Abstract
Background
Malaria is a major public health concern in Ethiopia, and its incidence could worsen with the spread of the invasive mosquito species Anopheles stephensi in the country. This study aimed to provide updates on the distribution of An. stephensi and likely household exposure in Ethiopia.
Methods
Entomological surveillance was performed in 26 urban settings in Ethiopia from 2021 to 2023. A kilometer-by-kilometer quadrant was established per town, and approximately 20 structures per quadrant were surveyed every 3 months. Additional extensive sampling was conducted in 50 randomly selected structures in four urban centers in 2022 and 2023 to assess households’ exposure to An. stephensi. Prokopack aspirators and CDC light traps were used to collect adult mosquitoes, and standard dippers were used to collect immature stages. The collected mosquitoes were identified to species level by morphological keys and molecular methods. PCR assays were used to assess Plasmodium infection and mosquito blood meal source.
Results
Catches of adult An. stephensi were generally low (mean: 0.15 per trap), with eight positive sites among the 26 surveyed. This mosquito species was reported for the first time in Assosa, western Ethiopia. Anopheles stephensi was the predominant species in four of the eight positive sites, accounting for 75–100% relative abundance of the adult Anopheles catches. Household-level exposure, defined as the percentage of households with a peridomestic presence of An. stephensi, ranged from 18% in Metehara to 30% in Danan. Anopheles arabiensis was the predominant species in 20 of the 26 sites, accounting for 42.9–100% of the Anopheles catches. Bovine blood index, ovine blood index and human blood index values were 69.2%, 32.3% and 24.6%, respectively, for An. stephensi, and 65.4%, 46.7% and 35.8%, respectively, for An. arabiensis. None of the 197 An. stephensi mosquitoes assayed tested positive for Plasmodium sporozoite, while of the 1434 An. arabiensis mosquitoes assayed, 62 were positive for Plasmodium (10 for P. falciparum and 52 for P. vivax).
Conclusions
This study shows that the geographical range of An. stephensi has expanded to western Ethiopia. Strongly zoophagic behavior coupled with low adult catches might explain the absence of Plasmodium infection. The level of household exposure to An. stephensi in this study varied across positive sites. Further research is needed to better understand the bionomics and contribution of An. stephensi to malaria transmission.
Graphical Abstract

Similar content being viewed by others
Background
Malaria remains a threat to global public health, with 249 million cases and 608,000 deaths in 2022 [1]. The WHO African region is disproportionately affected, and approximately 78% of malaria-related deaths in the region were in children aged < 5 years [1,2,3,4]. More than half of all Ethiopians, mainly those in rural areas, are at risk of contracting malaria [5,6,7]. Unlike most African countries, clinical malaria of public health importance in Ethiopia is caused by both Plasmodium falciparum (P. falciparum) and P. vivax, which co-occur in all malarious areas, with the prevalence attributable to each parasite dependent on ecological settings and seasons [7, 8]. The transmission of malaria is highly variable due to the diverse eco-topography and climate conditions and, in general, transmission is bimodal, mainly occurring following the rainy seasons, “Kiremt” and “Belg,” which are associated with major (long) and minor (short) transmission periods, respectively [8]. Since the 2000s, comprehensive preventative and case management interventions, including improved coverage of long-lasting insecticide-treated bed-nets and indoor residual spraying, the rollout and scale-up of artemisinin-based combination therapy, the deployment of a more sensitive and specific rapid diagnostic test (histidine-rich protein-2/3 [HRP2/3]-based test) and treatment at the grassroots level through health extension programs have achieved successive reductions in malaria burden [7, 9,10,11]. As a consequence, the aim of Ethiopia is to achieve zero indigenous malaria cases by 2030 [8, 12]. However, the country has experienced a nationwide resurgence in recent years and an unprecedented increase in case burden [1, 13]. Possible contributing factors include insecticide resistance in the primary malaria vector, the COVID-19 pandemic, the emergence of the HRP2/3 deletion, deterioration of the healthcare system, internal conflicts and invasion by the exotic malaria vector, Anopheles stephensi [14,15,16,17].
Until recently, about 46 Anopheles species and subspecies were recorded in Ethiopia [18, 19]. However, only a few Anopheles species, including An. arabiensis, An. pharoensis, An. funestus and An. nili, were incriminated as vectors of malaria [20]. Anopheles arabiensis is the primary malaria vector in most malaria-endemic areas [21,22,23]. However, its abundance, host preference and Plasmodium sporozoite rate vary across ecological gradients and epidemiological settings [22,23,24,25]. Anopheles pharoensis is of secondary importance [20, 23], with An. funestus and An. nili playing lesser roles in malaria transmission [22, 23].
Anopheles stephensi is an efficient urban malaria vector in southeast Asia and the Gulf Region [26] but is currently expanding its geographical range in Africa, where there have been reports of its presence in Djibouti [27], Ethiopia [17], Sudan [28], Somalia [29] and, more recently, Nigeria, Eritrea, Ghana and Kenya [30]. Anopheles stephensi is known to readily invade urban environments, and immature stages thrive in artificial aquatic habitats, with the consequent potential to increase malaria incidence in cities [27, 31] or reintroduce the disease into regions where it has been successfully eliminated [32,33,34,35].
In Ethiopia, since the first detection of An. stephensi in Kebri Dehar, Somali region [17], surveillance has confirmed its presence in the central, northeast, northwest and southwest parts of the country [36, 37]. Recent studies have shown that An. stephensi is a permissive host to P. falciparum and P. vivax infection [16, 37]. The results of a study from Dire Dawa City, eastern Ethiopia, suggest that An. stephensi was responsible, at least in part, for a malaria outbreak [38]. Similarly, in Djibouti, an upsurge in malaria incidence was observed following the detection of An. stephensi, providing further evidence for the potential for increased risk [39, 40]. In line with the WHO’s call for strengthened entomological surveillance of An. stephensi [41], this study aims to update current data on the distribution of An. stephensi across Ethiopia and to increase understanding of the patterns of household exposure to An. stephensi across the country.
