiol. 1996;143(7):658–64.
White PA, Oliveira RC, Oliveira AP, Serafini MR, Araujo AA, Gelain DP, et al. Antioxidant activity and mechanisms of action of natural compounds isolated from lichens: a systematic review. Molecules. 2014;19(9):14496–527.
Singh R, Devi S, Gollen R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: larger-than-life. Diabetes Metab Res Rev. 2015;31(2):113–26.
Tabatabaei-Malazy O, Nikfar S, Larijani B, Abdollahi M. Influence of ascorbic acid supplementation on type 2 diabetes mellitus in observational and randomized controlled trials; a systematic review with meta-analysis. J Pharm Pharm Sci. 2014;17(4):554–82.
Montero D, Walther G, Stehouwer CD, Houben AJ, Beckman JA, Vinet A. Effect of antioxidant vitamin supplementation on endothelial function in type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2014;15(2):107–16.
Sarmento RA, Silva FM, Sbruzzi G, Schaan BD, Almeida JC. Antioxidant micronutrients and cardiovascular risk in patients with diabetes: a systematic review. Arq Bras Cardiol. 2013;101(3):240–8.
George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012;29(8):e142–50.
Xu R, Zhang S, Tao A, Chen G, Zhang M. Influence of vitamin E supplementation on glycaemic control: a meta-analysis of randomised controlled trials. PLoS ONE. 2014;9(4):e95008.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0: Cochrane. London: The cochrane collaboration; 2011.
Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Aghamohammadi V, Gargari BP, Aliasgharzadeh A. Effect of folic acid supplementation on homocysteine, serum total antioxidant capacity, and malondialdehyde in patients with type 2 diabetes mellitus. J Am Coll Nutr. 2011;30(3):210–5.
Anderson RA, Evans LM, Ellis GR, Khan N, Morris K, Jackson SK, et al. Prolonged deterioration of endothelial dysfunction in response to postprandial lipaemia is attenuated by vitamin C in type 2 diabetes. Diabet Med. 2006;23(3):258–64.
Antoniades C, Tousoulis D, Tountas C, Tentolouris C, Toutouza M, Vasiliadou C, et al. Vascular endothelium and inflammatory process, in patients with combined Type 2 diabetes mellitus and coronary atherosclerosis: the effects of vitamin C. Diabet Med. 2004;21(6):552–8.
Ble-Castillo JL, Carmona-Diaz E, Mendez JD, Larios-Medina FJ, Medina-Santillan R, Cleva-Villanueva G, et al. Effect of alpha-tocopherol on the metabolic control and oxidative stress in female type 2 diabetics. Biomed Pharmacother. 2005;59(6):290–5.
Chen H, Karne RJ, Hall G, Campia U, Panza JA, Cannon RO 3rd, et al. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am J Physiol Heart Circ Physiol. 2006;290(1):H137–45.
Dalan R, Liew H, Assam PN, Chan ES, Siddiqui FJ, Tan AW, et al. A randomised controlled trial evaluating the impact of targeted vitamin D supplementation on endothelial function in type 2 diabetes mellitus: the DIMENSION trial. Diabetes Vasc Dis Res. 2016;13(3):192–200.
Gariballa S, Afandi B, Abu Haltem M, Yassin J, Alessa A. Effect of antioxidants and B-group vitamins on risk of infections in patients with type 2 diabetes mellitus. Nutrients. 2013;5(3):711–24.
Haghighat NVM, Eghtesadi S, Heidari I, Hosseini A, Rostami A. The Effects of tocotrienols added to canola oil on microalbuminuria, inflammation, and nitrosative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Int J Prev Med. 2014;5(5):617–23.
Hejazi N, Dabbaghmanesh M, Mazloom Z, Dashtabi A. Effects of vitamin E on fasting and postprandial oxidative stress, inflammatory markers, glucose status, insulin resistance, blood pressure and pulse rate in type-2 diabetic patients: a randomized clinical trial. Galen Med J. 2015;4(3):67–74.
Jamalan M, Rezazadeh M, Zeinali M, Ghaffari MA. Effect of ascorbic acid and alpha-tocopherol supplementations on serum leptin, tumor necrosis factor alpha, and serum amyloid A levels in individuals with type 2 diabetes mellitus. Avicenna J Phytomed. 2015;5(6):531–9.
Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr. 2009;48(6):349–54.