Methods
Study area
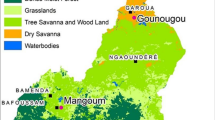
Twenty-six urban centers were selected for this study (Fig. 1). A range of variables, including ecological setting, presence of dry ports and major transportation corridors, An. stephensi habitat suitability modeling and malaria endemicity, were considered when selecting the sites [36, 42]. The study sites (Additional file 1: Table S1) are located at altitudes between 339 and 2355 m a.s.l. and range from hot-desert lowland to humid highland environments. The mean annual temperature ranges from 30.9 °C in Afambo, the northeastern tip of the country, to 15.6 °C in Akaki, central Ethiopia, and the mean annual rainfall ranges from 224 to 1883 mm2 [43].
Study design
Spatiotemporal distribution of An. stephensi
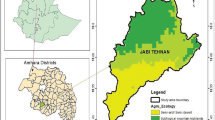
Four rounds of adult and immature stage collections were conducted at approximately 3-month intervals from November 2021 to January 2023. At each of the 26 study sites, a preliminary survey was first conducted to locate potential aquatic habitats of Anopheles mosquitoes outside compound/property limit of human dwellings/households. Based on the availability of aquatic habitats, we delineated a 1- × 1-km quadrant for entomological sampling (Fig. 2). In each quadrant, 20 households were selected for adult mosquito collection, of which four households were purposively selected based on their proximity to a major aquatic habitat and four were randomly selected in different directions from each of the four purposively chosen dwellings. Immature-stage mosquitoes were collected from all potential aquatic habitats within the compounds/property limits of the households selected for adult collection as well as from the purposively identified aquatic habitats within the quadrant beyond the compounds/property limits of the selected dwellings. Catches from the immature-stage collections were pooled by habitat type (either artificial or natural) and reared to adults for morphological species identification as described below. Written informed consent was obtained from the head of each household prior to mosquito collection. To increase the probability of detecting An. stephensi, adaptive sampling was employed [44, 45]. Thus, 50% of the households for adult collection were replaced randomly in subsequent collection rounds.
Household exposure to An. stephensi
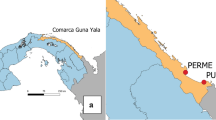
Four urban centers where An. stephensi was detected were selected for more detailed study: Awash Sebat Kilo (Afar Regional State), Danan (Somali Regional State), Metehara (Oromia Regional State) and Jiga (Amhara Regional State). At each selected site, adult mosquitoes were collected from 50 randomly selected households and their surroundings in December 2022 and February 2023. Using a map of urban centers, a household located toward the center of the town was selected first, and then four additional households were selected (at approximately a distance of 100 m, with each of these four dwellings located in a different direction from the first household). This approach was repeated 9 times, to select the remaining 45 households systematically (Fig. 3). Adult Anopheles collections were conducted both indoors and outdoors from the selected households using Centers for Disease Control and Prevention light traps (CDC LTs) and from any structures serving as potential resting places within the household’s property compound using a Prokopack aspirator (John W. Hock Company, Gainesville, FL, USA).
Diagrammatic representation of household selection for studying exposure to An. stephensi. Large shaded circles indicate the selected households together with additional structures within a 50-m radius for entomological collection. Shaded circle designated with A indicates the first selected household (in red) located toward the center of the town. Shaded circles designated with B–E indicate 4 additional households that were selected by moving 100 m (blue dotted line) in four directions from the first selected household. The remaining 45 households were selected by repeating this approach 9 times. The numbers in the blue circle indicate different types of structures within a 50-m radius of the selected household for adult or immature-stage mosquito collection
Adult mosquito collections
Trained field workers collected adult mosquitoes from 06:00 to 08:00 h and from 17:30 to 19:00 h with Prokopack aspirators. Indoor resting mosquito collections were conducted along the walls and ceilings of houses and under or behind household furniture. Outdoor collections were made from vegetation, tree trunks or walls of water containers located outside of the selected structures. The collections were made for 15–30 min in each structure, with the time scaled to the number and size of the structures/areas. The head of the household was requested to avoid activities that could repel mosquitoes on the night before collection, such as smoking inside the house, using repellents or spraying aerosol insecticides [46, 47].
Host-seeking adult mosquitoes were collected both indoors and outdoors using CDC LTs. Indoor traps were set near a sleeping space at the foot edge next to an existing bed-net, and corresponding outdoor traps were set within 5–10 m of each household selected for the indoor collection. Traps were set approximately 1.5 m from the ground, both indoors and outdoors. At approximately 18:00 h, the battery was connected to run the trap, and the following morning, at 06:30 h, the trap was removed. The collection cup was tied off securely prior to the trap being switched off and then labeled with the associated household code, whether an indoor/outdoor collection and with the date of collection before being removed from the trap and the mosquitoes retrieved.
Immature-stage mosquito collection and rearing
Aquatic habitats within a 50-m radius of the selected households were surveyed for immature stages, with the collections made using dippers and pipettes [48]. The collected immature-stage mosquitoes were subsequently transported to temporary field insectaries in plastic jars where they were transferred into enamel trays containing water from their respective aquatic habitat and larval food; the trays were check each day for pupation. Pupae were collected into beakers containing water from the respective aquatic habitat and placed in rearing cages with cotton balls soaked in a 10% sugar solution at the top of the cages. When all the pupae emerged, the beakers were removed from the rearing cages.
Morphological identification and preservation of mosquito samples
All wild-caught adults and those reared from immature stages were sorted by genus and sex after anesthetization with 70% alcohol. Only female Anopheles and Aedes mosquitoes were counted and recorded. Female mosquitoes belonging to the genus Anopheles were identified morphologically to the species level. Anopheles mosquitoes with speckled legs, with maxillary palpus with two apical pale bands very broad, with speckling on palpus segment three and with second main dark area on wing vein one with two pale interruptions were identified as An. stephensi [49]. Wild-caught female adult Anopheles mosquitoes were sorted based on their abdominal status as freshly fed or unfed, then preserved individually in a 1.5-ml Eppendorf tube with holes at the top and sealed in Ziplock bags containing silica gel. The immature-stage (larvae and pupae) collections were pooled by the household and preserved in absolute ethanol for molecular identification of An. stephensi.
Molecular procedures
The head and thorax of mosquitoes were separated from the abdomen for molecular analyses carried out at the Armauer Hansen Research Institute (AHRI) and Jimma University (JU) Tropical Infectious Diseases Research Center (TIDRC) laboratories. The bisected parts or pooled immature-stage materials were homogenized using a BioSpec BeadBeater apparatus (Bead Homogenizer 96 Microplate; Biospec Products, Bartlesville, USA) in 150 µl of molecular-grade water with 0.2 mg of beads (diameter: 1.0 mm; composition: zirconia/silica; BioSpec Products) at 3800 rpm for 20 s. DNA was extracted from 50- and 100-µl samples of homogenate according to the manufacturer's instructions (Qiagen GmbH, Hilden, Germany) and used for the detection of Plasmodium infection (head-thorax), blood meal analysis (abdomen), molecular species identification (immature stages) and confirmation of morphological identification (adult stage).
A PCR endpoint assay targeting the internal transcribed spacer 2 region (ITS2) was performed for species identification of An. stephensi as described elsewhere [50]. Briefly, the PCR was performed using the ST-F (5’CGTATCTTTCCTCGCATCCA3’), U5.8S-F (5’ATCACTCGGCTCATGGATCG3’) and UD2-R (5’GCACTATCAAGCAACACGACT3’) universal primers in a total reaction mixture of 25 µl containing 1 µl of DNA template, 0.25 µM each of ST-F and U5.8S-F primers, 0.37 µM of the UD2-R primer, 0.25 µM of dNTPs, 1.5 µM of Mg and 0.5 µM of Taq polymerase). PCR cycling conditions were: 95 °C for 30 s; 30 cycles of 95 °C for 30 s, 55 °C for 30 s and 68 °C for 7 min; with a final extension step at 68 °C for 7 min. The identification of specimens as An. stephensi was based on the visualization of a 438-bp band following gel electrophoresis of the PCR products.
Detection of Plasmodium infection
Plasmodium parasites in wild-caught Anopheles mosquitoes were identified either by PCR (AHRI laboratory) or enzyme-linked immunosorbent assay (ELISA) (Jimma University TIDRC laboratory). The PCR used to detect Plasmodium parasites targeted the cytochrome c oxidase subunit 1 (COXI) mitochondrial gene, and the presence of the parasites was based on the visualization of a 540-bp region following gel electrophoresis of the PCR products, as described elsewhere [51, 52]. Briefly, the PCR was performed in a total reaction volume of 25 µl containing 3 µl of DNA template from the head and thorax, primers (0.25 µM), dNTPs (0.2 µM), Mg (1.5 µM) and 1 unit of Taq polymerase. For those samples which tested PCR-positive for COXI, a nested PCR was run targeting the small 18S subunit of P. falciparum and P. vivax [53]. The first amplification reaction (nested-1) was performed in a final reaction volume of 25 µl containing 3 µl of DNA template, and the second reaction was performed by using a 5 µl amplicon of nested-1. The presence of P. falciparum and P. vivax was confirmed upon visualization of 205- and 120-bp bands, respectively, following electrophoresis of the PCR products on 1% agarose gel.
To detect Plasmodium via ELISA, the head-thorax of each mosquito was separated from the abdomen and ground in blocking buffer containing IGEPAL CA-630 (Sigma-Aldrich, St. Louis, MO, USA) in a 1.5-ml grinding tube. Antibodies against the circumsporozoite protein (CSP) of P. falciparum, P. vivax-210 (Pv-210) and P. vivax-247 (Pv-247) were detected using a sandwich CSP-ELISA [54, 55]. More specifically, 50 μl of species-specific capture monoclonal antibody (mAb) was added to each well of a micro-ELISA plate. The binding sites were blocked by adding 200 µl of blocking buffer and incubating the plate for 1 h at room temperature. Then, 50 μl of mosquito homogenate or control sample was added to the respective labeled wells. The plates were subsequently covered and incubated for 2 h at room temperature, following which 50 μl of peroxidase-linked mAb were then added to the wells and the plates incubated for 1 h at room temperature. Finally, 100 µl of peroxidase substrate solution (ABTS [2,2’-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid]; Kirkegaard and Perry Laboratories [KPL], Gaithersburg, MD, USA) was added and the plate incubated for 30 min for P. falciparum and 60 min for P. vivax. Between the addition of each reagent or sample and the incubation, the wells were shaken and washed. Absorbance was read at 405–411 nm using an ELISA reader (model ELX800; BioTek, Winooski, VT, USA) after 30 and 60 min of incubation for P. falciparum and P. vivax, respectively. Samples with a value higher than twofold of the mean absorbance value of the negative controls were considered to be positive for Plasmodium parasites.
Detection of blood meal sources
The blood meal source of wild-caught, freshly blood-fed female Anopheles mosquitoes was analyzed by PCR (AHRI laboratory) or ELISA (Jimma University TIDRC laboratory). For the PCR, the extracted DNA from the abdominal region of female Anopheles was analyzed by a multiplex PCR assay as previously described [56]. The PCR assay was performed in a reaction volume of 25 µl containing universal vertebrate-specific and species-specific primers for pigs, humans, goats, dogs and cows [0.2 μM of each primer], 1× GoTAQ (Promega, Madison, WI, USA), 1.5 mM MgCl2, 0.2 mM dNTPs and 13.85 µl of molecular-grade water. The PCR amplification conditions were: 95 °C for 5 min; 40 cycles of 95 °C for 60 s, 57 °C for 60 s and 72 °C for 60 s; with a final extension of 72 °C for 7 min. The PCR products were confirmed by gel electrophoresis.