Lai MH. Antioxidant effects and insulin resistance improvement of chromium combined with vitamin C and e supplementation for type 2 diabetes mellitus. J Clin Biochem Nutr. 2008;43(3):191–8.
Lu Q, Bjorkhem I, Wretlind B, Diczfalusy U, Henriksson P, Freyschuss A. Effect of ascorbic acid on microcirculation in patients with type II diabetes: a randomized placebo-controlled cross-over study. Clin Sci. 2005;108(6):507–13.
Mahmoudabadi MM, Rahbar AR. Effect of EPA and vitamin C on superoxide dismutase, glutathione peroxidase, total antioxidant capacity and malondialdehyde in type 2 diabetic patients. Oman Med J. 2014;29(1):39–45.
Manzella D, Barbieri M, Ragno E, Paolisso G. Chronic administration of pharmacologic doses of vitamin E improves the cardiac autonomic nervous system in patients with type 2 diabetes. Am J Clin Nutr. 2001;73(6):1052–7.
Mason SA, Della Gatta PA, Snow RJ, Russell AP, Wadley GD. Ascorbic acid supplementation improves skeletal muscle oxidative stress and insulin sensitivity in people with type 2 diabetes: findings of a randomized controlled study. Free Radic Biol Med. 2016;93:227–38.
Mazloom ZHN, Dabbaghmanesh MH, Tabatabaei HR, Ahmadi A, Ansar H. Effect of vitamin C supplementation on postprandial oxidative stress and lipid profile in type 2 diabetic patients. Pak J Biol Sci. 2011;14(19):900–4.
Mullan BA, Young IS, Fee H, McCance DR. Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension. 2002;40(6):804–9.
Nikooyeh B, Neyestani TR, Farvid M, Alavi-Majd H, Houshiarrad A, Kalayi A, et al. Daily consumption of vitamin D − or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr. 2011;93(4):764–71.
Nikooyeh B, Neyestani TR, Tayebinejad N, Alavi-Majd H, Shariatzadeh N, Kalayi A, et al. Daily intake of vitamin D − or calcium-vitamin D-fortified Persian yogurt drink (doogh) attenuates diabetes-induced oxidative stress: evidence for antioxidative properties of vitamin D. J Hum Nutr Diet. 2014;27(Suppl 2):276–83.
Paolisso G, Tagliamonte MR, Barbieri M, Zito GA, Gambardella A, Varricchio G, et al. Chronic vitamin E administration improves brachial reactivity and increases intracellular magnesium concentration in type II diabetic patients. J Clin Endocrinol Metab. 2000;85(1):109–15.
Park S, Choi SB. Effects of alpha-tocopherol supplementation and continuous subcutaneous insulin infusion on oxidative stress in Korean patients with type 2 diabetes. Am J Clin Nutr. 2002;75(4):728–33.
Reaven PD, Herold DA, Barnett J, Edelman S. Effects of Vitamin E on susceptibility of low-density lipoprotein and low-density lipoprotein subfractions to oxidation and on protein glycation in NIDDM. Diabetes Care. 1995;18(6):807–16.
Shab-Bidar SNTR, Djazayery A. The interactive effect of improvement of vitamin D status and VDR FokI variants on oxidative stress in type 2 diabetic subjects: a randomized controlled trial. Eur J Clin Nutr. 2015;69(2):216–22.
Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25(3):320–5.
Tessier DM, Khalil A, Trottier L, Fulop T. Effects of vitamin C supplementation on antioxidants and lipid peroxidation markers in elderly subjects with type 2 diabetes. Arch Gerontol Geriatr. 2009;48(1):67–72.
Vafa MHN, Moslehi N, Eghtesadi S, Heydari I. Effect of tocotrienols enriched canola oil on glycemic control and oxidative status in patients with type 2 diabetes mellitus: a randomized double-blind placebo-controlled clinical trial. J Res Med Sci. 2015;20(6):540–7.
Winterbone MS, Sampson MJ, Saha S, Hughes JC, Hughes DA. Pro-oxidant effect of alpha-tocopherol in patients with type 2 diabetes after an oral glucose tolerance test—a randomised controlled trial. Cardiovasc Diabetol. 2007;6:8.
Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53(10):2112–9.
Wu JH, Ward NC, Indrawan AP, Almeida CA, Hodgson JM, Proudfoot JM, et al. Effects of alpha-tocopherol and mixed tocopherol supplementation on markers of oxidative stress and inflammation in type 2 diabetes. Clin Chem. 2007;53(3):511–9.