For the ELISA, the abdomens of freshly fed female Anopheles mosquitoes were homogenized in phosphate-buffered saline (PBS) in a 1.5-ml grinding tube following a standard protocol [55]. More specifically, 100 μl of homogenized sample or control was loaded added into a well of an ELISA plate and the plate incubated for 2 h at room temperature, following which the wells were washed 3 times with 200 µl of PBS-Tween 20 solution. Then, 50 µl of host-specific peroxidase-labeled mAb of human, bovine and goat (Sigma-Aldrich) was added and incubated for 1 h in the dark, followed by 3 washes with 200 µl of PBS–Tween 20, with the plate shaken 5 times with each wash. Finally, 100 µl of ABTS was added to each well as the substrate for the peroxidase enzyme, and the mixture was incubated for 30 min. The plates were observed visually, and their optical density was read at 405–414 nm using an ELISA reader (model ELX800; BioTek). Samples with a value higher than twofold the mean absorbance value of the negative controls were considered to be positive for Plasmodium parasites.
Data management and statistical analysis
The data were collected using tablets with data forms developed in REDCap [57, 58] and uploaded to the AHRI data server on a daily basis. Data were downloaded and cleaned using Microsoft Excel (Microsoft Corp., Redmond, WA, USA), and Microsoft Excel and Stata software release 14 (StataCorp LLC, College Station, TX, USA) were used for analysis. Only Anopheles mosquito catches identified to species level were included in the statistical analyses. Site positivity proportion was determined by dividing the number of sites at which Anopheles species were identified in at least one round of entomological surveys by the number of sites surveyed. To estimate catches per method of collection, method-specific catches of species were divided by the total number of that species caught. Relative abundance of Anopheles species was investigated per site as catches of specific species divided by the total adult-stage collections per site. The mean number of catches per trap was determined by considering the number of traps in all collection rounds. Household exposure was defined as the presence of either immature or adult stages of An. stephensi in a 50-m radius of surveyed households. The level of household-exposure was estimated as the fraction of the surveyed households per number of households detected with An. stephensi. Mixed blood meal sources were included in the nominator to determine the blood meal indices. Sporozoite rate was determined as the fraction of Anopheles species tested per that species detected with Plasmodium parasite by PCR and ELISA.
Results
Anopheles mosquito fauna and abundance
Among the study sites, 96.2% (25/26) were positive for Anopheles mosquitoes, including An. stephensi, and 99.2% (5353/5398) of the total catches were identified to species level. Of the total adult Anopheles catches, 86.6% (1612/1862) were collected by CDC LTs, and 13.4% (250/1862) were collected by Prokopack aspirators (Fig. 4), with 79.5% of adult catches identified as An. arabeinsis, 7.7% as An. stephensi and 7.4% as An. pharoensis. Anopheles coustani, An. tenebrosus, An. fuenstus and An. rufipes accounted for only 2.5%, 1.8%, 1.1% and 0.1% of adult catches, respectively. Anopheles stephensi was predominant in four sites of the 26 surveyed (Babile, Kebri Dehar, Danan and Modjo), with 77.3–100% relative abundance. Among the immature-stage collections, An. stephensi was predominant in five sites of the 26 surveyed (Babile, Kebri Dehar, Danan, Dubti, and Modjo), with 75–100% relative abundance. Anopheles arabiensis was the predominant species across most sites (20/26) (Table 1). In two study sites, Anopheles mosquitoes were either not found (Kebri Beyah) or not identified morphologically (Yabelo) due to damage during collections.
Spatiotemporal distribution of An. stephensi
In four rounds of entomological surveys, 30.8% (8/26) of the sites were positive for An. stephensi, immature and/or adult stages. Both adult and immature stages of An. stephensi were detected at six of these positive sites (Babile, Danan, Dubti, Jiga, Kebri Dehar, and Modjo), while at the remaining two sites either adults (Assosa) or immature stages (Ataye) were detected (Table 2). In four of the An. stephensi-positive sites, adult or immature stages were recorded every round. The mean number of wild-caught adult An. stephensi per trap was 0.15 (CDC LT: 0.04 per trap night; Prokopack aspirator: 0.38 per collection) (Additional file 1: Table S2). Anopheles stephensi larvae were collected from a range of artificial and natural aquatic habitats.
Household exposure to An. stephensi
During additional extensive entomological sampling in and around households, An. stephensi was detected in three of the four urban centers, namely Danan, Awash Sebat Kilo and Metehara. Among the 50 surveyed households, 30% (15/50) were positive for An. stephensi in Danan. The household positivity rates were 26% (13/50) and 18% (9/50) in Awash Sebat Kilo and Metehara, respectively. Anopheles stephensi was not detected in any of the sampled households in Jiga during the sampling period (Table 3).
Species identification by molecular methods
A total of 80 field-caught adult mosquitoes identified as An. stephensi based on morphological characteristics and 28 pooled immature-stage mosquito samples were screened using molecular methods to confirm species identity. Of the 80 adult samples identified morphologically as An. stephensi, the molecular assays confirmed the identity as An. stephensi in 78 samples; in the remaining two samples, the products were either not amplified or were found to be non-An. stephensi. Among the pooled immature-stage mosquito samples, 82.1% (23/28 pools) were confirmed to contain An. stephensi.
Detection of blood meal sources
Blood meals were analyzed in 784 Anopheles mosquitoes, of which 386 samples were assayed by multiplex PCR (Additional file 1: Fig. S1) and 398 specimens were analyzed using an ELISA. Among Anopheles mosquitoes identified with a blood meal source, 76.9% (50/65) of An. stephensi and 56.2% (181/322) of An. arabiensis were found with single host blood. The blood meal indices for An. stephensi were 69.2% for bovines (BBI), 32.3% for ovines (OBI) and 24.6% for humans (HBI), including the mixed blood meal sources (Table 4); for An. arabiensis, the blood meal indices were 65.4% for bovines, 46.7% for ovines and 35.8% for humans.
Detection of Plasmodium infection
Among the 1847 Anopheles mosquitoes examined for Plasmodium infection, 69.4% (1282/1847) and 30.6% (565/1847) were assessed using PCR (Additional file 1: Fig. S2) and ELISA, respectively (Table 5). None of the 197 samples identified as An. stephensi were found to be infected with Plasmodium parasites. Overall, the Plasmodium sporozoite rate of An. arabiensis was 4.3% (62/1434), of which 16.1% (10/62) were P. falciparum and 83.9% (52/62) were P. vivax. In An. pharoensis, the Plasmodium sporozoite rate was 6.7% (8/119), of which 12.5% (1/8) were P. falciparum and 87.5% (7/8) were P. vivax. Two other Anopheles mosquito species were detected with sporozoites of P. vivax: An. coustani (12.8%, 5/39) and An. funestus (11.5%, 3/26).
Discussion
The results of the present study add to the body of available evidence on the distribution and abundance of invasive An. stephensi in Ethiopia. Entomological surveillance in 26 urban centers between 2021 and 2023 revealed that An. arabiensis was the predominant Anopheles species in the catches, accounting for 79.5% of all collections, followed by An. stephensi, accounting for 7.7% of the total Anopheles catches. The relative abundance of adult An. stephensi was greater than that of An. arabiensis in Babile, Kebri Dehar, Danan and Modjo. Modjo is located along the main ground transportation route or corridor that connects Ethiopia to Djibouti [36]. Generally, adult An. stephensi collections were low (mean: 0.15 catches/trap), and most of the immature-stage collections were from artificial aquatic habitats.
Since the first detection of An. stephensi in eastern Ethiopia in 2016, new positive sites have been identified in subsequent surveys [36, 37, 59]. In line with these findings, we detected An. stephensi in western Ethiopia (Assosa), in an area bordering Sudan, which might indicate the continued spread of this species. We also found An. stephensi at all previously reported sites, as well as at new sites along its purported invasion route. However, the site positivity of An. stephensi was relatively low (8/26 sampled sites) compared to that reported in previous studies. A study in 2020 that covered 10 sites in eastern Ethiopia reported the presence of An. stephensi at all sites [36]. Similarly, sampling at 21 sites between 2018 and 2020 revealed 61.9% positivity for An. stephensi [37]. Another study conducted by the PMI Vector Link Ethiopia project showed the presence of An. stephensi in 16 urban settings, of which nine (56.3%) sites were newly positive for An. stephensi [60]. One explanation for the differences in results might be the selection of study sites; most of the collection points in these previous studies were purposefully chosen to detect An. stephensi, while the current study followed substantial random steps in the selection of study sites and collection points within sites. Our approach has the advantage of providing unbiased distribution estimates, but it does reduce the probability of detection. In addition, some of our study sites were located far from major transportation corridors [61], which are considered as the main invasion routes.
In line with the findings of other studies [16, 36], we also noted that An. stephensi was more readily detectable as immature stages than as adults in most of the positive sites. The highest proportion of An. stephensi collections (85.7%) was obtained as immature stages (larvae and pupae) in aquatic habitats. A range of aquatic habitats were positive; for example, in Dubti, immature stages of An. stephensi were detected in both artificial habitats (water tanks, barrels, buckets, tires) and natural habitats (ponds, streams, swamps and marshes). It has been reported that An. stephensi can breed in various aquatic habitats with differing physicochemical characteristics, such as salinity and turbidity [62]. In Modjo, Danan, Kebri Dehar and Babile, immature stages of An. stephensi were detected only in artificial habitats. This variation highlights how larval source management of An. stephensi, which has been recommended by the WHO [63] and is being implemented by PMI VectorLink and others, will be more complex than simply targeting container habitats.
In the current study, An. arabiensis was the most abundant species at 20/26 sites, which is in line with the findings of other studies showing that this species is still the predominant malaria vector in different eco-epidemiological settings of Ethiopia [20, 25, 64]. Even though An. arabiensis is considered less adapted to urban ecology [65], our findings suggest that it is likely to be the primary malaria vector in urban centers in Ethiopia. The other Anopheles species collected in this study were An. pharoensis, An. coustani, An. funestus, An. tenebrosus and An. rufipes, which together accounted for 12.8% of the total adult Anopheles catches. Of these five species, An. pharoensis and An. funestus have been reported to be secondary or suspected malaria vectors in Ethiopia [66]. We detected An. pharoensis infected with P. falciparum or P. vivax at one and five of our study sites, respectively, while An. coustani and An. funestus were detected with P. vivax sporozoites across four and three of the study sites, respectively.
The level of household exposure to An. stephensi was heterogeneous across the study sites, with household positivity for both (adults and immature) stages ranging from 18% in Metehara to 30% in Danan. The level of household exposure to adult An. stephensi was highest in the region where this species was first reported as an invasive species (Danan) and lower in more central parts of the country (Awash Sebat Kilo and Metehara). A similar trend was observed for household exposure to the immature stages of An. stephensi. These results could be due to well-established populations of An. stephensi in areas where it was first reported since its invasion.
Our findings reveal that An. stephensi prefers non-human vertebrate hosts for their blood meal. The most prevalent blood meal among An. stephensi detected with sources of blood was cattle (69.2%), followed by goats (32.3%). These findings are consistent with results previously reported in Ethiopia [16, 37] and India [67], which showed that most An. stephensi fed on livestock. In the present study, one-third of An. stephensi fed on unidentified blood meal sources, which might be due to a lack of host antibodies or primers for blood meal analysis. It is noteworthy that at some of the study sites, especially in eastern Ethiopia, the most readily available animals were camels and that these sites were where most of the tested An. stephensi were collected. Despite the relatively high non-human vertebrate host blood meal indices, 24.6% of An. stephensi were found with human blood, including mixed blood meal sources. The blood meal source of vectors might be affected by multiple factors, including host availability and proximity, possibly explaining why 76.9% of the An. stephensi and 56.2% of An. arabiensis fed on a single blood meal source of either an animal or a human host.
Of the 197 screened An. stephensi, none were detected with Plasmodium parasites. These findings are similar to those of another study in which none of the tested An. stephensi was positive for Plasmodium [36]. However, a study conducted in 2019 in Awash Sebat Kilo reported an infection rate of 2.8% for P. vivax and 1.4% for P. falciparum, based on analysis of homogenates of whole mosquitoes [16]. The authors of another study in which the heads and thoraxes was used to detect Plasmodium by ELISA reported that the sporozoite rate was 0.5% in Dire Dawa and 0.3% in Kebri Dehar for P. vivax [37]. The most recent study, from Dire Dawa, implicated An. stephensi in an outbreak and detected a P. falciparum sporozoite rate of 1.2% [38]. It should be noted that our study might be limited in its ability to elucidate An. stephensi sporozoite infection, as a large proportion of the adult catches (91/146) were from a single aquatic site using the Prokopack aspirator.