Yiu YF, Yiu KH, Siu CW, Chan YH, Li SW, Wong LY, et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis. 2013;227(1):140–6.
Ashor AW, Siervo M, Lara J, Oggioni C, Afshar S, Mathers JC. Effect of vitamin C and vitamin E supplementation on endothelial function: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr. 2015;113(8):1182–94.
Brigelius-Flohe R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A. The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr. 2002;76(4):703–16.
Mahreen R, Mohsin M, Nasreen Z, Siraj M, Ishaq M. Significantly increased levels of serum malondialdehyde in type 2 diabetics with myocardial infarction. Int J Diabetes Dev Ctries. 2010;30(1):49–51.
Rani AJ, Mythili SV. Study on total antioxidant status in relation to oxidative stress in type 2 diabetes mellitus. J Clin Diagn Res. 2014;8(3):108–10.
Fenercioglu AK, Saler T, Genc E, Sabuncu H, Altuntas Y. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in Type 2 diabetes mellitus without complications. J Endocrinol Invest. 2010;33(2):118–24.
Khodaeian M, Tabatabaei-Malazy O, Qorbani M, Farzadfar F, Amini P, Larijani B. Effect of vitamins C and E on insulin resistance in diabetes: a meta-analysis study. Eur J Clin Invest. 2015;45(11):1161–74.
Rafighi ZSA, Arab S, Mohd Yousof R. Association of dietary vitamin C and e intake and antioxidant enzymes in type 2 diabetes mellitus patients. Glob J Health Sci. 2013;5(3):183–7.
Church TS, Earnest CP, Wood KA, Kampert JB. Reduction of C-reactive protein levels through use of a multivitamin. Am J Med. 2003;115(9):702–7.
Gey KF, Moser UK, Jordan P, Stahelin HB, Eichholzer M, Ludin E. Increased risk of cardiovascular disease at suboptimal plasma concentrations of essential antioxidants: an epidemiological update with special attention to carotene and vitamin C. Am J Clin Nutr. 1993;57(5 Suppl):787S–97S.
Gey KF, Puska P, Jordan P, Moser UK. Inverse correlation between plasma vitamin E and mortality from ischemic heart disease in cross-cultural epidemiology. Am J Clin Nutr. 1991;53(1 Suppl):326S–34S.
Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017–29.
Mokhtari Z, Hekmatdoost A, Nourian M. Antioxidant efficacy of vitamin D. J Parathyr Dis. 2017;5(1):11–6.
Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673–9.
Krul-Poel YH, Ter Wee M, Lips P, Simsek S. Management of endocrine disease: The effect of vitamin D supplementation on glycaemic control in patients with Type 2 Diabetes Mellitus: a systematic review and meta-analysis. Eur J Endocrinol. 2017;176(1):R1–14.
Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175(5):745–54.
Liu RH. Dietary bioactive compounds and their health implications. J Food Sci. 2013;78(Suppl 1):A18–25.
Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53(6):1841–56.
Jones CW, Keil LG, Holland WC, Caughey MC, Platts-Mills TF. Comparison of registered and published outcomes in randomized controlled trials: a systematic review. BMC Med. 2015;13:282.
Authors’ contributions
RP, FFL and AW designed the study and wrote the protocol. MEB, FST, AMM, and HHB screened, updated and abstracted publications. MEB, FST and AMM evaluated methodological quality. FST, HHB and FFL statistically analyzed data. MEB and FST wrote the manuscript, with editorial contributions from AMM, HHB, AW, FFL and RP. All authors reviewed the manuscript for accuracy and scientific content. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data supporting the conclusions of this article are included within the article and its additional files. Other information is available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the Brazilian National Council of Technological and Scientific Development (CNPq), and the Coordination for the Improvement of Higher Education Personnel (CAPES). The funding sources had no role in the study design, data collection, data analyses, data interpretation, or writing of the report. The corresponding author had full access to all of the data in the study and was responsible for making the final decision to submit the manuscript for publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1.
Search strategies and Quality assessment.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Balbi, M.E., Tonin, F.S., Mendes, A.M. et al. Antioxidant effects of vitamins in type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetol Metab Syndr 10, 18 (2018). https://doi.org/10.1186/s13098-018-0318-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-018-0318-5