There are a number of limitations to our study. The limited number of An. stephensi adults caught indicates the need for further studies to investigate more efficient trapping methods. We employed both PCR and ELISA for detecting blood meal sources and Plasmodium infection in Anopheles mosquitoes, which might limit the direct comparability of our findings across study sites. The collection rounds did not directly coincide with the malaria transmission seasons in Ethiopia, and some of the sites were excluded due to civil unrest.
Conclusions
Our findings reveal an expansion of An. stephensi into a new western geographical range and its transition to predominant species status in some areas where it was first detected. These results highlight the need for enhanced entomological surveillance with efficient traps to determine the bionomics and relative contribution of An. stephensi for malaria transmission in the region. In the meantime, the plan set forth to limit the spread and contain An. stephensi establishment should be put into action.
Availability of data and materials
The datasets reported herein were shared with stakeholders, including the Ministry of Health and the WHO. All the data from the CEASE project will be made publicly available upon completion of the study.
Abbreviations
- AHRI:
-
Armauer Hansen Research Institute
- BBI:
-
Bovine blood index
- CDC LTs:
-
U.S. Centers for Disease Control and Prevention light traps
- COXI:
-
Cytochrome c oxidase subunit 1
- CSP:
-
Circumsporozoite protein
- ELISA:
-
Enzyme-linked immunosorbent assay
- HBI:
-
Human blood index
- ITS2:
-
Internal transcribed spacer 2 region
- mAb:
-
Monoclonal antibody
- OBI:
-
Ovine blood index
- PBS:
-
Phosphate-buffered saline
- REDCap:
-
Research Electronic Data Capture
References
WHO. World malaria report 2023. 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023. Accessed 10 Dec 2023.
WHO. World malaria report 2020: 20 years of global progress and challenges. 2020. https://www.who.int/publications/i/item/9789240015791. Accessed 22 Dec 2021.
WHO. World malaria report 2021. 2021. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021. Accessed 23 Feb 2022.
WHO. World malaria report 2022. 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022. Accessed 15 Feb 2023.
U.S. President's Malaria Initiative. Ethiopia Malaria Operational Plan FY 2019. 2019. Washington, DC. https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2021/03/fy-2019-ethiopia-malaria-operational-plan.pdf. Accessed 30 Feb 2022.
Yalew AW. Achievements, gaps, and emerging challenges in controlling malaria in Ethiopia. Front Trop Dis. 2022. https://doi.org/10.3389/fitd.2021.771030.
Taffese HS, Hemming-Schroeder E, Koepfli C, Tesfaye G, Lee MC, Kazura J, et al. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect Dis Poverty. 2018;7:103.
Federal Democratic Republic of Ethiopia Ministry of Health. Ethiopia malaria elimination strategic plan: 2021–2025. 2020. http://repository.iifphc.org/handle/123456789/1526. Accessed 11 Feb 2021.
Tefera S, Bekele T, Getahun K, Negash A, Ketema T. The changing malaria trend and control efforts in Oromia special zone, Amhara Regional State, northeast Ethiopia. Malar J. 2022;21:128.
Mulugeta A, Assefa A, Eshetie A, Asmare B, Birhanie M, Gelaw Y. Six-year trend analysis of malaria prevalence at University of Gondar Specialized Referral Hospital, northwest Ethiopia, from 2014 to 2019. Sci Rep. 2022;12.
Teka H, Golassa L, Medhin G, Balkew M, Sisay C, Gadisa E, et al. Trend analysis of malaria in urban settings in Ethiopia from 2014 to 2019. Malar J. 2023;22:235.
Federal Democratic Republic of Ethiopia Ministry of Health. National malaria elimination roadmap. 2017. http://repository.iifphc.org/bitstream/handle/123456789/1438/Malaria-Elimination-Roadmap-Ethiopia%202017.pdf. Accessed 11 Feb 2021.
Debash H, Nigatie M, Bisetegn H, Feleke DG, Tesfaw G, Amha A, et al. Malaria surveillance, outbreak investigation, response and its determinant factors in Waghemra zone, northeast Ethiopia: unmatched case-control study. Sci Rep. 2023;13:9938.https://doi.org/10.1038/s41598-023-36918-3.
Messenger LA, Shililu J, Irish SR, Anshebo GY, Tesfaye AG, Ye-Ebiyo Y, et al. Insecticide resistance in Anopheles arabiensis from Ethiopia (2012–2016): a nationwide study for insecticide resistance monitoring. Malar J. 2017;16:469.
Balkew M, Elhassen I, Ibrahim M, Gebre-Michael T, Engers H. Very high DDT-resistant population of Anopheles pharoensis Theobald (Diptera: Culicidae) from Gorgora, northern Ethiopia. Parasite. 2006;13:327–9.
Tadesse FG, Ashine T, Teka H, Esayas E, Messenger LA, Chali W, et al. Anopheles stephensi mosquitoes as vectors of Plasmodium vivax and P. falciparum, Horn of Africa, 2019. Emerg Infect Dis. 2021;27:603–7.
Carter TE, Yared S, Gebresilassie A, Bonnell V, Damodaran L, Lopez K, et al. First detection of Anopheles stephensi Liston, 1901 (Diptera: culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018;188:180–6.
Kyalo D, Amratia P, Mundia CW, Mbogo CM, Coetzee M, Snow RW. A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898–2016. Wellcome Open Res. 2017;2:57.
Irish SR, Kyalo D, Snow RW, Coetzee M. Updated list of Anopheles species (Diptera: Culicidae) by country in the Afrotropical Region and associated islands. Zootaxa. 2020;4747:3.
Adugna F, Wale M, Nibret E. Review of Anopheles mosquito species, abundance, and distribution in Ethiopia. J Trop Med. 2021;2021:7.
Massebo F, Balkew M, Gebre-Michael T, Lindtjorn B. Blood meal origins and insecticide susceptibility of Anopheles arabiensis from Chano in southwest Ethiopia. Parasit Vectors. 2013;6:44.
Animut A, Balkew M, Gebre-Michael T, Lindtjorn B. Blood meal sources and entomological inoculation rates of anophelines along a highland altitudinal transect in south-central Ethiopia. Malar J. 2013;12:76.
Kibret S, Wilson GG, Tekie H, Petros B. Increased malaria transmission around irrigation schemes in Ethiopia and the potential of canal water management for malaria vector control. Malar J. 2014;13:360.
Kenea O, Balkew M, Tekie H, Gebre-Michael T, Deressa W, Loha E, et al. Human-biting activities of Anopheles species in south-central Ethiopia. Parasit Vectors. 2016;9:527.
Massebo F, Balkew M, Gebre-Michael T, Lindtjorn B. Entomologic inoculation rates of Anopheles arabiensis in southwestern Ethiopia. Am J Trop Med Hyg. 2013;89:466–73.
Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89.
Faulde MK, Rueda LM, Khaireh BA. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop. 2014;139:39–43.
Ahmed A, Pignatelli P, Elaagip A, Abdel Hamid MM, Alrahman OF, Weetman D. Invasive malaria vector Anopheles stephensi mosquitoes in Sudan, 2016–2018. Emerg Infect Dis. 2021;27:2952–4.
Ali S, Samake JN, Spear J, Carter TE. Morphological identification and genetic characterization of Anopheles stephensi in Somaliland. Parasit Vectors. 2022;15:247.
WHO. Partners convening: a regional response to the invasion of Anopheles stephensi in Africa: meeting report, 8–10 March 2023. 2023. https://www.who.int/publications/i/item/9789240075535. Accessed 19 Jul 2023.
Sorichetta A, Bird TJ, Ruktanonchai NW, Zu Erbach-Schoenberg E, Pezzulo C, Tejedor N, et al. Mapping internal connectivity through human migration in malaria endemic countries. Sci Data. 2016;3:160066. https://doi.org/10.1038/sdata.2016.66.
Wesolowski A, Eagle N, Tatem AJ, Smith DL, Noor AM, Snow RW, et al. Quantifying the impact of human mobility on malaria. Science. 2012;338:267–70.
Guerra CA, Citron DT, Garcia GA, Smith DL. Characterising malaria connectivity using malaria indicator survey data. Malar J. 2019;18:440.
Gayan Dharmasiri AG, Perera AY, Harishchandra J, Herath H, Aravindan K, Jayasooriya HTR, et al. First record of Anopheles stephensi in Sri Lanka: a potential challenge for prevention of malaria reintroduction. Malar J. 2017;16:326.
Regional Office for South-East Asia—WHO. Malaria-free Sri Lanka. 2016. https://iris.who.int/bitstream/handle/10665/251824/9789290225423-eng.pdf?sequence=1. Accessed 30 Dec 2019.
Balkew M, Mumba P, Dengela D, Yohannes G, Getachew D, Yared S, et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit Vectors. 2020;13:35.
Balkew M, Mumba P, Yohannes G, Abiy E, Getachew D, Yared S, et al. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018–2020. Malar J. 2021;20:263.
Emiru T, Getachew D, Murphy M, Sedda L, Ejigu LA, Bulto MG, et al. Evidence for a role of Anopheles stephensi in the spread of drug and diagnosis-resistant malaria in Africa. Nat Med. 2023;29:3203–11.
Seyfarth M, Khaireh BA, Abdi AA, Bouh SM, Faulde MK. Five years following first detection of Anopheles stephensi (Diptera: Culicidae) in Djibouti, Horn of Africa: populations established-malaria emerging. Parasitol Res. 2019;118:725–32.
Santi VPD, Khaireh BA, Chiniard T, Pradines B, Taudon N, Larreche S, et al. Role of Anopheles stephensi mosquitoes in malaria outbreak, Djibouti, 2019. Emerg Infect Dis. 2021;27:1697–700.
WHO. Vector alert: Anopheles stephensi invasion and spread. 2019. https://www.who.int/news/item/26-08-2019-vector-alert-anopheles-stephensi-invasion-and-spread. Accessed 23 Sept 2019.
Sinka ME, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, et al. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci USA. 2020;117:24900–8.
Climate: Ethiopia, 2023. https://en.climate-data.org/africa/ethiopia-249/. Accessed 24 Oct 2023.
Sedda L, Lucas ER, Djogbenou LS, Edi AVC, Egyir-Yawson A, Kabula BI, et al. Improved spatial ecological sampling using open data and standardization: an example from malaria mosquito surveillance. J R Soc Interface. 2019;163:20180941. https://doi.org/10.1098/rsif.2018.0941.
Monteiro GM, Djogbénou LS, Donnelly MJ, Sedda L. Development and deployment of an improved Anopheles gambiae s.l. field surveillance by adaptive spatial sampling design. Front Ecol Evol. 2024;11. https://doi.org/10.3389/fevo.2023.1241617.
Maia MF, Robinson A, John A, Mgando J, Simfukwe E, Moore SJ. Comparison of the CDC Backpack aspirator and the Prokopack aspirator for sampling indoor- and outdoor-resting mosquitoes in southern Tanzania. Parasit Vectors. 2011;4:124.
Charlwood JD, Kessy E, Yohannes K, Protopopoff N, Rowland M, LeClair C. Studies on the resting behaviour and host choice of Anopheles gambiae and An. arabiensis from Muleba. Tanzania Med Vet Entomol. 2018;32:263–70.
WHO–Arican Region. Larval source management: a supplementary measure for malaria vector control: an operational manual. 2013. https://www.afro.who.int/publications/larval-source-management-supplementary-measure-malaria-vector-controloperational. Accessed 12 Dec 2016.
Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 2020;19:70.
Djadid ND, Gholizadeh S, Aghajari M, Zehi AH, Raeisi A, Zakeri S. Genetic analysis of rDNA-ITS2 and RAPD loci in field populations of the malaria vector, Anopheles stephensi (Diptera: Culicidae): implications for the control program in Iran. Acta Trop. 2006;97:65–74.
Snounou G, Singh B. Malaria methods and protocols: nested PCR analysis of Plasmodium parasites. In: Doolan DL, editor. Methods in molecular medicine, vol. 72. Totowa: Humana Press; 2002. p. 189–203.
Echeverry DF, Deason NA, Makuru V, Davidson J, Xiao H, Niedbalski J, et al. Fast and robust single PCR for Plasmodium sporozoite detection in mosquitoes using the cytochrome oxidase I gene. Malar J. 2017;16:230.
Schriefer ME, Sacci JB Jr, Wirtz RA, Azad AF. Detection of polymerase chain reaction-amplified malarial DNA in infected blood and individual mosquitoes. Exp Parasitol. 1991;73:311–31.
Wirtz RA, Sattabongkot J, Hall T, Burkot TR, Rosenberg R. Development and evaluation of an enzyme-linked immunosorbent assay for Plasmodium vivax-VK247 sporozoites. J Med Entomol. 1992;29:854–7.
Beier JC, Perkins PV, Wirtz RA, Whitmire RE, Mugambi M, Hockmeyer WT. Field evaluation of an enzyme-linked immunosorbent assay (ELISA) for Plasmodium falciparum sporozoite detection in anopheline mosquitoes from Kenya. Am J Trop Med Hyg. 1987;36:459–68.
Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005;73:336–42.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Balkew M, Mumba P, Yohannes G, Abiy E, Getachew D, Yared S, et al. Correction to: an update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018–2020. Malar J. 2021;20:331.
Project TPV. Anopheles stephensi in Ethiopia: potential impact and mitigation. Rockville: The PMI VectorLink Project/Abt Associates Inc; 2021.
Samake JN, Lavretsky P, Gunarathna I, Follis M, Brown JI, Ali S, et al. Population genomic analyses reveal population structure and major hubs of invasive Anopheles stephensi in the Horn of Africa. Mol Ecol. 2023;32:5695–708.
Jude P, Gunathilaka P, Fernando S, Premaratne P, Wickremasinghe A, Udayanga N, et al., editors. The range of salinity tolerance by Anopheles stephensi in Sri Lanka. Research Conference in Health Sciences, March 2021. Gangodawila, Nugegoda: Faculty of Allied Health Sciences at the University of Sri Jayewardenepura.
WHO. Vector alert: Anopheles stephensiinvasion and spread: Horn of Africa, the Republic of the Sudan and surrounding geographical areas, and Sri Lanka: information note. Geneva: WHO; 2019. https://iris.who.int/handle/10665/326595. Accessed 11 Feb 2021.
Eba K, Habtewold T, Yewhalaw D, Christophides GK, Duchateau L. Anopheles arabiensis hotspots along intermittent rivers drive malaria dynamics in semi-arid areas of central Ethiopia. Malar J. 2021;20:154.
Doumbe-Belisse P, Kopya E, Ngadjeu CS, Sonhafouo-Chiana N, Talipouo A, Djamouko-Djonkam L, et al. Urban malaria in sub-saharan Africa: dynamic of the vectorial system and the entomological inoculation rate. Malar J. 2021;20:364.
Tsegaye A, Demissew A, Hawaria D, Getachew H, Habtamu K, Asale A, et al. Susceptibility of primary, secondary and suspected vectors to Plasmodium vivax and Plasmodium falciparum infection in Ethiopia. Parasit Vectors. 2022;15:384.
Thomas S, Ravishankaran S, Justin NA, Asokan A, Mathai MT, Valecha N, et al. Resting and feeding preferences of Anopheles stephensi in an urban setting, perennial for malaria. Malar J. 2017;16:111.
Acknowledgements
The authors would like to thank the study households and the field research team, including the entomology technicians, the community facilitators and the drivers at AHRI and Jimma University, for their support during sample collection and transportation. We are also grateful to the federal, regional and district health officers for their collaboration during the study period.
Funding
This work was supported by the National Institute for Health Research (NIHR) (using the UK’s Official Development Assistance [ODA] Funding) and Wellcome (220870_Z_20_Z) under the National Institutes of Health-Wellcome Partnership for Global Health Research. The views expressed are those of the authors and not necessarily those of Wellcome, the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
MJD, DW, EG, DY, ALW, TA and AE conceived the study. MJD, DW, EG, DY, ALW, AR, YA, TA, AE, ES, EZ, FM, LS, AEp and AZ designed the study. TA and AE drafted the manuscript. MJD, DW, EG, DY, ALW, AR, AEp, YA, ES, FM, EE, NN, AZ, GA, DD and HS contributed to the finalization of the manuscript. TA, AE, YA, AK, NN, EE, AD, EA, KW, EZ and MA conducted the field data collection and morphological identification of the mosquitoes. TA, AE, YA, NN, TT, SWB, AD and JDD conducted the molecular laboratory work. TA, AE, MGB, BL and FAK performed the data management and analysis. AR, YA, KW and ES managed the project. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was received from the Institutional Review Board (IRB) of the Institute of Health of Jimma University (JUIH/IRB/575/23), AHRI/ALERT ethics committee (PO/16/21), the National Research Ethics Review Committee (NRERC) of the Federal Ministry of Education of Ethiopia (Reference: 7/1-229/m259/35) and the Liverpool School of Tropical Medicine (Reference: 21-013). Written informed consent to participate in the study was obtained from the heads of household (or their designates) for all participating households.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Study sites in Ethiopia, 2021–2023. Table S2: Occurrence and abundance of An. stephensi across eight positive urban centers by round and stage of collection, Ethiopia, 2021–2023. Figure S1. Multiplex PCR for blood meal source detection in freshly fed wild-caught female Anopheles mosquitoes. Figure S2. Gel images of COXI and nested PCR for detecting Plasmodium infection.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ashine, T., Eyasu, A., Asmamaw, Y. et al. Spatiotemporal distribution and bionomics of Anopheles stephensi in different eco-epidemiological settings in Ethiopia. Parasites Vectors 17, 166 (2024). https://doi.org/10.1186/s13071-024-06243-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06243-3